Influence of the Inclusion of Chestnut (Castanea sativa Miller) in the Finishing Diet and Cooking Technique on the Physicochemical Parameters and Volatile Profile of Biceps femoris Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Slaughtered
2.2. Sample Preparation and Cooking Process
2.3. Analysis of Proximate Composition
2.4. Colour Analysis
2.5. Cooking Loss
2.6. Texture Measurement
2.7. Lipid Oxidation
2.8. Volatile Compound Profile
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Colour Parameters
3.3. Cooking Loss
3.4. Texture Analysis
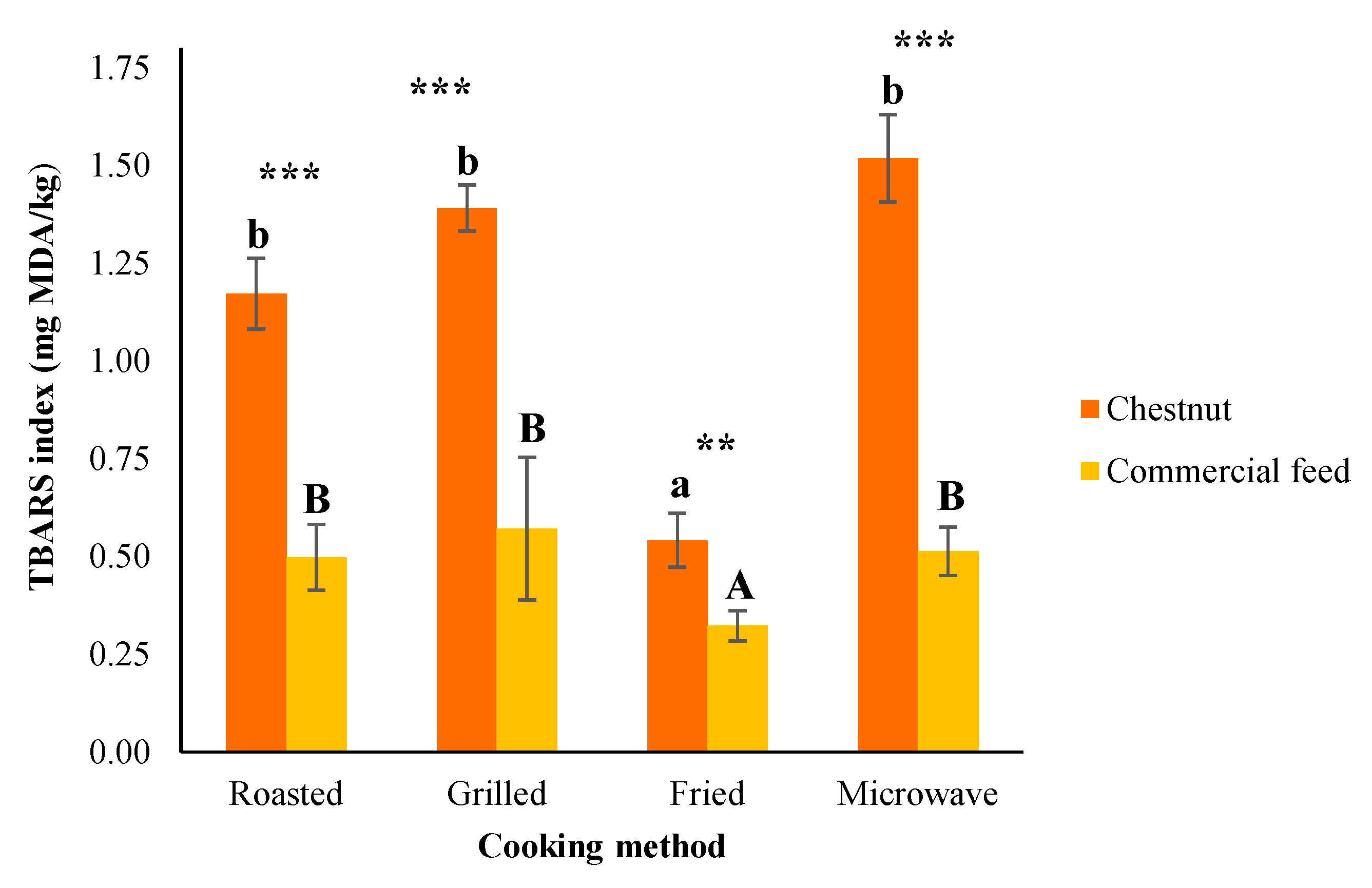
3.5. TBARS Values
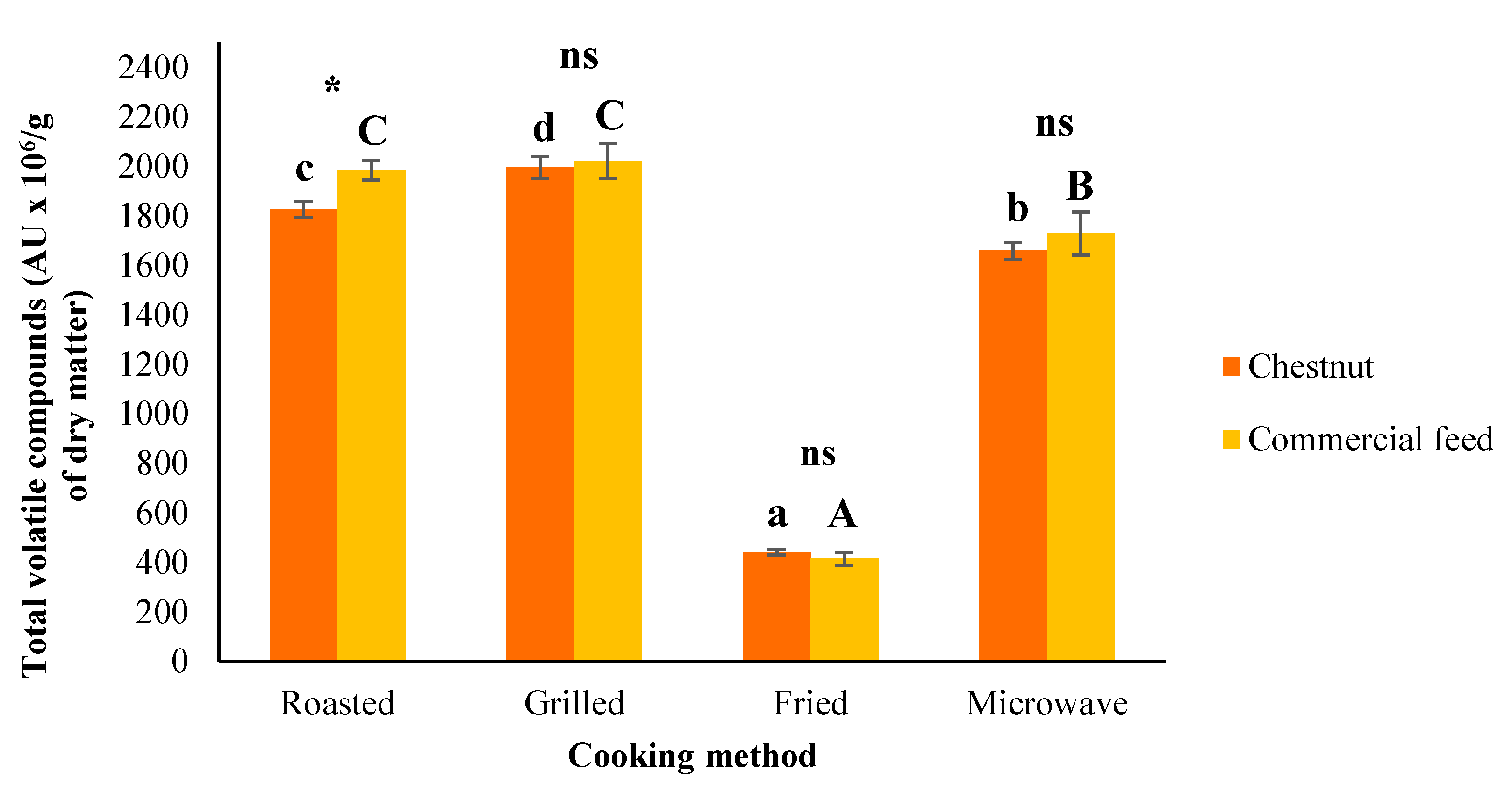
3.6. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simonetti, A.; Perna, A.; Gambacorta, E. Comparison of antioxidant compounds in pig meat from Italian autochthonous pig Suino Nero Lucano and a modern crossbred pig before and after cooking. Food Chem. 2019, 292, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Montes, R.; Purriños, L.; Cobas, N.; Franco, D. Fatty acid composition of Celta pig breed as influenced by sex and location of fat in the carcass. J. Sci. Food Agric. 2012, 92, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Díaz-Hernández, M.B.; Ciordia-Ara, M.; Ríos-Mesa, D. Chemical composition of chestnut cultivars from Spain. Sci. Hortic. (Amsterdam) 2006, 107, 306–314. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, I.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of inclusion of chestnut in the finishing diet on fatty acid profile of dry-cured ham from Celta pig breed. Meat Sci. 2012, 92, 394–399. [Google Scholar] [CrossRef]
- Temperan, S.; Lorenzo, J.M.; Castiñeiras, B.D.; Franco, I.; Carballo, J. Carcass and meat quality traits of Celta heavy pigs. Effect of the inclusion of chestnuts in the finishing diet. Spanish J. Agric. Res. 2014, 12, 694–707. [Google Scholar] [CrossRef]
- De Jesús, M.C.; Domínguez, R.; Cantalapiedra, J.; Iglesias, A.; Lorenzo, J.M.; Lorenzo, J.M. Effect of the amount of chestnuts in the diet of Celta pigs on the fatty acid profile of dry-cured lacon. Grasas y Aceites 2016, 67, e119. [Google Scholar]
