Methyl Jasmonate Treatment of Broccoli Enhanced Glucosinolate Concentration, Which Was Retained after Boiling, Steaming, or Microwaving
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broccoli Cultivation and Methyl Jasmonate Treatment
2.2. Broccoli Preparation and Cooking Process
2.2.1. Broccoli Sample Preparation
2.2.2. Boiling Treatment
2.2.3. Steaming Treatment
2.2.4. Microwaving Treatment
2.2.5. Cooking Water Volume Adjustjment for Quantification
2.3. Quantification of Glucosinolates (GSLs) via UHPLC-DAD
2.4. Quantification of GSL Hydrolysis Products via GC-MS
2.5. Quantification of Myrosinase Activity and Nitrile Formation via GC-MS
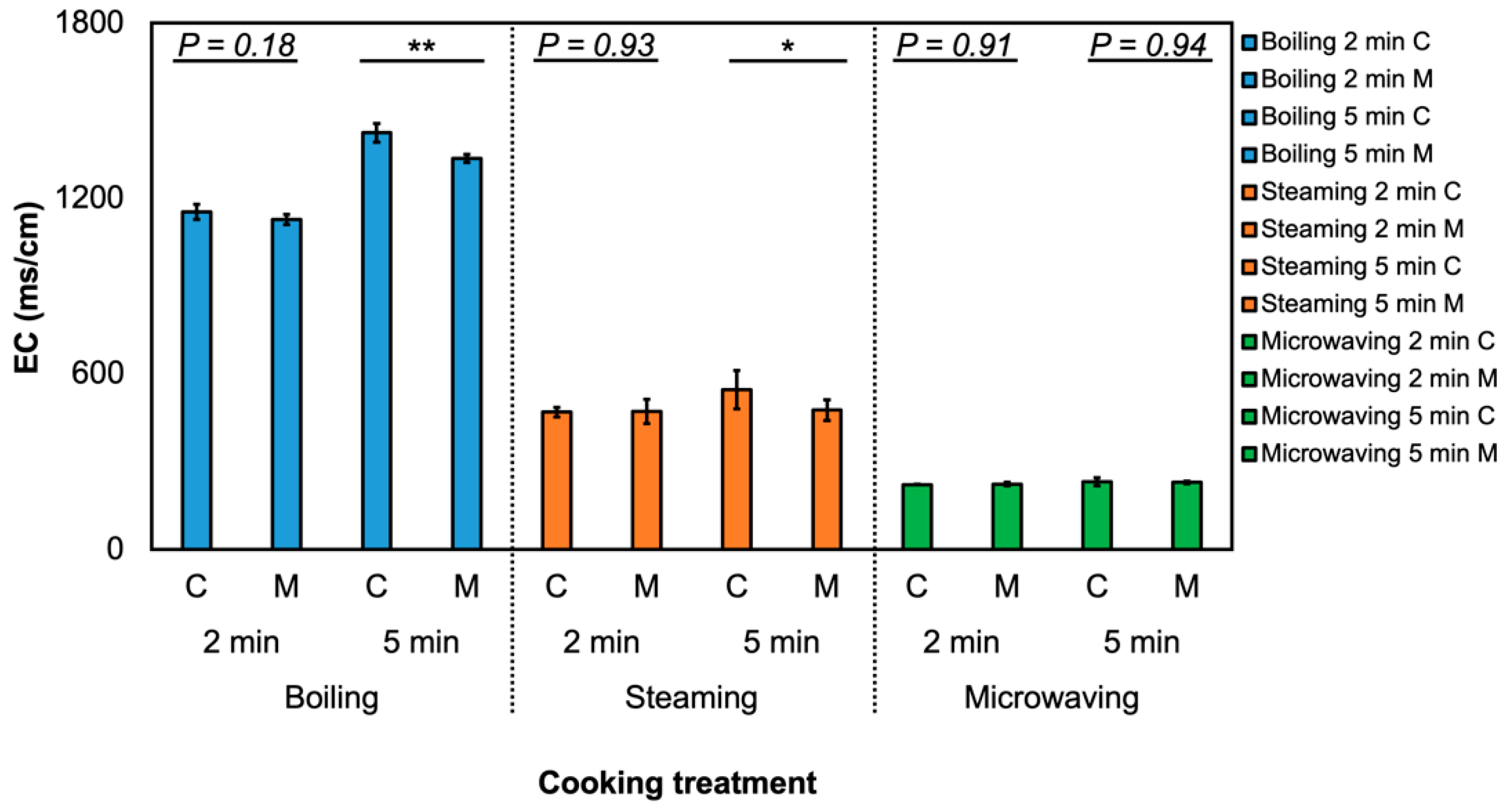
2.6. Measurement of Electrical Conductivity in Cooking Water
2.7. Untargeted Primary Metabolites by GC-MS
2.8. Univariate and Multivariate Analyses
3. Results and Discussion
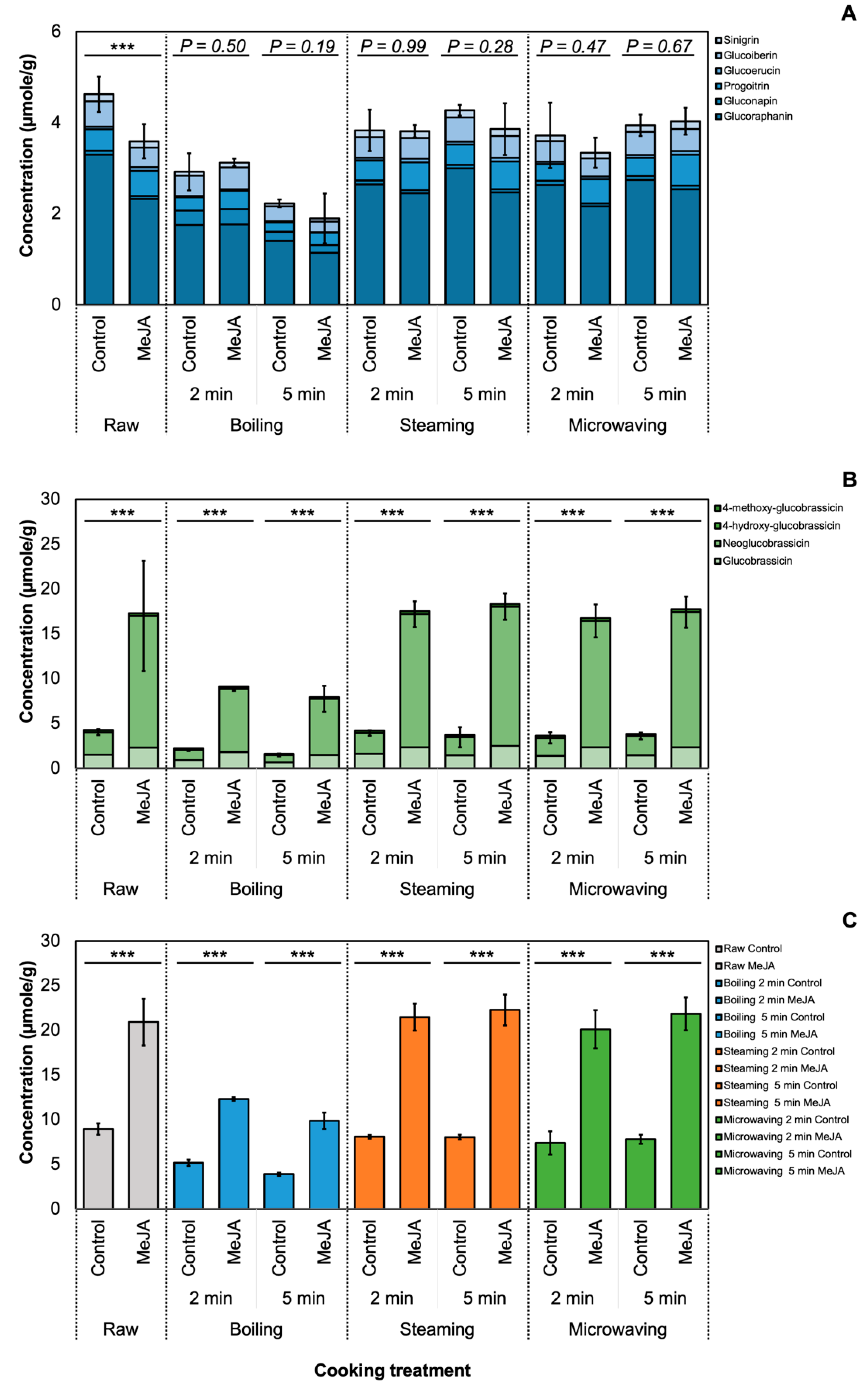
3.1. Effect of MeJA and Cooking Method on Glucosinolate Profile
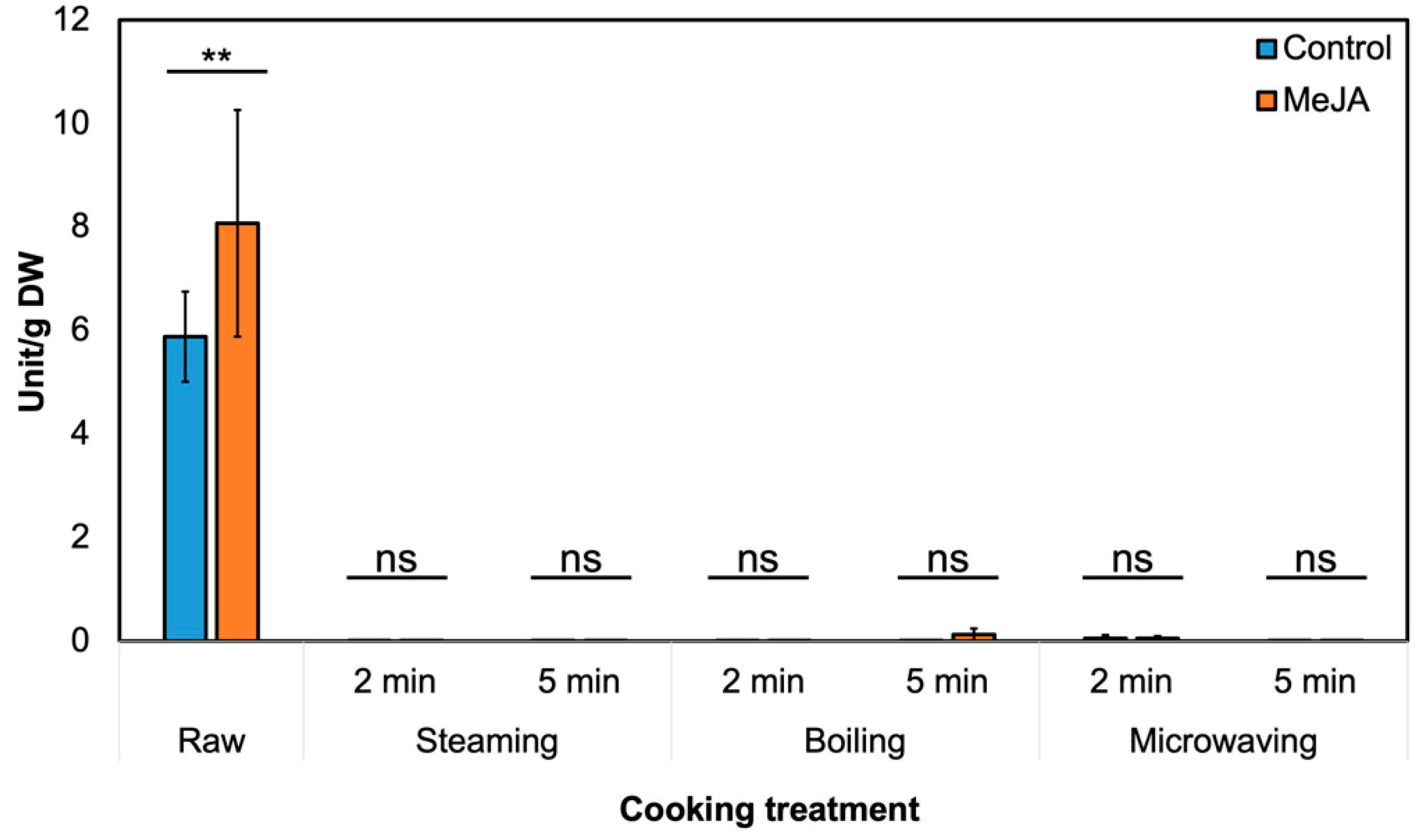
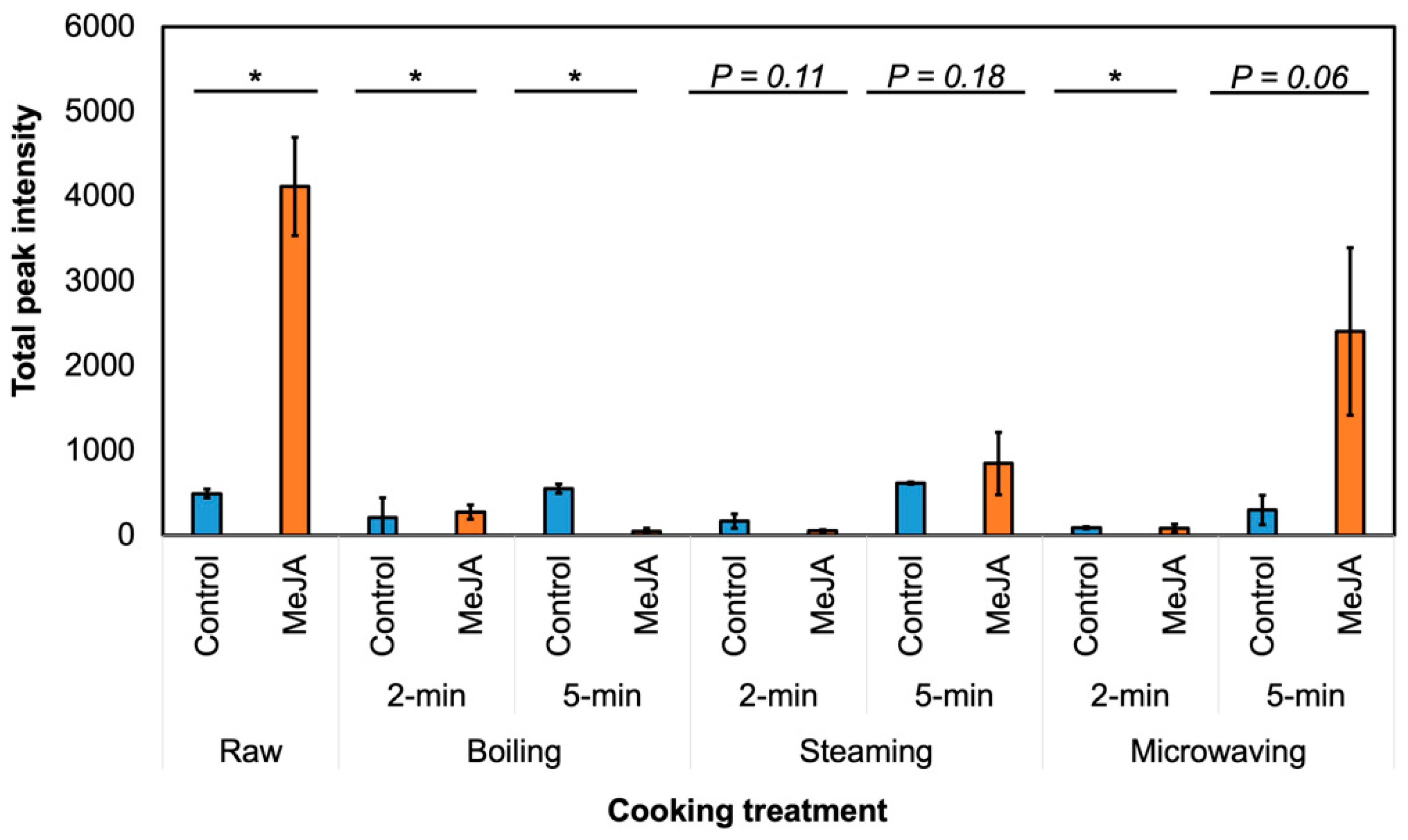
3.2. Effect of MeJA Treatment and Cooking Method on Myrosinase Activity and GSL Hydrolysis Products in ‘Green Magic’ Broccoli
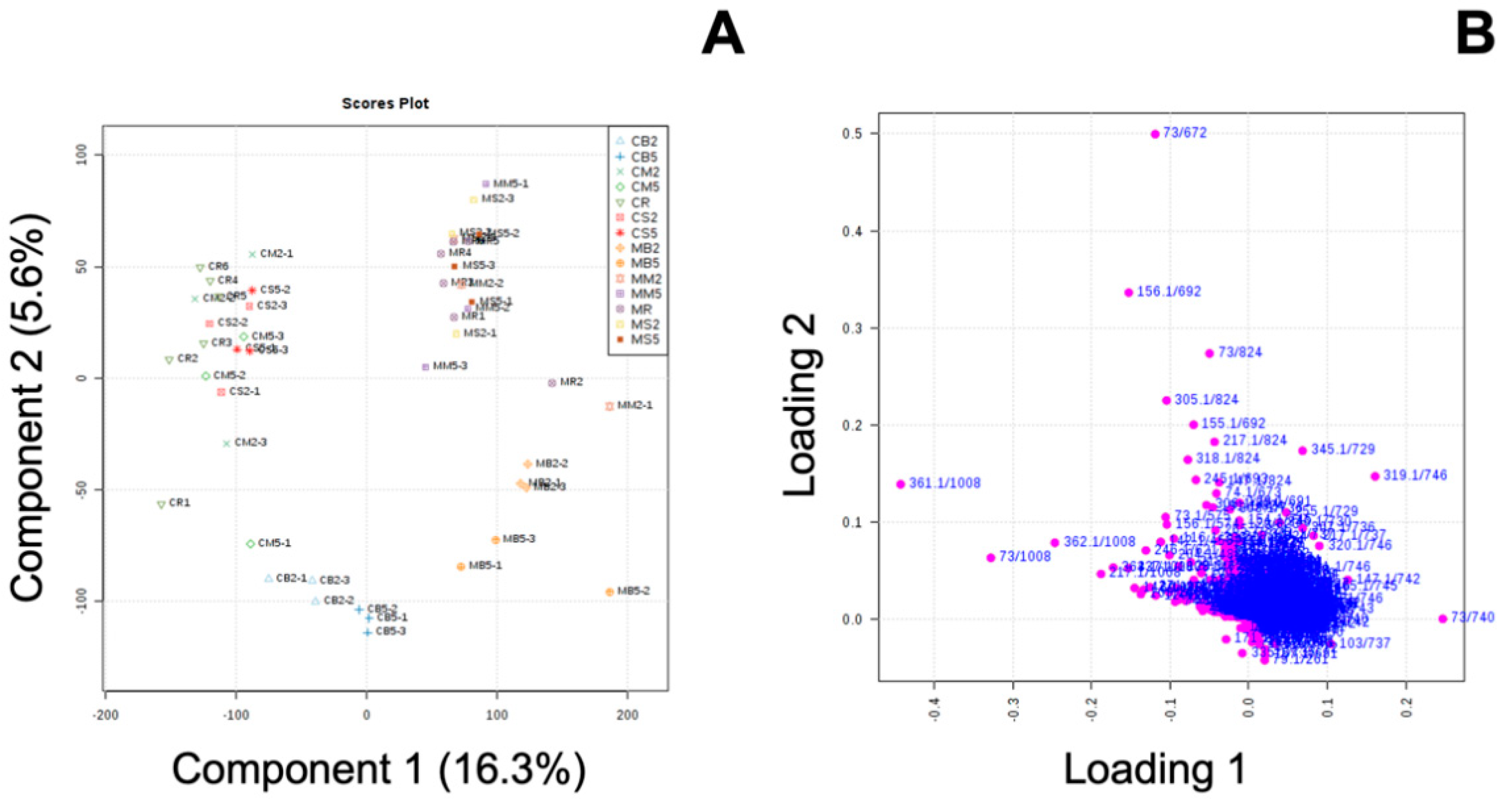
3.3. Effect of MeJA and Cooking Method on Primary Metabolites in ‘Green Magic’ Broccoli with or without MeJA Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ambrosone, C.B.; McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Zhang, Y.; Shields, P.G. Breast Cancer Risk in Premenopausal Women Is Inversely Associated with Consumption of Broccoli, a Source of Isothiocyanates, but Is Not Modified by GST Genotype. J. Nutr. 2004, 134, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Wallig, M.A.; Jeffery, E.H. Dietary Broccoli Lessens Development of Fatty Liver and Liver Cancer in Mice Given Diethylnitrosamine and Fed a Western or Control Diet. J. Nutr. 2016, 146, 542–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.-A.; Jung, Y.-H.; Lim, S.-H.; Park, S.U.; Kim, J.K. Metabolic Profiling in Chinese Cabbage (Brassica rapa L. subsp. pekinensis) Cultivars Reveals that Glucosinolate Content Is Correlated with Carotenoid Content. J. Agric. Food Chem. 2016, 64, 4426–4434. [Google Scholar] [CrossRef] [PubMed]
