One-Pot Synthesis of Lactose Derivatives from Whey Permeate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
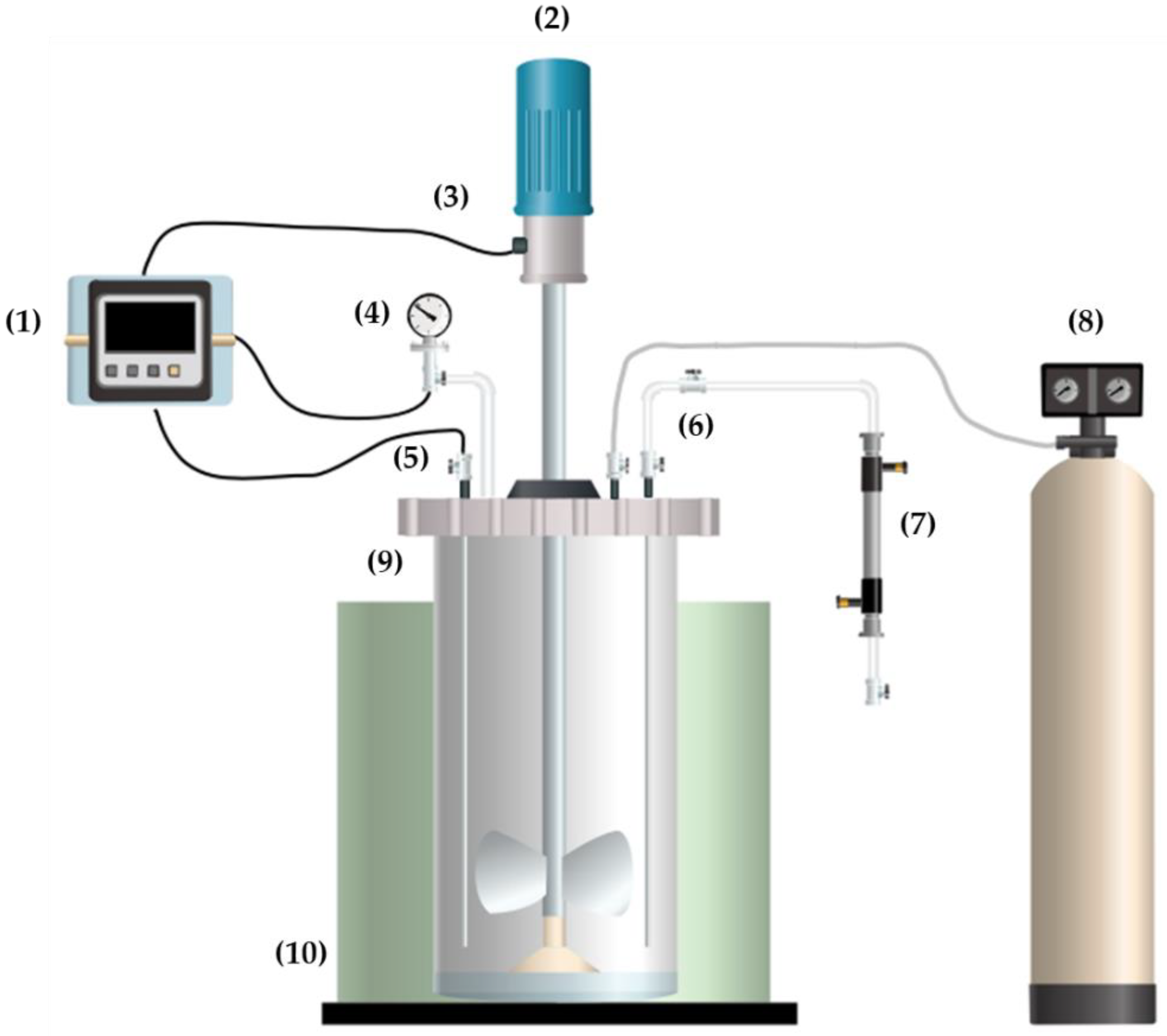
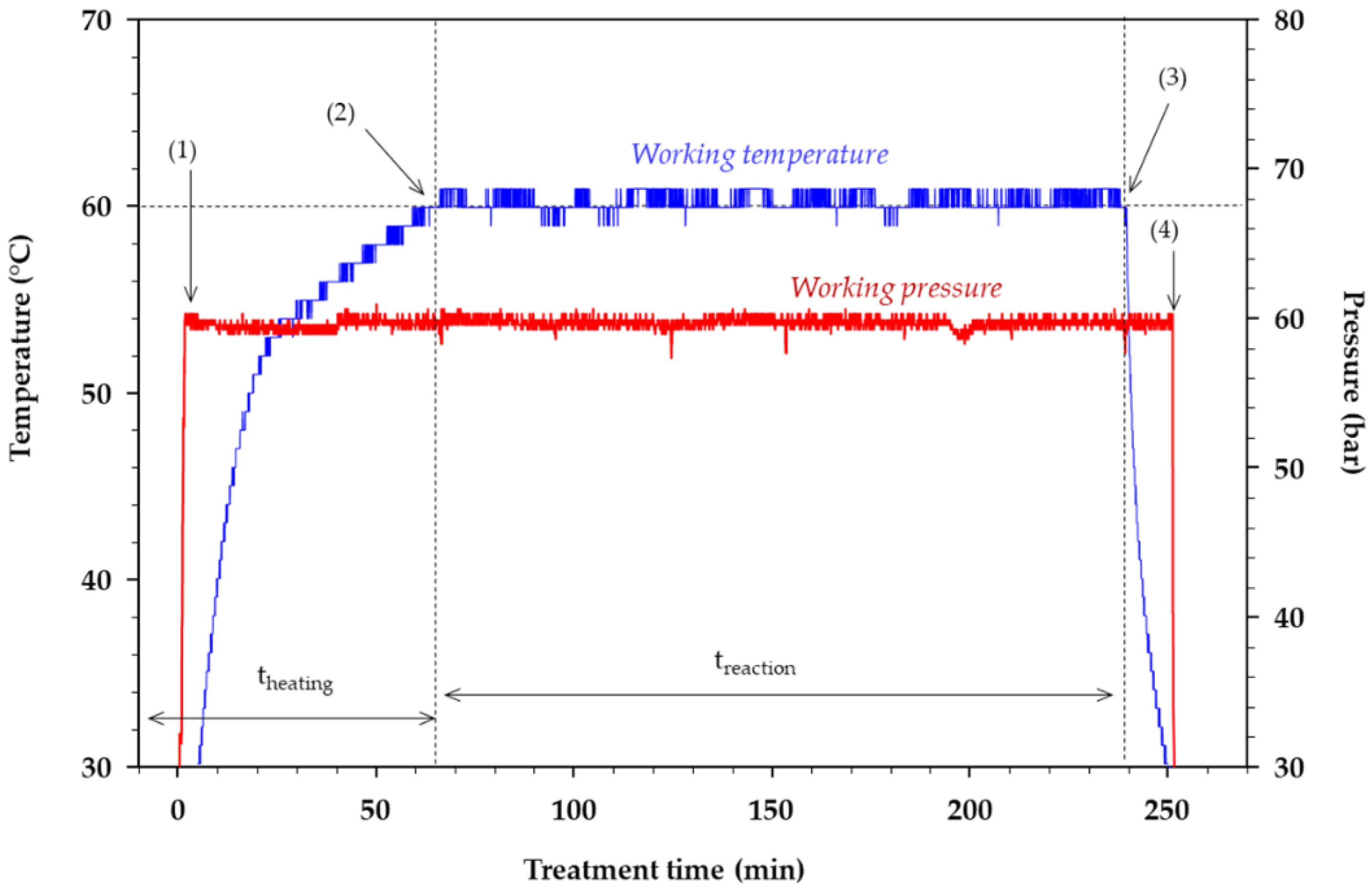
2.3. Experimental Treatments
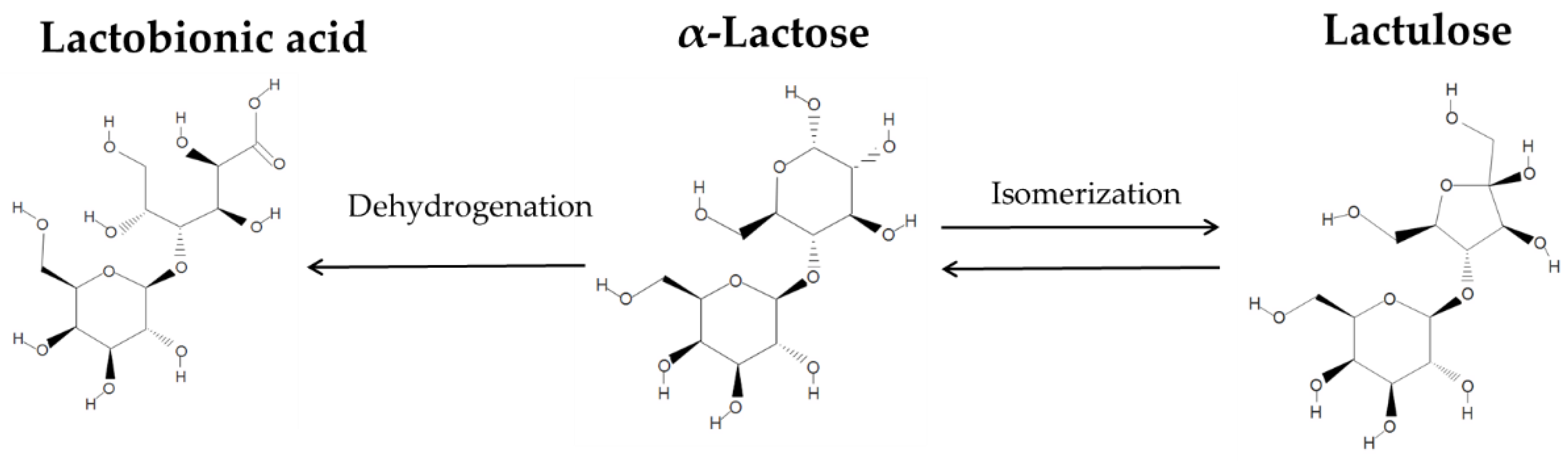
2.4. One-Pot Synthesis
2.5. Quantification of Reaction Products
2.6. Quantification of Organic Acids
2.7. Data Analysis
3. Results and Discussion
3.1. Reaction Conditions
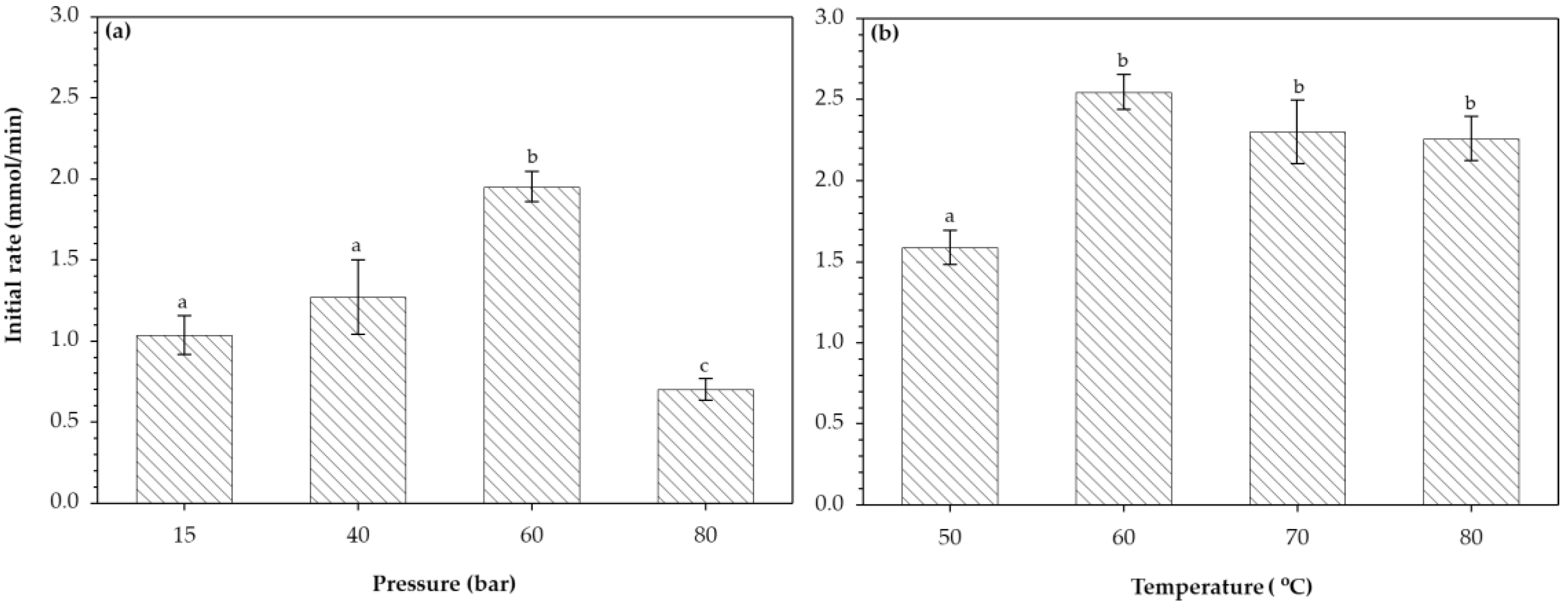
3.2. Initial Rates
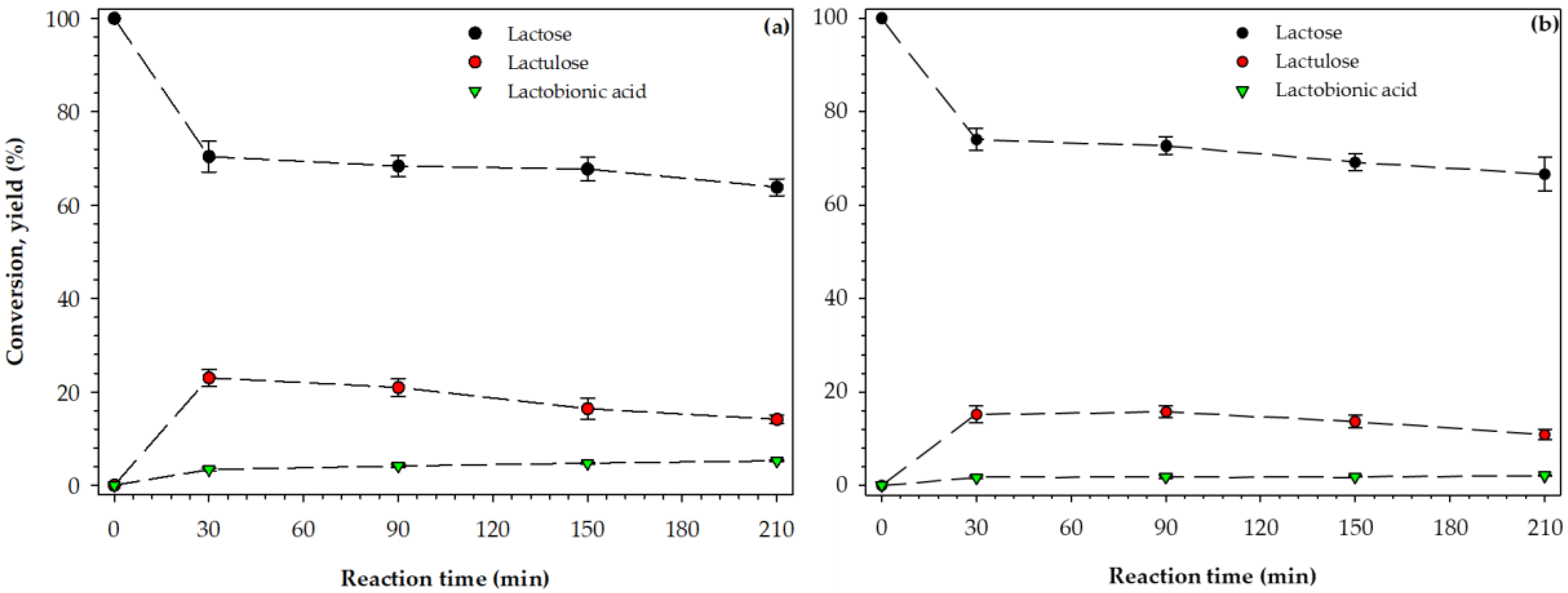
3.3. Conversion and Yield
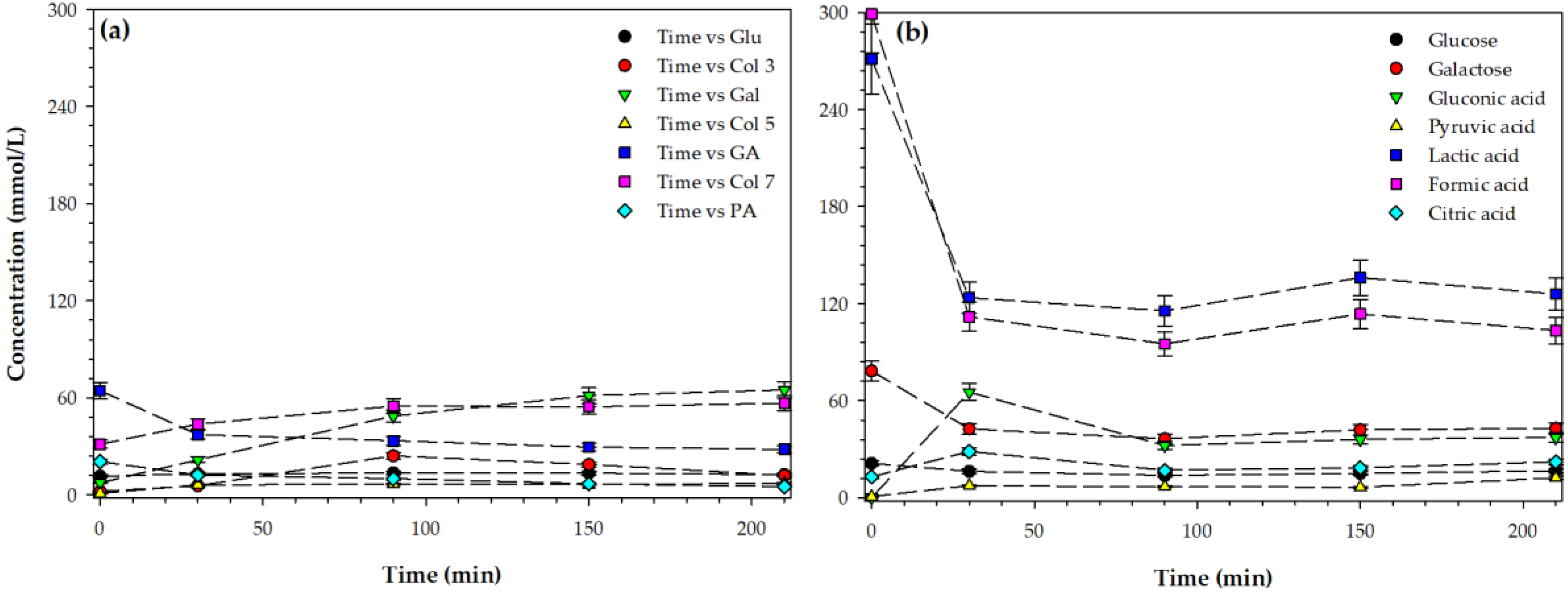
3.4. Formation of Glucose, Galactose, and Organic Acids
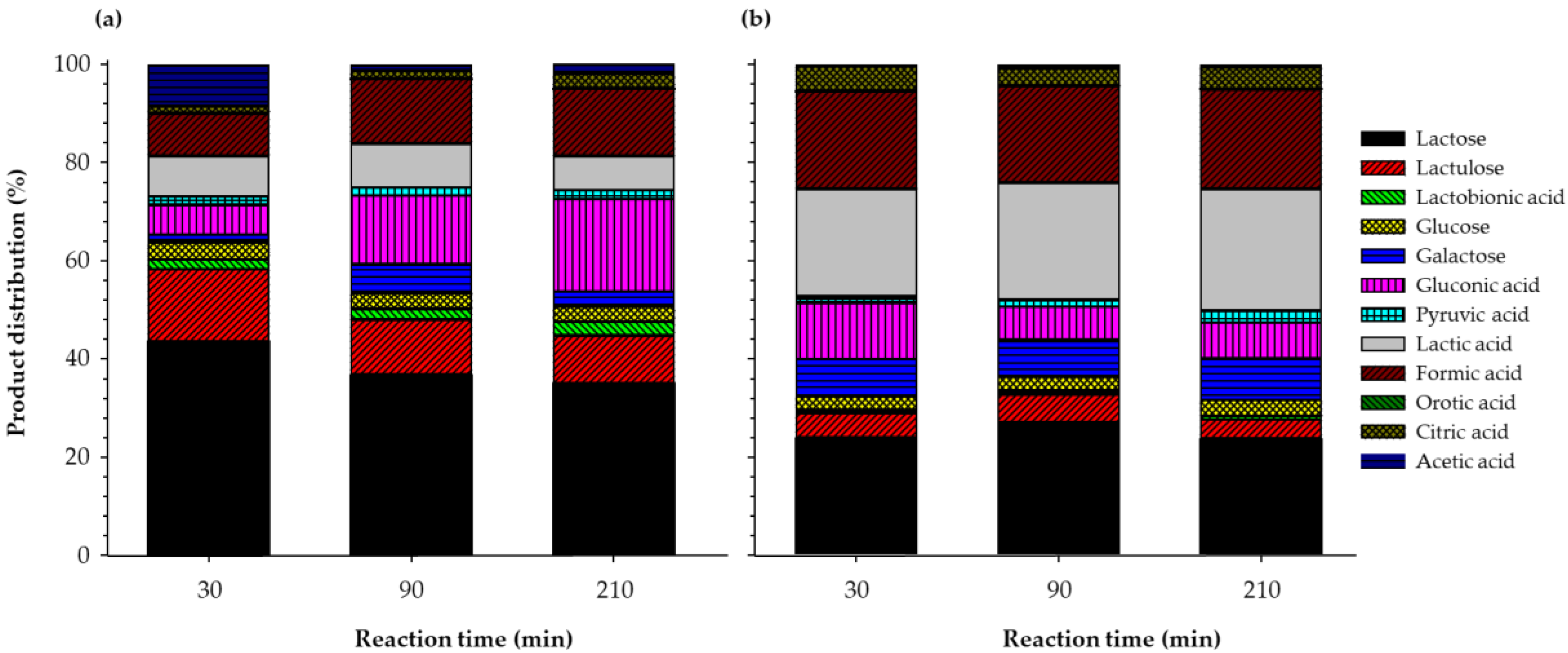
3.5. Product Distribution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nath, A.; Verasztó, B.; Basak, S.; Koris, A.; Kovacs, Z.; Vatai, G. Synthesis of lactose-derived nutraceuticals from dairy waste whey—A review. Food Bioprocess Technol. 2016, 9, 16–48. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey-ing up the options—Yesterday, today and tomorrow. Int. Dairy J. 2015, 48, 2–14. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Cheng, S.; Martínez-Monteagudo, S.I. Hydrogenation of lactose for the production of lactitol. Asia-Pac. J. Chem. Eng. 2019, 14, e2275. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Haase, G.; Jelen, P. Lactose: Crystallization, hydrolysis and value-added derivatives. Int. Dairy J. 2008, 18, 685–694. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Enteshari, M.; Metzger, L. Lactitol: Production, properties, and applications. Trends Food Sci. Technol. 2019, 83, 18–191. [Google Scholar] [CrossRef]
- Seki, N.; Saito, H. Lactose as a source for lactulose and other functional lactose derivatives. Int. Dairy J. 2012, 22, 110–115. [Google Scholar] [CrossRef]
- Schuster-Wolff-Bühring, R.; Fischer, L.; Hinrichs, J. Production and physiological action of the disaccharide lactulose. Int. Dairy J. 2010, 20, 731–741. [Google Scholar] [CrossRef]
- Aider, M.; de Halleux, D. Isomerization of lactose and lactulose production: Review. Trends Food Sci. Technol. 2007, 18, 356–364. [Google Scholar] [CrossRef]
- Gutiérrez, L.-F.; Hamoudi, S.; Belkacemi, K. Lactobionic acid: A high value-added lactose derivative for food and pharmaceutical applications. Int. Dairy J. 2012, 26, 103–111. [Google Scholar] [CrossRef]
- Chia, Y.N.; Latusek, M.P.; Holles, J.H. Catalytic wet oxidation of lactose. Ind. Eng. Chem. Res. 2008, 47, 4049–4055. [Google Scholar] [CrossRef][Green Version]
- Cheng, S.; Metzger, L.E.; Martínez-Monteagudo, S.I. One-pot synthesis of sweetening syrup from lactose. Sci. Rep. 2020, 10, 2730. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Hummel, M.; Dahal, B.; Gu, Z.; Kharel, P.; Martínez-Monteagudo, S.I. A two-step process for the synthesis of sweetening syrup from aqueous lactose. LWT 2020, 117, 108659. [Google Scholar] [CrossRef]
- Zaccheria, F.; Mariani, M.; Scotti, N.; Psaro, R.; Ravasio, N. Catalytic upgrading of lactose: A rest raw material from the dairy industry. Green Chem. 2017, 19, 1904–1910. [Google Scholar] [CrossRef]