- Broncano, J.M.; Petrón, M.J.; Parra, V.; Timón, M.L. Effect of different cooking methods on lipid oxidation and formation of free cholesterol oxidation products (COPs) in Latissimus dorsi muscle of Iberian pigs. Meat Sci. 2009, 83, 431–437. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef]
- Domínguez, R.; Borrajo, P.; Lorenzo, J.M. The effect of cooking methods on nutritional value of foal meat. J. Food Compos. Anal. 2015, 43, 61–67. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.R. Analysis of visible reflectance spectra of stored, cooked and diseased chicken meats. Meat Sci. 2001, 58, 395–401. [Google Scholar] [CrossRef]
- Chiavaro, E.; Rinaldi, M.; Vittadini, E.; Barbanti, D. Cooking of pork Longissimus dorsi at different temperature and relative humidity values: Effects on selected physico-chemical properties. J. Food Eng. 2009, 93, 158–165. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Domínguez, R. Cooking losses, lipid oxidation and formation of volatile compounds in foal meat as affected by cooking procedure. Flavour Fragr. J. 2014, 29, 240–248. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT-Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef]
- European Union Council Directive 2008/120/EC laying down minimum standards for the protection of pigs. Off. J. Eur. Union 2008, 47, 5–13.
- Domínguez, R.; Martínez, S.; Gómez, M.; Carballo, J.; Franco, I. Fatty acids, retinol and cholesterol composition in various fatty tissues of Celta pig breed: Effect of the use of chestnuts in the finishing diet. J. Food Compos. Anal. 2015, 37, 104–111. [Google Scholar] [CrossRef]
- International Standards Meat and Meat Products-Determination of Moisture Content; International Organization for Standarization: Geneva, Switzerland, 1997; ISO 1442.
- International Standardsmeat Andmeat Products-Determination of Nitrogen Content; International Organization for Standarization: Geneva, Switzerland, 1978; ISO 937.
- International Standards Meat and Meat Products-Determination of Ash Content; International Organization for Standarization: Geneva, Switzerland, 1998; ISO 936.