- Klopsch, R.; Witzel, K.; Artemyeva, A.; Ruppel, S.; Hanschen, F.S. Genotypic Variation of Glucosinolates and Their Breakdown Products in Leaves of Brassica rapa. J. Agric. Food Chem. 2018, 66, 5481–5490. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.M.; Choi, J.H.; Kim, H.S.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Methyl Jasmonate and 1-Methylcyclopropene Treatment Effects on Quinone Reductase Inducing Activity and Post-Harvest Quality of Broccoli. PLoS ONE 2013, 8, e77127. [Google Scholar] [CrossRef]
- Hu, R.; Xu, C.; Shen, G.; Jain, M.R.; Khor, T.O.; Gopalkrishnan, A.; Lin, W.; Reddy, B.; Chan, J.Y.; Kong, A.-N.T. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006, 243, 170–192. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Jeffery, E.H.; Klein, B.P.; Wallig, M.A.; Kushad, M.M.; Juvik, J.A. Glucosinolate Profiles in Broccoli: Variation in Levels and Implications in Breeding for Cancer Chemoprotection. J. Am. Soc. Hortic. Sci. 2002, 127, 807–813. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur. Food Res. Technol. 2002, 215, 310–316. [Google Scholar] [CrossRef]
- Jones, R.B.; Frisina, C.L.; Winkler, S.; Imsic, M.; Tomkins, R.B. Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chem. 2010, 123, 237–242. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kusznierewicz, B.; Leszczyńska, T.; Borczak, B. Effect of cooking on the contents of glucosinolates and their degradation products in selected Brassica vegetables. J. Funct. Foods 2016, 23, 412–422. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Vermeulen, M.; Dekker, M. In vivo formation and bioavailability of isothiocyanates from glucosinolates in broccoli as affected by processing conditions. Mol. Nutr. Food Res. 2014, 58, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Optimization of methyl jasmonate application to broccoli florets to enhance health-promoting phytochemical content: Meja enhancement of health-promoting phytochemicals in broccoli. J. Sci. Food Agric. 2014, 94, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, L.; Baldwin, I.T. Methyl jasmonate-elicited herbivore resistance: Does MeJA function as a signal without being hydrolyzed to JA? Planta 2008, 227, 1161–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamogami, S.; Noge, K.; Abe, M.; Agrawal, G.K.; Rakwal, R. Methyl jasmonate is transported to distal leaves via vascular process metabolizing itself into JA-Ile and triggering VOCs emission as defensive metabolites. Plant Signal. Behav. 2012, 7, 1378–1381. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Juvik, J.A. Effect of Selenium Fertilization and Methyl Jasmonate Treatment on Glucosinolate Accumulation in Broccoli Florets. J. Am. Soc. Hortic. Sci. 2011, 136, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Frerigmann, H.; Gigolashvili, T. MYB34, MYB51, and MYB122 Distinctly Regulate Indolic Glucosinolate Biosynthesis in Arabidopsis thaliana. Mol. Plant 2014, 7, 814–828. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.S.; Stout, M.J.; Karban, R.; Duffey, S.S. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 1996, 22, 1767–1781. [Google Scholar] [CrossRef]
- Campos, M.L.; Yoshida, Y.; Major, I.T.; de Ferreira, D.O.; Weraduwage, S.M.; Froehlich, J.E.; Johnson, B.F.; Kramer, D.M.; Jander, G.; Sharkey, T.D.; et al. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 2016, 7, 12570. [Google Scholar] [CrossRef] [Green Version]