- Gallezot, P. Alternative Value Chains for Biomass Conversion to Chemicals. Top. Catal. 2010, 53, 1209–1213. [Google Scholar] [CrossRef]
- Murzina, E.V.; Tokarev, A.V.; Kordás, K.; Karhu, H.; Mikkola, J.P.; Murzin, D. D-lactose oxidation over gold catalysts. Catal. Today 2008, 131, 385–392. [Google Scholar] [CrossRef]
- Enteshari, M.; Martínez-Monteagudo, S.I. Catalytic synthesis of lactose derivatives from whey permeate. J. Dairy Sci. 2019, 102 (Suppl. S1), 146. [Google Scholar]
- AOAC International. International Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Enteshari, M.; Martínez-Monteagudo, S.I. Subcritical hydrolysis of ice-cream wastewater: Modeling and functional properties of hydrolysate. Food Bioprod. Process. 2018, 111, 104–113. [Google Scholar] [CrossRef]
- Zeppa, G.; Conterno, L.; Gerbi, V. Determination of Organic Acids, Sugars, Diacetyl, and Acetoin in Cheese by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2001, 49, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Monteagudo, S.I.; Saldaña, M.D.A. Chemical Reactions in Food Systems at High Hydrostatic Pressure. Food Eng. Rev. 2014, 6, 105–127. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Murzina, E.V.; Campo, B.; Heikkilä, T.; Leino, A.R.; Kordas, K.; Wolf, D.; Tokarev, A.V.; Murzin, D.Y. The effect of palladium dispersion and promoters on lactose oxidation kinetics. Res. Chem. Intermed. 2010, 36, 423–442. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Tokarev, A.V.; Murzina, E.V.; Campo, B.; Heikkilä, T.; Brozinski, J.M.; Wolf, D.; Murzin, D.Y. Kinetics of lactose and rhamnose oxidation over supported metal catalysts. Phys. Chem. Chem. Phys. 2011, 13, 9268–9280. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Park, G.W.; Han, J.-I. Efficient lactulose production from cheese whey using sodium carbonate. Food Chem. 2015, 173, 1167–1171. [Google Scholar] [CrossRef]