- AOCS. Rapid Determination of oil/Fat Utilizing High Temperature Solvent Extraction; AOCS Official Procedure Am5-04; American Oil Chemists Society: Urbana, IL, USA, 2005. [Google Scholar]
- Vyncke, W. Evaluation of the direct thiobarbituric acid extraction method for determining oxidative rancidity in mackerel. Fette Seifen Anstrichm. 1975, 77, 239–240. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Acciaioli, A.; Bozzi, R.; Campodoni, G.; Franci, O. Quality of fresh and seasoned fat of Cinta Senese pigs as affected by fattening with chestnut. Meat Sci. 2013, 93, 92–97. [Google Scholar] [CrossRef]
- Serrano, A.; Librelotto, J.; Cofrades, S.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. Composition and physicochemical characteristics of restructured beef steaks containing walnuts as affected by cooking method. Meat Sci. 2007, 77, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Parvin, R.; Yim, D.G.; Zahid, M.A.; Yang, H.S. Effects on quality properties of cooked pork sausages with Caesalpinia sappan L. extract during cold storage. J. Food Sci. Technol. 2019, 56, 4946–4955. [Google Scholar] [CrossRef] [PubMed]
- Latoch, A.; Libera, J.; Stasiak, D.M. Physicochemical properties of pork loin marinated in Kefir, yoghurt or buttermilk and cooked sous vide. Acta Sci. Pol. Technol. Aliment. 2019, 18, 163–171. [Google Scholar] [PubMed]
- Zhang, X.; Xu, Y.; Xue, H.; Jiang, G.; Liu, X. Antioxidant activity of vine tea (Ampelopsis grossedentata) extract on lipid and protein oxidation in cooked mixed pork patties during refrigerated storage. Food Sci. Nutr. 2019, 7, 1735–1745. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Ikonić, P.; Tomović, V.; Ikonić, B.; Zeković, Z.; Kocić-Tanackov, S.; Jokanović, M.; Škaljac, S.; Ivić, M. Coriander essential oil as natural food additive improves quality and safety of cooked pork sausages with different nitrite levels. Meat Sci. 2019, 157. [Google Scholar] [CrossRef]
- Muscle Foods: Meat, Poultry and Seafood Technology. Kinsman, D.M.; Kotula, A.W.; Breidenstein, B.C. (Eds.) Springer: New York, NY, USA, 1994; ISBN 9781475759358. [Google Scholar]
- Vittadini, E.; Rinaldi, M.; Chiavaro, E.; Barbanti, D.; Massini, R. The effect of different convection cooking methods on the instrumental quality and yield of pork Longissimus dorsi. Meat Sci. 2005, 69, 749–756. [Google Scholar] [CrossRef]
- Bertola, N.C.; Bevilacqua, A.E.; Zaritzky, N.E. Heat treatment effect on texture changes and thermal denaturation of proteins in beef muscle. J. Food Process. Preserv. 1994, 18, 31–46. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Sensory properties and physicochemical changes in the biceps femoris muscle during processing of dry-cured ham from celta pigs. Effects of cross-breeding with duroc and landrace pigs. Ital. J. Food Sci. 2016, 29, 2017–2023. [Google Scholar]
- Lorenzo, J.M.J.M.; Cittadini, A.; Munekata, P.E.P.E.; Domínguez, R. Physicochemical properties of foal meat as affected by cooking methods. Meat Sci. 2015, 108, 50–54. [Google Scholar] [CrossRef]
- García-Segovia, P.; Andrés-Bello, A.; Martínez-Monzó, J. Effect of cooking method on mechanical properties, color and structure of beef muscle (M. pectoralis). J. Food Eng. 2007, 80, 813–821. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, D.-W. Factors affecting the water holding capacity of red meat products: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2008, 48, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.G.; Jo, C.; Mahabbat, A.; Park, J.Y.; Lee, S.Y.; Nam, K.C. Combined effect of aging and irradiation on physicochemical quality of pork shoulder. Food Sci. Anim. Resour. 2019, 39, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhou, L.; Huang, Y.; Zheng, M.; Liu, X.; Zhang, Y.; Huang, C.; Peng, S.; Zeng, Q.; Zhong, L.; et al. A whole-genome sequence based association study on pork eating quality traits and cooking loss in a specially designed heterogeneous F6 pig population. Meat Sci. 2018, 146, 160–167. [Google Scholar] [CrossRef]
- Alfaia, C.M.M.; Alves, S.P.; Lopes, A.F.; Fernandes, M.J.E.; Costa, A.S.H.; Fontes, C.M.G.A.; Castro, M.L.F.; Bessa, R.J.B.; Prates, J.A.M. Effect of cooking methods on fatty acids, conjugated isomers of linoleic acid and nutritional quality of beef intramuscular fat. Meat Sci. 2010, 84, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Feng, J.; Cao, A.; Zhang, Y.; Lv, Y.; Li, J. Denaturation kinetics and aggregation mechanism of the sarcoplasmic and myofibril proteins from grass carp during microwave processing. Food Bioprocess Technol. 2018, 11, 417–426. [Google Scholar] [CrossRef]
- Juárez, M.; Failla, S.; Ficco, A.; Peña, F.; Avilés, C.; Polvillo, O. Buffalo meat composition as affected by different cooking methods. Food Bioprod. Process. 2009, 88, 145–148. [Google Scholar] [CrossRef]
- Hearne, L.; Penfield, M.; Goertz, G. Heating effects on bovine semitendinosus: Shear, muscle fiber measurements, and cooking losses. J. Food Sci. 2006, 43, 10–12. [Google Scholar] [CrossRef]
- Hovenier, R.; Kanis, E.; Verhoeven, J.A.M. Repeatability of taste panel tenderness scores and their relationships to objective pig meat quality traits. J. Anim. Sci. 1993, 71, 2018–2025. [Google Scholar] [CrossRef]