- Becker, T.M.; Juvik, J.A. The Role of Glucosinolate Hydrolysis Products from Brassica Vegetable Consumption in Inducing Antioxidant Activity and Reducing Cancer Incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Jump, S.M.; Kung, J.; Staub, R.; Kinseth, M.A.; Cram, E.J.; Yudina, L.N.; Preobrazhenskaya, M.N.; Bjeldanes, L.F.; Firestone, G.L. N-Alkoxy derivatization of indole-3-carbinol increases the efficacy of the G1 cell cycle arrest and of I3C-specific regulation of cell cycle gene transcription and activity in human breast cancer cells. Biochem. Pharmacol. 2008, 75, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, F.H.; Li, Y. Indole-3-Carbinol and Prostate Cancer. J. Nutr. 2004, 134, 3493S–3498S. [Google Scholar] [CrossRef]
- Neave, A.S.; Sarup, S.M.; Seidelin, M.; Duus, F.; Vang, O. Characterization of the N-methoxyindole-3-carbinol (NI3C)–Induced Cell Cycle Arrest in Human Colon Cancer Cell Lines. Toxicol. Sci. 2005, 83, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesner, M.; Schreiner, M.; Glatt, H. High mutagenic activity of juice from pak choi (Brassica rapa ssp. chinensis) sprouts due to its content of 1-methoxy-3-indolylmethyl glucosinolate, and its enhancement by elicitation with methyl jasmonate. Food Chem. Toxicol. 2014, 67, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; Vos, M.D.; Kim, J.H.; Jander, G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2008, 8, 101–120. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Influence of Seasonal Variation and Methyl Jasmonate Mediated Induction of Glucosinolate Biosynthesis on Quinone Reductase Activity in Broccoli Florets. J. Agric. Food Chem. 2013, 61, 9623–9631. [Google Scholar] [CrossRef]
- Ku, K.M.; Juvik, J.A. Environmental Stress and Methyl Jasmonate-mediated Changes in Flavonoid Concentrations and Antioxidant Activity in Broccoli Florets and Kale Leaf Tissues. HortScience 2013, 48, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Juvik, J.A.; Ku, K.-M. Targeted Metabolomic and Transcriptomic Analyses of “Red Russian” Kale (Brassicae napus var. pabularia) Following Methyl Jasmonate Treatment and Larval Infestation by the Cabbage Looper (Trichoplusia ni Hübner). Int. J. Mol. Sci. 2018, 19, 1058. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Chiu, Y.-C.; Kim, N.K.; Park, H.M.; Lee, C.H.; Juvik, J.A.; Ku, K.-M. Cultivar-Specific Changes in Primary and Secondary Metabolites in Pak Choi (Brassica Rapa, Chinensis Group) by Methyl Jasmonate. Int. J. Mol. Sci. 2017, 18, 1004. [Google Scholar] [CrossRef] [Green Version]
- Pfalz, M.; Mukhaimar, M.; Perreau, F.; Kirk, J.; Hansen, C.I.C.; Olsen, C.E.; Agerbirk, N.; Kroymann, J. Methyl Transfer in Glucosinolate Biosynthesis Mediated by Indole Glucosinolate O -Methyltransferase 5. Plant Physiol. 2016, 172, 2190–2203. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhao, M.; Wang, X.; Tong, C.; Huang, S.; Tehrim, S.; Liu, Y.; Hua, W.; Liu, S. Bolbase: A comprehensive genomics database for Brassica oleracea. BMC Genom. 2013, 14, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusznierewicz, B.; Iori, R.; Piekarska, A.; Namieśnik, J.; Bartoszek, A. Convenient identification of desulfoglucosinolates on the basis of mass spectra obtained during liquid chromatography–diode array–electrospray ionisation mass spectrometry analysis: Method verification for sprouts of different Brassicaceae species extracts. J. Chromatogr. A 2013, 1278, 108–115. [Google Scholar] [CrossRef]
- Kim, M.J.; Chiu, Y.-C.; Ku, K.-M. Glucosinolates, Carotenoids, and Vitamins E and K Variation from Selected Kale and Collard Cultivars. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A.; Kushad, M.M. Correlation of quinone reductase activity and allyl isothiocyanate formation among different genotypes and grades of horseradish roots. J. Agric. Food Chem. 2015, 63, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Diedrich, J.; Chu, Y.-Y.; Yates, J.R. Extracting accurate precursor information for tandem mass spectra by rawconverter. Anal. Chem. 2015, 87, 11361–11367. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarvan, I.; Verkerk, R.; van Boekel, M.; Dekker, M. Comparison of the degradation and leaching kinetics of glucosinolates during processing of four Brassicaceae (broccoli, red cabbage, white cabbage, Brussels sprouts). Innov. Food Sci. Emerg. Technol. 2014, 25, 58–66. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Brüggemann, N.; Brodehl, A.; Mewis, I.; Schreiner, M.; Rohn, S.; Kroh, L.W. Characterization of Products from the Reaction of Glucosinolate-Derived Isothiocyanates with Cysteine and Lysine Derivatives Formed in Either Model Systems or Broccoli Sprouts. J. Agric. Food Chem. 2012, 60, 7735–7745. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Kim, G.-H. Effects of various heating methods on glucosinolate, carotenoid and tocopherol concentrations in broccoli. Int. J. Food Sci. Nutr. 2013, 64, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Thornalley, P.J. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem. Toxicol. 2007, 45, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.-J.; Choi, Y.D. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003, 19, 409–413. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2010, 118, 641–647. [Google Scholar] [CrossRef]
- Boonyaritthongchai, P.; Chimvaree, C.; Buanong, M.; Uthairatanakij, A.; Jitareerat, P. Effect of Methyl Jasmonate on Physical and Chemical Properties of Mango Fruit cv. Nam Dok Mai. Horticulturae 2016, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Napoleão, T.A.; Soares, G.; Vital, C.E.; Bastos, C.; Castro, R.; Loureiro, M.E.; Giordano, A. Methyl jasmonate and salicylic acid are able to modify cell wall but only salicylic acid alters biomass digestibility in the model grass Brachypodium distachyon. Plant Sci. 2017, 263, 46–54. [Google Scholar] [CrossRef]