- Hajek, J.; Murzin, D.Y.; Salmi, T.; Mikkola, J.P. Interconversion of Lactose to Lactulose in Alkaline Environment: Comparison of Different Catalysis Concepts. Top. Catal. 2013, 56, 839–845. [Google Scholar] [CrossRef]
- Tokarev, A.V.; Murzina, E.V.; Mikkola, J.P.; Kuusisto, J.; Kustov, L.M.; Murzin, D.Y. Application of in situ catalyst potential measurements for estimation of reaction performance: Lactose oxidation over Au and Pd catalysts. Chem. Eng. J. 2007, 134, 153–161. [Google Scholar] [CrossRef]
- Gutierrez, L.F.; Hamoudi, S.; Belkacemi, K. Belkacemi, Selective production of lactobionic acid by aerobic oxidation of lactose over gold crystallites supported on mesoporous silica. Appl. Catal. A Gen. 2011, 402, 94–103. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Selective oxidation of alcohols and aldehydes on metal catalysts. Catal. Today 2000, 57, 127–141. [Google Scholar] [CrossRef]
- Talebi, S.; Suarez, F.; Chen, G.Q.; Chen, X.; Bathurst, K.; Kentish, S.E. Pilot Study on the Removal of Lactic Acid and Minerals from Acid Whey Using Membrane Technology. ACS Sustain. Chem. Eng. 2020, 8, 2742–2752. [Google Scholar] [CrossRef]