- Hamilton, D.N.; Ellis, M.; Wolter, B.F.; McKeith, F.K.; Wilson, E.R. Carcass and meat quality characteristics of the progeny of two swine sire lines reared under differing environmental conditions. Meat Sci. 2003, 63, 257–263. [Google Scholar] [CrossRef]
- Olsson, V.; Andersson, K.; Hansson, I.; Lundström, K. Differences in meat quality between organically and conventionally produced pigs. Meat Sci. 2003, 64, 287–297. [Google Scholar] [CrossRef]
- Swigert, K.S.; McKeith, F.K.; Carr, T.C.; Brewer, M.S.; Culbertson, M. Effects of dietary vitamin D3, vitamin E, and magnesium supplementation on pork quality. Meat Sci. 2004, 67, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Destefanis, G.; Brugiapaglia, A.; Barge, M.T.; Dal Molin, E. Relationship between beef consumer tenderness perception and Warner-Bratzler shear force. Meat Sci. 2008, 78, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska-Kapituła, M.; Dabrowska, E.; Jankowska, B.; Kwiatkowska, A.; Cierach, M. The effect of muscle, cooking method and final internal temperature on quality parameters of beef roast. Meat Sci. 2012, 91, 195–202. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Bakhsh, A.; Joo, S.T. The alternative approach of low temperature-long time cooking on bovine semitendinosus meat quality. Asian-Australas. J. Anim. Sci. 2019, 32, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, X.; Hayat, K.; Liu, P.; Jia, C.; Xia, S.; Xiao, Z.; Tian, H.; Niu, Y. Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 2011, 124, 203–209. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Cobos, Á.; Veiga, A.; Díaz, O. Influencia de un pienso con castañas y pulpa de remolacha azucarera en la composición lipídica del lacón gallego. Grasas y Aceites 2008, 59, 121–127. [Google Scholar] [CrossRef]
- Díaz, O.; Ros, C.; Veiga, A.; Cobos, Á. Including chestnuts and sugar beet pulp in diets for pigs: The effects on the quality of pork meat and the sensory properties of dry-cured sausage (chorizo gallego). J. Muscle Foods 2009, 20, 449–464. [Google Scholar] [CrossRef]
- Sirtori, F.; Pugliese, C.; Meo Zilio, D.; Campodoni, G.; Pianaccioli, L.; Bonelli, A. Characteristics of cured lard of Cinta senese pig fed acorn and chestnut. Ital. J. Anim. Sci. 2005, 4, 467–469. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; D’Adorante, S.; Parenti, S.; Rey, A.; Lopez-Bote, C.; Franci, O. Effect of pasture in oak and chestnut groves on chemical and sensorial traits of cured lard of Cinta Senese pigs. Ital. J. Anim. Sci. 2009, 8, 131–142. [Google Scholar] [CrossRef]
- Gómez, M.; Fonseca, S.; Cachaldora, A.; Carballo, J.; Franco, I. Effect of chestnuts intake by Celta pigs on lipolytic, oxidative and fatty acid profile changes during ripening and vacuum-packed storage of Galician “0022chorizo”. J. Food Compos. Anal. 2017, 56, 73–83. [Google Scholar] [CrossRef]
- Echegaray, N.; Domínguez, R.; Franco, D.; Lorenzo, J.M.; Carballo, J. Effect of the use of chestnuts (Castanea sativa Miller) in the finishing diet of Celta pig breed on the shelf-life of meat refrigerated and frozen. Food Res. Int. 2018, 114, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Gai, F.; Gasco, L.; Brugiapaglia, A.; Lussiana, C.; Guo, K.J.; Tong, J.M.; Zoccarato, I. Effects of chestnut tannins on carcass characteristics, meat quality, lipid oxidation and fatty acid composition of rabbits. Meat Sci. 2009, 83, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Bian, W.; Ye, L.; Yang, X.; Song, X. Preservation of traditional Chinese pork balls supplemented with essential oil microemulsion in a phase-change material package. J. Sci. Food Agric. 2020, 100, 2288–2295. [Google Scholar] [CrossRef]
- Hernández, P.; Navarro, J.L.; Toldrá, F. Lipids of pork meat as affected by various cooking techniques. Food Sci. Technol. Int. 1999, 5, 501–508. [Google Scholar] [CrossRef]
- O’Grady, M.N.; Carpenter, R.; Lynch, P.B.; O’Brien, N.M.; Kerry, J.P. Addition of grape seed extract and bearberry to porcine diets: Influence on quality attributes of raw and cooked pork. Meat Sci. 2008, 78, 438–446. [Google Scholar] [CrossRef]
- Park, K.C.; Pyo, H.S.; Kim, W.S.; Yoon, K.S. Effects of cooking methods and tea marinades on the formation of benzo[a]pyrene in grilled pork belly (Samgyeopsal). Meat Sci. 2017, 129, 1–8. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Jokanović, M.; Ikonić, P.; Džinić, N.; Kocić-Tanackov, S.; Popović, L.; Tasić, T.; Savanović, J.; Šojić, N.Ž. Antioxidant activity of Juniperus communis L. essential oil in cooked pork sausages. Czech J. Food Sci. 2017, 35, 189–193. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Greene, B.E.; Cumuze, T.H. Relationship Between TBA Numbers and Inexperienced Panelists’Assessments of Oxidized Flavor in Cooked Beef. J. Food Sci. 1982, 47, 52–54. [Google Scholar] [CrossRef]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.P.; Shand, P.; Dugan, M.E.R.; Gariépy, C.; Aalhus, J.L.; Estévez, M.; Juárez, M. Influence of cooking methods and storage time on lipid and protein oxidation and heterocyclic aromatic amines production in bacon. Food Res. Int. 2017, 99, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Bochi, V.C.; Ribeiro, C.P.; Victório, A.d.M.; Emanuelli, T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Saguy, I.S.; Dana, D. Integrated approach to deep fat frying: Engineering, nutrition, health and consumer aspects. J. Food Eng. 2003, 56, 143–152. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Mylona, A.; Ioannou, M.S.; Andrikopoulos, N.K. Recovery and distribution of natural antioxidants (α-tocopherol, polyphenols and terpenic acids) after panfrying of Mediterranean finfish in virgin olive oil. Food Chem. 2007, 100, 509–517. [Google Scholar] [CrossRef]