- Tian, S.; Liu, X.; Lei, P.; Zhang, X.; Shan, Y. Microbiota: A mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. J. Sci. Food Agric. 2018, 98, 1255–1260. [Google Scholar] [CrossRef]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.C.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Erez, A.; Donia, M.S.; Sonnenburg, J.L.; Sattely, E.S. A Metabolic Pathway for Activation of Dietary Glucosinolates by a Human Gut Symbiont. Cell 2020, 180, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.-N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Antioxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharm. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephensen, P.U.; Bonnesen, C.; Schaldach, C.; Andersen, O.; Bjeldanes, L.F.; Vang, O. N-Methoxyindole-3-Carbinol Is a More Efficient Inducer of Cytochrome P-450 1A1 in Cultured Cells Than Indol-3-Carbinol. Nutr. Cancer 2000, 36, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Kaufmann, M.; Kupke, F.; Hackl, T.; Kroh, L.W.; Rohn, S.; Schreiner, M. Brassica vegetables as sources of epithionitriles: Novel secondary products formed during cooking. Food Chem. 2018, 245, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Matak, K.; Ku, K.-M. Methyl jasmonate treated broccoli: Impact on the production of glucosinolates and consumer preferences. Food Chem. 2019, 299, 125099. [Google Scholar] [CrossRef] [PubMed]
- Dosz, E.B.; Jeffery, E.H. Commercially produced frozen broccoli lacks the ability to form sulforaphane. J. Funct. Foods 2013, 5, 987–990. [Google Scholar] [CrossRef]
- Havko, N.E.; Major, I.T.; Jewell, J.B.; Attaran, E.; Browse, J.; Howe, G.A. Control of Carbon Assimilation and Partitioning by Jasmonate: An Accounting of Growth–Defense Tradeoffs. Plants 2016, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- Beck, T.K.; Jensen, S.; Bjoern, G.K.; Kidmose, U. The Masking Effect of Sucrose on Perception of Bitter Compounds in Brassica Vegetables. J. Sens. Stud. 2014, 29, 190–200. [Google Scholar] [CrossRef]
- Chadwick, M.; Gawthrop, F.; Michelmore, R.W.; Wagstaff, C.; Methven, L. Perception of bitterness, sweetness and liking of different genotypes of lettuce. Food Chem. 2016, 197, 66–74. [Google Scholar] [CrossRef]