- Pal, P.; Kumar, R.; Banerjee, S. Manufacture of gluconic acid: A review towards process intensification for green production. Chem. Eng. Process. Process Intensif. 2016, 104, 160–171. [Google Scholar] [CrossRef]
- Comotti, M.; Della Pinam, C.; Falletta, E.; Rossi, M. Aerobic oxidation of glucose with gold catalyst: Hydrogen peroxide as intermediate and reagent. Adv. Synth. Catal. 2006, 348, 313–316. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, D.; Sun, Y.; Zhou, Z.; Du, Y.; Du, Y.; Li, Y.; Liu, M.; Zhang, Y.; Shen, J.; et al. Structural sensitivity of heterogeneous catalysts for sustainable chemical synthesis of gluconic acid from glucose. Chin. J. Catal. 2020, 41, 1320–1336. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
| Parameters | Sweet Whey Permeate | Acid Whey Permeate |
|---|---|---|
| pH | 6.23 ± 0.01 | 4.38 ± 0.02 |
| Total solids (g/L) | 87.38 ± 0.25 | 85.35 ± 0.06 |
| Total non-volatile solids (g/L) | 8.23 ± 0.17 | 9.22 ± 0.16 |
| Fat (g/L) | 0.85 ± 0.23 | 1.81 ± 0.10 |
| Total protein (g/L) | 3.02 ± 0.01 | 3.95 ± 0.08 |
| Lactose (g/L) | 76.42 ± 4.3 | 61.73 ± 6.29 |
| Organic acids (g/L) | 7.11 ± 0.35 | 17.91 ± 0.89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enteshari, M.; Martínez-Monteagudo, S.I. One-Pot Synthesis of Lactose Derivatives from Whey Permeate. Foods 2020, 9, 784. https://doi.org/10.3390/foods9060784
Enteshari M, Martínez-Monteagudo SI. One-Pot Synthesis of Lactose Derivatives from Whey Permeate. Foods. 2020; 9(6):784. https://doi.org/10.3390/foods9060784
Chicago/Turabian StyleEnteshari, Maryam, and Sergio I. Martínez-Monteagudo. 2020. "One-Pot Synthesis of Lactose Derivatives from Whey Permeate" Foods 9, no. 6: 784. https://doi.org/10.3390/foods9060784
APA StyleEnteshari, M., & Martínez-Monteagudo, S. I. (2020). One-Pot Synthesis of Lactose Derivatives from Whey Permeate. Foods, 9(6), 784. https://doi.org/10.3390/foods9060784