- Moroney, N.C.; O’Grady, M.N.; O’Doherty, J.V.; Kerry, J.P. Addition of seaweed (Laminaria digitata) extracts containing laminarin and fucoidan to porcine diets: Influence on the quality and shelf-life of fresh pork. Meat Sci. 2012, 92, 423–429. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Argemí-Armengol, I.; Villalba, D.; Tor, M.; Pérez-Santaescolástica, C.; Purriños, L.; Lorenzo, J.M.; Álvarez-Rodríguez, J. The extent to which genetics and lean grade affect fatty acid profiles and volatile compounds in organic pork. Peer J. 2019, 7, e7322. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of Volatile Compounds of Dry-Cured Meat Products Using HS-SPME-GC/MS Technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Lu, P.; Li, D.; Yin, J.; Zhang, L.; Wang, Z. Flavour differences of cooked longissimus muscle from Chinese indigenous pig breeds and hybrid pig breed (Duroc × Landrace × Large White). Food Chem. 2008, 107, 1529–1537. [Google Scholar] [CrossRef]
- Peterson, R.J.; Izzo, H.K.; Jungermann, E.; Chang, S. Changes in volatile flavor compounds during the retorting of canned beef stew. J. Food Sci. 2006, 40, 948–954. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Ramírez, R.; Cava, R. Influence of the addition of rosemary essential oil on the volatiles pattern of porcine frankfurters. J. Agric. Food Chem. 2005, 53, 8317–8324. [Google Scholar] [CrossRef] [PubMed]
- Van, H.; Hwang, I.; Jeong, D.; Touseef, A. Principle of Meat Aroma Flavors and Future Prospect. In Latest Research into Quality Control; Akyar, I., Ed.; IntechOpen Limited: London, UK, 2012; pp. 145–176. [Google Scholar]
- Du, X. Determination of flavor substances in fermented pork by GC-MS. Meat Res. 2012, 26, 34–36. [Google Scholar]
- Maggiolino, A.; Lorenzo, J.M.; Marino, R.; della Malva, A.; Centoducati, P.; De Palo, P. Foal meat volatile compounds: Effect of vacuum ageing on semimembranosus muscle. J. Sci. Food Agric. 2019, 99, 1660–1667. [Google Scholar] [CrossRef]

| Roasted | Grilled | Fried | Microwaved | SEM | T | FxT | |
|---|---|---|---|---|---|---|---|
| Moisture (%) | |||||||
| Chestnut | 58.24 bc | 60.89 c | 54.58 ab | 53.41 a | 0.903 | ** | ns |
| Commercial feed | 60.50 | 62.46 | 59.45 | 58.96 | 0.542 | ns | |
| SEM | 0.828 | 0.730 | 1.145 | 1.260 | |||
| F | ns | ns | * | * | |||
| Intramuscular Fat (%) | |||||||
| Chestnut | 6.08 | 4.22 | 6.82 | 7.69 | 0.524 | ns | ns |
| Commercial feed | 2.67 a | 2.68 a | 4.07 b | 2.83 a | 0.904 | ** | |
| SEM | 0.785 | 0.360 | 0.516 | 0.971 | |||
| F | * | * | ** | ** | |||
| Protein (%) | |||||||
| Chestnut | 32.92 a | 32.06 a | 36.33 b | 36.07 b | 0.580 | ** | ns |
| Commercial feed | 34.14 ab | 32.40 a | 34.00 ab | 35.99 b | 0.447 | * | |
| SEM | 0.548 | 0.566 | 0.752 | 0.528 | |||
| F | ns | ns | ns | ns | |||
| Ash (%) | |||||||
| Chestnut | 1.66 a | 1.59 a | 1.79 b | 1.63 a | 0.026 | * | ns |
| Commercial feed | 1.83 c | 1.61 a | 1.75 bc | 1.70 ab | 0.026 | * | |
| SEM | 0.039 | 0.019 | 0.032 | 0.036 | |||
| F | * | ns | ns | ns |
| Roasted | Grilled | Fried | Microwaved | SEM | T | FxT | |
|---|---|---|---|---|---|---|---|
| Color parameters | |||||||
| Lightness (L*) | |||||||
| Chestnut | 49.97 b | 51.13 b | 34.89 a | 51.22 b | 1.663 | *** | ns |
| Commercial feed | 49.25 b | 52.32 b | 40.96 a | 51.33 b | 1.125 | *** | |
| SEM | 1.113 | 0.824 | 1.611 | 0.981 | |||
| F | ns | ns | ns | ns | |||
| Redness (a*) | |||||||
| Chestnut | 7.15 b | 6.31 a,b | 9.34 c | 5.89 a | 0.333 | *** | ns |
| Commercial feed | 7.52 a | 6.39 a | 10.32 b | 6.34 a | 0.404 | *** | |
| SEM | 0.298 | 0.260 | 0.416 | 0.224 | |||
| F | ns | ns | ns | ns | |||
| Yellowness (b*) | |||||||
| Chestnut | 18.95 | 17.54 | 15.81 | 16.87 | 0.460 | ns | * |
| Commercial feed | 18.63 b,c | 17.53 a,b | 19.63 c | 17.40 a | 0.263 | ** | |
| SEM | 0.242 | 0.175 | 0.924 | 0.431 | |||
| F | ns | ns | * | ns | |||
| Water holding capacity | |||||||
| Cooking loss (%) | |||||||
| Chestnut | 39.34 b | 35.90 a | 42.93 c | 45.43 c | 0.911 | *** | ns |
| Commercial feed | 35.02 a | 33.46 a | 37.55 a,b | 40.66 b | 0.973 | * | |
| SEM | 1.048 | 1.235 | 1.364 | 0.981 | |||
| F | * | ns | * | ** | |||
| Textural parameters | |||||||
| Shear force (N) | |||||||
| Chestnut | 73.95 | 73.50 | 81.18 | 77.09 | 2.893 | ns | ns |
| Commercial feed | 86.92 | 71.43 | 80.90 | 77.57 | 3.672 | ns | |
| SEM | 5.816 | 3.892 | 5.892 | 2.044 | |||
| F | ns | ns | ns | ns | |||