| Cooking Method | Amino Acids † | Sugar and Sugar Derivatives | Organic Acids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxo- Proline | Glu | Val | Pro | Ser | Ile | Ala | Fructose | Glucose | Sucrose | myo-Inositol | Quinic Acid | ||
| Raw | 0.58 * | 0.42 * | 0.94 | 1.06 | 0.28 * | 0.91 * | 1.35 | ||||||
| Boiling | 2 min | 0.77 * | 0.35 * | 0.96 * | 1.09 * | 0.26 * | 0.90 * | 1.35 * | |||||
| 5 min | 0.95 | 0.43 * | 0.80 | 1.09 | 0.37 * | 0.84 | 1.73 | ||||||
| Steaming | 2 min | 0.71 * | 0.40 * | 0.49 * | 0.40 * | 0.39 * | 0.38 * | 1.05 | 0.32 * | 0.74 * | 1.72 * | ||
| 5 min | 0.70 * | 0.35 * | 0.43 * | 0.35 * | 0.40 * | 0.26 * | 0.90 * | 0.26 * | 0.59 * | 1.54 * | |||
| Micro- waving | 2 min | 0.46 * | 0.43 * | 0.48 | 0.37 * | 0.36 * | 0.69 * | 0.98 | 1.05 * | 0.26 * | 0.76 * | 2.04 * | |
| 5 min | 0.62 * | 0.57 * | 0.57 * | 0.57 * | 0.56 * | 0.86 * | 1.31 * | 1.38* | 0.49 * | 1.02 | 2.33 * | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-C.; Matak, K.; Ku, K.-M. Methyl Jasmonate Treatment of Broccoli Enhanced Glucosinolate Concentration, Which Was Retained after Boiling, Steaming, or Microwaving. Foods 2020, 9, 758. https://doi.org/10.3390/foods9060758
Chiu Y-C, Matak K, Ku K-M. Methyl Jasmonate Treatment of Broccoli Enhanced Glucosinolate Concentration, Which Was Retained after Boiling, Steaming, or Microwaving. Foods. 2020; 9(6):758. https://doi.org/10.3390/foods9060758
Chicago/Turabian StyleChiu, Yu-Chun, Kristen Matak, and Kang-Mo Ku. 2020. "Methyl Jasmonate Treatment of Broccoli Enhanced Glucosinolate Concentration, Which Was Retained after Boiling, Steaming, or Microwaving" Foods 9, no. 6: 758. https://doi.org/10.3390/foods9060758
APA StyleChiu, Y.-C., Matak, K., & Ku, K.-M. (2020). Methyl Jasmonate Treatment of Broccoli Enhanced Glucosinolate Concentration, Which Was Retained after Boiling, Steaming, or Microwaving. Foods, 9(6), 758. https://doi.org/10.3390/foods9060758