| Roasted | Grilled | Fried | Microwaved | SEM | T | FxT | |
|---|---|---|---|---|---|---|---|
| Aliphatic hydrocarbons | |||||||
| Chestnut | 256.42 a | 216.93 a | 371.16 c | 300.98 b | 15.932 | *** | ns |
| Commercial feed | 91.09 a | 81.56 a | 185.56 c | 117.23 b | 10.632 | *** | |
| SEM | 31.332 | 26.260 | 35.717 | 35.807 | |||
| F | *** | *** | *** | *** | |||
| Aromatic and alicyclic hydrocarbons | |||||||
| Chestnut | 12.29 b | 10.87 b | 5.98 a | 4.35 a | 0.895 | *** | *** |
| Commercial feed | 3.47 a | 3.77 a | 10.78 c | 8.18 b | 0.824 | *** | |
| SEM | 1.709 | 1.384 | 1.028 | 0.759 | |||
| F | *** | *** | ** | *** | |||
| Alcohols | |||||||
| Chestnut | 19.70 | 35.12 | 18.16 | 35.45 | 3.282 | ns | *** |
| Commercial feed | 53.05 b | 50.49 b | 8.51 a | 22.20 a | 5.374 | *** | |
| SEM | 7.354 | 4.291 | 4.515 | 3.739 | |||
| F | ** | ns | ns | ns | |||
| Aldehydes | |||||||
| Chestnut | 1332.71 c | 1439.59 d | 45.84 a | 1127.46 b | 143.844 | *** | ns |
| Commercial feed | 1554.76 c | 1601.21 c | 206.17 a | 1321.84 b | 147.954 | *** | |
| SEM | 45.510 | 44.048 | 32.297 | 51.907 | |||
| F | ** | ns | *** | * | |||
| Others | |||||||
| Chestnut | 204.04 b | 293.27 c | 0.44 a | 189.90 b | 28.085 | *** | ** |
| Commercial feed | 281.80 b | 284.61 b | 3.33 a | 259.83 b | 31.391 | *** | |
| SEM | 16.486 | 13.688 | 0.550 | 17.218 | |||
| F | ** | ns | *** | * | |||
| Total volatile compounds | |||||||
| Chestnut | 1825.15 c | 1995.78 d | 441.58 a | 1658.13 b | 158.561 | *** | ns |
| Commercial feed | 1984.17 c | 2021.63 c | 414.36 a | 1729.28 b | 172.060 | *** | |
| SEM | 38.422 | 38.508 | 13.812 | 45.688 | |||
| F | * | ns | ns | ns | |||
| Roasted | Grilled | Fried | Microwaved | SEM | T | FxT | |
|---|---|---|---|---|---|---|---|
| Aliphatic hydrocarbons | |||||||
| 2,2,4,4-Tetramethyloctane | |||||||
| Chestnut | 7.48 | 7.30 | 8.31 | 8.94 | 0.335 | ns | ns |
| Commercial feed | 5.06 b | 4.59 b | 3.84 a | 4.80 b | 0.156 | * | |
| SEM | 0.532 | 0.723 | 0.855 | 0.817 | |||
| F | ** | * | *** | *** | |||
| 3-Tridecene, (E)- | |||||||
| Chestnut | 0.00 a | 0.00 a | 0.00 a | 3.01 b | 0.369 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 0.000 | 0.649 | |||
| F | ns | ns | ns | ** | |||
| Decane | |||||||
| Chestnut | 18.9 b | 7.10 a | 26.75 c | 18.53 b | 1.930 | *** | *** |
| Commercial feed | 3.93 | 4.76 | 5.80 | 4.27 | 0.547 | ns | |
| SEM | 2.884 | 1.002 | 4.126 | 2.804 | |||
| F | *** | ns | *** | *** | |||
| Decane, 2,3,5-trimethyl- | |||||||
| Chestnut | 13.59 c | 9.48 b | 0.00 a | 13.33 c | 1.519 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 2.588 | 2.041 | 0.000 | 2.557 | |||
| F | *** | ** | ns | *** | |||
| Dodecane | |||||||
| Chestnut | 32.54 b | 17.40 a | 32.02 b | 33.67 b | 1.909 | *** | ns |
| Commercial feed | 13.18 b | 4.32 a | 16.15 b | 18.83 c | 1.596 | *** | |
| SEM | 3.772 | 2.629 | 3.169 | 3.239 | |||
| F | *** | *** | *** | ** | |||
| Dodecane, 2,6,11-trimethyl- | |||||||
| Chestnut | 0.00 a | 0.00 a | 0.00 a | 5.66 b | 0.675 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 0.000 | 1.176 | |||
| F | ns | ns | ns | ** | |||
| Dodecane, 3-methyl- | |||||||
| Chestnut | 3.26 b | 0.00 a | 0.00 a | 6.51 c | 0.747 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.723 | 0.000 | 0.000 | 1.296 | |||
| F | ** | ns | ns | *** | |||
| Heptane | |||||||
| Chestnut | 2.17 b | 0.00 a | 0.00 a | 0.00 a | 0.250 | *** | *** |
| Commercial feed | 0.00 a | 0.00 a | 9.38 b | 0.00 a | 1.067 | *** | |
| SEM | 0.429 | 0.000 | 1.820 | 0.000 | |||
| F | *** | ns | *** | ns | |||
| Heptane, 2,2,4,6,6-pentamethyl- | |||||||
| Chestnut | 44.06 a | 40.42 a,b | 52.85 c | 47.25 b | 1.408 | ** | *** |
| Commercial feed | 34.44 b | 34.46 b | 31.00 a | 29.86 a | 0.621 | ** | |
| SEM | 2.087 | 1.363 | 4.161 | 3.449 | |||
| F | ** | * | *** | *** | |||
| Heptane, 2,2,4-trimethyl- | |||||||
| Chestnut | 0.00 a | 0.00 a | 16.88 b | 0.00 a | 2.037 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 3.564 | 0.000 | |||
| F | ns | ns | ** | ns | |||
| Heptane, 3,3,5-trimethyl- | |||||||
| Chestnut | 9.76 b | 0.00 a | 15.27 b | 0.00 a | 2.001 | ** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 2.006 | 0.000 | 3.549 | 0.000 | |||
| F | ** | ns | * | ns | |||
| Heptane, 3-ethyl- | |||||||
| Chestnut | 4.42 a | 4.19 a | 8.61b | 8.51 b | 0.771 | * | ns |
| Commercial feed | 0.00 a | 1.84 b | 1.92 b | 2.45 b | 0.308 | ** | |
| SEM | 0.888 | 0.733 | 1.447 | 1.343 | |||
| F | *** | ns | ** | ** | |||
| Heptane, 3-methylene- | |||||||
| Chestnut | 8.14 b | 2.79 a | 6.99 b | 6.62 b | 0.690 | * | *** |
| Commercial feed | 0.00 a | 2.92 b | 0.00 a | 3.36 b | 0.481 | ** | |
| SEM | 1.646 | 0.471 | 1.412 | 0.855 | |||
| F | *** | ns | *** | * | |||
| Hexane | |||||||
| Chestnut | 11.36 a | 73.73 c | 50.92 b | 8.67 a | 7.155 | *** | *** |
| Commercial feed | 8.91 a | 8.31 a | 56.58 b | 11.69 a | 5.353 | *** | |
| SEM | 1.273 | 12.495 | 1.721 | 1.799 | |||
| F | ns | *** | ns | ns | |||
| Hexane, 2,2,4-trimethyl- | |||||||
| Chestnut | 0.00 a | 0.00 a | 9.86 b | 0.00 a | 1.199 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 2.105 | 0.000 | |||
| F | ns | ns | ** | ns | |||
| Hexane, 2,2,5-trimethyl- | |||||||
| Chestnut | 2.42 a | 2.53 a | 17.49 b | 10.55 a,b | 2.051 | ** | ** |
| Commercial feed | 1.06 b | 0.00 a | 1.92 c | 1.45 b,c | 0.211 | *** | |
| SEM | 0.434 | 0.550 | 3.832 | 1.889 | |||
| F | ns | ** | * | ** | |||
| Hexane, 3,3-dimethyl- | |||||||
| Chestnut | 7.40 b | 0.00 a | 0.00 a | 0.00 a | 0.896 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 1.569 | 0.000 | 0.000 | 0.000 | |||
| F | ** | ns | ns | ns | |||
| Nonadecane | |||||||
| Chestnut | 1.50 b | 0.00 a | 2.73 c | 0.00 a | 0.314 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.287 | 0.000 | 0.559 | 0.000 | |||
| F | *** | ns | ** | ns | |||
| Nonane, 3-methyl- | |||||||
| Chestnut | 5.21 b | 3.15 a | 7.74 c | 5.12 b | 0.508 | ** | *** |
| Commercial feed | 0.00 a | 0.00 a | 0.00 a | 2.26 b | 0.262 | *** | |
| SEM | 1.003 | 0.707 | 1.504 | 0.596 | |||
| F | *** | *** | *** | ** | |||
| Octane | |||||||
| Chestnut | 7.88 | 5.80 | 8.18 | 5.91 | 0.542 | ns | ns |
| Commercial feed | 6.52 | 5.04 | 11.20 | 4.26 | 1.078 | ns | |
| SEM | 0.678 | 0.850 | 1.686 | 0.559 | |||
| F | ns | ns | ns | ns | |||
| Octane, 2,2-dimethyl- | |||||||
| Chestnut | 0.00 a | 0.00 a | 0.00 a | 15.54 b | 1.904 | *** | *** |
| Commercial feed | 0.00 a | 0.00 a | 3.94 b | 0.00 a | 0.500 | *** | |
| SEM | 0.000 | 0.000 | 0.892 | 3.350 | |||
| F | ns | ns | * | ** | |||
| Octane, 3-methyl-6-methylene- | |||||||
| Chestnut | 8.53 b | 0.00 a | 10.31 b | 0.00 a | 1.375 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 1.842 | 0.000 | 2.157 | 0.000 | |||
| F | ** | ns | ** | ns | |||
| Pentane, 2,3,3-trimethyl- | |||||||
| Chestnut | 9.21 a,b | 5.86 a | 13.83 b,c | 17.79 c | 1.435 | *** | ns |
| Commercial feed | 0.00 a | 0.00 a | 9.89 b | 7.12 b | 1.318 | *** | |
| SEM | 2.036 | 1.382 | 1.693 | 2.220 | |||
| F | ** | * | ns | ** | |||
| Pentane, 2,3,4-trimethyl- | |||||||
| Chestnut | 2.89 a | 1.16 a | 8.04 b | 4.79 a,b | 0.860 | ** | * |
| Commercial feed | 1.00 | 1.51 | 2.00 | 1.96 | 0.194 | ns | |
| SEM | 0.469 | 0.315 | 1.581 | 0.571 | |||
| F | * | ns | ** | *** | |||
| Pentane, 3-ethyl-2-methyl- | |||||||
| Chestnut | 0.00 a | 0.00 a | 0.00 a | 12.48 b | 1.460 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 0.000 | 2.520 | |||
| F | ns | ns | ns | *** | |||
| Tridecane | |||||||
| Chestnut | 8.67 b | 6.04 a | 6.98 a | 6.42 a | 0.334 | ** | *** |
| Commercial feed | 2.98 a | 2.47 a | 4.11 b | 4.88 b | 0.292 | ** | |
| SEM | 1.109 | 0.717 | 0.605 | 0.424 | |||
| F | *** | *** | ** | ns | |||
| Undecane | |||||||
| Chestnut | 47.02 b | 19.38 a | 57.02 c | 50.92 b,c | 3.841 | *** | *** |
| Commercial feed | 11.02 a | 7.68 a | 23.99 b | 20.03 b | 2.141 | ** | |
| SEM | 6.881 | 2.460 | 6.568 | 6.223 | |||
| F | *** | ** | *** | *** | |||
| Undecane, 3-methyl- | |||||||
| Chestnut | 0.00 a | 8.05 b | 10.40 b | 10.80 b | 1.238 | *** | *** |
| Commercial feed | 2.99 b | 3.68 b | 3.87 b | 0.00 a | 0.506 | ** | |
| SEM | 0.610 | 1.275 | 1.294 | 2.142 | |||
| F | *** | ns | *** | *** | |||
| Undecane, 4,6-dimethyl- | |||||||
| Chestnut | 0.00 a | 2.55 b | 0.00 a | 0.00 a | 0.327 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.586 | 0.000 | 0.000 | |||
| F | ns | * | ns | ns | |||
| Aromatic and cyclic hydrocarbons | |||||||
| .alpha.-Pinene | |||||||
| Chestnut | 0.00 a | 0.00 a | 0.00 a | 4.03 b | 0.457 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 0.000 | 0.778 | |||
| F | ns | ns | ns | *** | |||
| 1R-.alpha.-Pinene | |||||||
| Chestnut | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | *** |
| Commercial feed | 0.00 a | 0.00 a | 5.24 c | 2.39 b | 0.561 | *** | |
| SEM | 0.000 | 0.000 | 0.999 | 0.453 | |||
| F | ns | ns | *** | *** | |||
| Cyclopentane, nonyl- | |||||||
| Chestnut | 4.69 c | 2.35 b | 0.00 a | 0.00 a | 0.527 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.916 | 0.505 | 0.000 | 0.000 | |||
| F | *** | ** | ns | ns | |||
| Diethyl Phthalate | |||||||
| Chestnut | 0.00 a | 0.00 a | 0.31 b | 0.32 b | 0.049 | ** | ns |
| Commercial feed | 0.00 a | 0.00 a | 0.41 c | 0.24 b | 0.047 | *** | |
| SEM | 0.000 | 0.000 | 0.053 | 0.043 | |||
| F | ns | ns | ns | ns | |||
| Oxime-, methoxy-phenyl-_ | |||||||
| Chestnut | 7.59 b | 8.52 b | 0.00 a | 0.00 a | 1.064 | *** | *** |
| Commercial feed | 3.47 a | 3.77 a,b | 5.12 b,c | 5.55 c | 0.314 | * | |
| SEM | 0.884 | 0.933 | 1.036 | 1.056 | |||
| F | ** | *** | *** | *** | |||
| Pyrazine, 3-ethyl-2,5-dimethyl- | |||||||
| Chestnut | 0.00 a | 0.00 a | 5.68 b | 0.00 a | 0.648 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 1.105 | 0.000 | |||
| F | ns | ns | *** | ns | |||
| Alcohols | |||||||
| 1-Decanol, 2-ethyl- | |||||||
| Chestnut | 0.00 a | 1.54 b | 0.00 a | 0.00 a | 0.182 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.315 | 0.000 | 0.000 | |||
| F | ns | ** | ns | ns | |||
| 1-Hexanol | |||||||
| Chestnut | 0.00 a | 7.36 b | 0.00 a | 0.00 a | 0.837 | *** | *** |
| Commercial feed | 5.39 b | 4.71 b | 0.00 a | 0.00 a | 0.741 | *** | |
| SEM | 1.035 | 0.912 | 0.000 | 0.000 | |||
| F | *** | ns | ns | ns | |||
| 1-Octanol, 2-butyl- | |||||||
| Chestnut | 0.00 a | 0.00 a | 0.00 a | 6.09 b | 0.789 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 0.000 | 1.417 | |||
| F | ns | ns | ns | * | |||
| 1-Pentanol | |||||||
| Chestnut | 17.65 b | 26.23 c | 0.00 a | 16.68 b | 2.628 | *** | * |
| Commercial feed | 47.65 b | 45.77 b | 8.51 a | 22.20 a | 4.771 | *** | |
| SEM | 6.777 | 4.724 | 1.840 | 1.482 | |||
| F | ** | * | ** | ns | |||
| 2,3-Butanediol | |||||||
| Chestnut | 0.00 a | 0.00 a | 18.16 b | 12.68 a,b | 3.144 | * | ns |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.000 | 0.000 | 5.295 | 3.712 | |||
| F | ns | ns | ns | ns | |||
| 2-Isopropyl-5-methyl-1-heptanol | |||||||
| Chestnut | 2.05 b | 0.00 a | 0.00 a | 0.00 a | 0.247 | *** | *** |
| Commercial feed | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | |
| SEM | 0.432 | 0.000 | 0.000 | 0.000 | |||
| F | ** | ns | ns | ns | |||
| Aldehydes | |||||||
| 2-Hexenal, (E)- | |||||||
| Chestnut | 1.41 b | 1.48 b | 0.00 a | 0.00 a | 0.195 | *** | *** |
| Commercial feed | 1.36 c | 1.47 c | 0.00 a | 0.95 b | 0.154 | *** | |
| SEM | 0.088 | 0.088 | 0.000 | 0.191 | |||
| F | ns | ns | ns | *** | |||
| Butanal, 3-methyl- | |||||||
| Chestnut | 0.00 a | 0.00 a | 4.88 b | 0.00 a | 0.556 | *** | ns |
| Commercial feed | 0.00 a | 0.00 a | 4.88 b | 0.00 a | 0.575 | *** | |
| SEM | 0.000 | 0.000 | 0.433 | 0.000 | |||
| F | ns | ns | ns | ns | |||
| Heptanal | |||||||
| Chestnut | 28.01 b | 42.20 c | 11.46 a | 24.73 b | 3.002 | *** | ns |
| Commercial feed | 32.89 c | 36.09 c | 10.20 a | 24.81 b | 2.734 | *** | |
| SEM | 1.886 | 1.981 | 0.516 | 1.548 | |||
| F | ns | ns | ns | ns | |||
| Hexanal | |||||||
| Chestnut | 1178.88 c | 1268.93 c | 29.50 a | 1031.90 b | 128.940 | *** | ns |
| Commercial feed | 1412.07 c | 1475.43 c | 159.00 a | 1203.04 b | 138.668 | *** | |
| SEM | 47.538 | 49.643 | 27.288 | 46.649 | |||
| F | *** | * | ** | ns | |||
| Octanal | |||||||
| Chestnut | 40.04 b | 42.33 b | 0.00 a | 0.00 a | 5.380 | *** | *** |
| Commercial feed | 31.52 b,c | 33.60 c | 23.03 a | 25.67 a,b | 1.418 | ** | |
| SEM | 1.948 | 2.502 | 4.430 | 4.921 | |||
| F | * | ns | *** | *** | |||
| Pentanal | |||||||
| Chestnut | 84.36 c | 84.64 c | 0.00 a | 70.82 b | 9.094 | *** | *** |
| Commercial feed | 76.92 d | 54.62 b | 9.06 a | 67.38 c | 6.846 | *** | |
| SEM | 2.081 | 6.050 | 1.736 | 2.039 | |||
| F | ns | *** | *** | ns | |||
| Others | |||||||
| 2-Heptanone | |||||||
| Chestnut | 5.04 c | 4.68 c | 0.00 a | 3.78 b | 0.529 | *** | ns |
| Commercial feed | 5.59 c | 5.73 c | 1.12 a | 3.47 a | 0.521 | *** | |
| SEM | 0.219 | 0.293 | 0.213 | 0.358 | |||
| F | ns | ns | *** | ns | |||
| 4-Hydroxymandelic acid, ethyl ester, di-TMS | |||||||
| Chestnut | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | ns | *** |
| Commercial feed | 0.00 a | 0.00 a | 0.00 a | 2.59 b | 0.292 | *** | |
| SEM | 0.000 | 0.000 | 0.000 | 0.496 | |||
| F | ns | ns | ns | *** | |||
| Caprolactam | |||||||
| Chestnut | 0.00 a | 0.00 a | 0.44 b | 0.00 a | 0.049 | *** | *** |
| Commercial feed | 0.49 b | 0.00 a | 0.31 b | 0.00 a | 0.060 | *** | |
| SEM | 0.107 | 0.000 | 0.027 | 0.000 | |||
| F | ** | ns | ** | ns | |||
| Furan, 2-pentyl | |||||||
| Chestnut | 8.41 b | 7.98 b | 0.00 a | 8.91 b | 0.965 | *** | *** |
| Commercial feed | 6.98 c | 5.78 b | 1.91 a | 5.48 b | 0.513 | *** | |
| SEM | 0.385 | 0.490 | 0.364 | 0.728 | |||
| F | ns | ** | *** | ** | |||
| n-Caproic acid vinyl ester | |||||||
| Chestnut | 190.59 b | 280.61 c | 0.00 a | 177.21 b | 26.834 | *** | ** |
| Commercial feed | 268.75 b | 273.10 b | 0.00 a | 248.29 b | 30.423 | *** | |
| SEM | 16.577 | 13.704 | 0.000 | 17.178 | |||
| F | ** | ns | ns | * | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echegaray, N.; Pateiro, M.; Zhang, W.; Domínguez, R.; Campagnol, P.C.B.; Carballo, J.; Lorenzo, J.M. Influence of the Inclusion of Chestnut (Castanea sativa Miller) in the Finishing Diet and Cooking Technique on the Physicochemical Parameters and Volatile Profile of Biceps femoris Muscle. Foods 2020, 9, 754. https://doi.org/10.3390/foods9060754
Echegaray N, Pateiro M, Zhang W, Domínguez R, Campagnol PCB, Carballo J, Lorenzo JM. Influence of the Inclusion of Chestnut (Castanea sativa Miller) in the Finishing Diet and Cooking Technique on the Physicochemical Parameters and Volatile Profile of Biceps femoris Muscle. Foods. 2020; 9(6):754. https://doi.org/10.3390/foods9060754
Chicago/Turabian StyleEchegaray, Noemi, Mirian Pateiro, Wangang Zhang, Rubén Domínguez, Paulo C. B. Campagnol, Javier Carballo, and José M. Lorenzo. 2020. "Influence of the Inclusion of Chestnut (Castanea sativa Miller) in the Finishing Diet and Cooking Technique on the Physicochemical Parameters and Volatile Profile of Biceps femoris Muscle" Foods 9, no. 6: 754. https://doi.org/10.3390/foods9060754
APA StyleEchegaray, N., Pateiro, M., Zhang, W., Domínguez, R., Campagnol, P. C. B., Carballo, J., & Lorenzo, J. M. (2020). Influence of the Inclusion of Chestnut (Castanea sativa Miller) in the Finishing Diet and Cooking Technique on the Physicochemical Parameters and Volatile Profile of Biceps femoris Muscle. Foods, 9(6), 754. https://doi.org/10.3390/foods9060754










