Reducing Oil Separation in Ready-to-Use Therapeutic Food
Abstract
1. Introduction
2. Materials and Methods
2.1. RUTF Ingredients
2.2. Benchtop RUTF Preparation and Storage
2.3. Vegetable Oil Stabilizers to Reduce Oil Separation
2.4. Performance at Industrial Scale
- All dry ingredients were added into a large ribbon blender in the same sequence as was done in the benchtop method. No external heat was added to the mixture other than the preheating of the vegetable oil and stabilizers to 65 °C. The RUTF was mixed for 25 min.
- The RUTF was transferred from the ribbon blender to a 50 °C water-jacketed, constantly stirred holding tank. In the transfer process, the RUTF passes through a vertical, conical grinder with a 3 cm orifice and high-speed rotating bit. The purpose of the grinder is to disperse aggregates of milk powder or other dry matter into the lipid liquid.
- The passage of the heated, stirred mixture through a disk mill and then into the packaging machine. The temperature as the RUTF passed out of the disk mill was 70 °C.
2.5. Definition and Measurement of Oil Separation in RUTF
3. Results
3.1. Variability of Experimental Method and Dynamic Nature of Oil Separation
3.2. Effect of Vegetable Oil Stabilizers in RUTF to Reduce Oil Separation
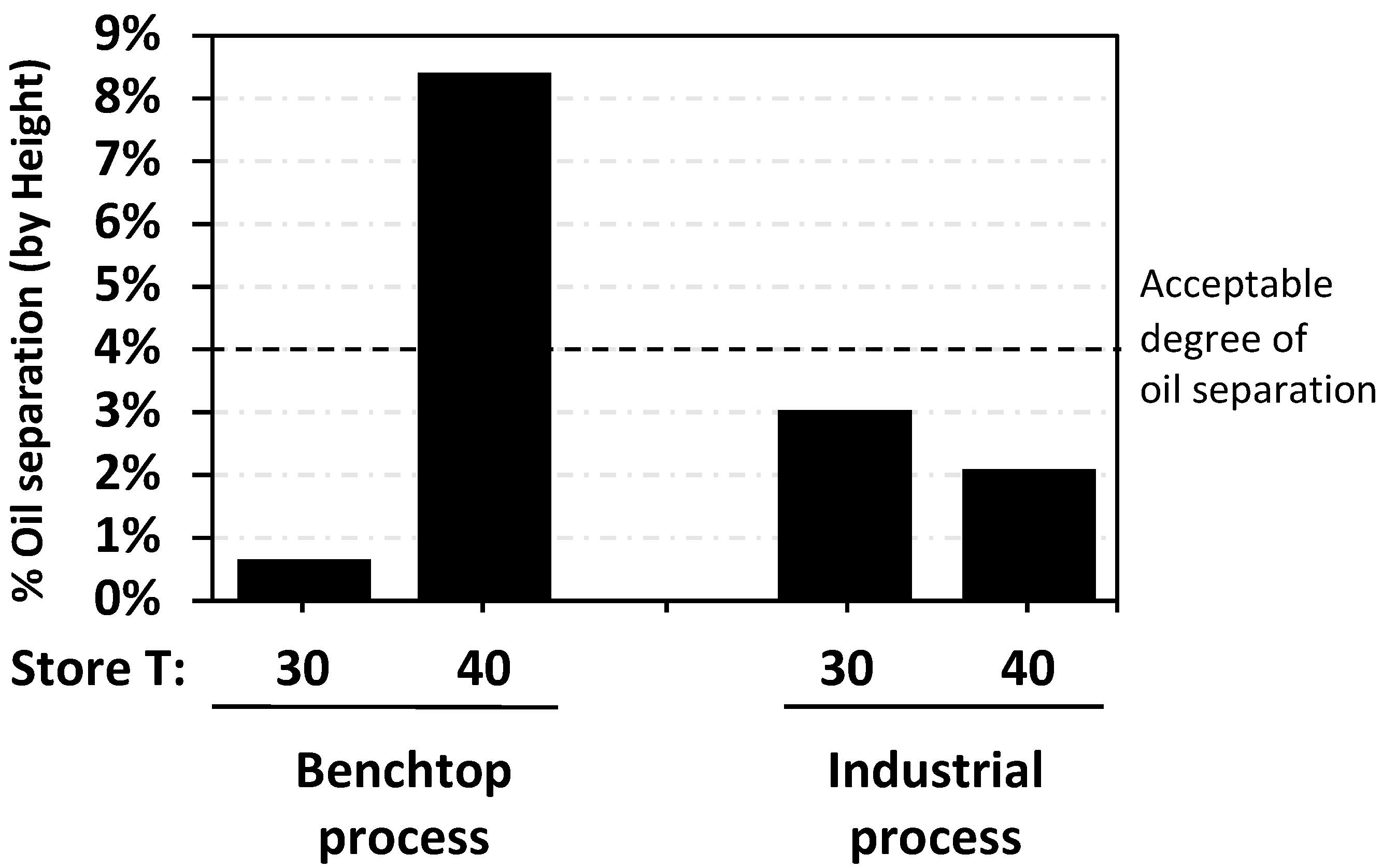
3.3. Comparison of Oil Separation in RUTF at 30 °C and 40 °C
3.4. Industrial Scale Performance of Best Performing Additive to Reduce Oil Separation
4. Discussion
5. Conclusions
- The dynamic nature of oil separation in RUTF follows a Michaelis–Menton pattern with a maximum separation seen after 60 d.
- Triglyceride and 50% monoglyceride stabilizers made from hydrogenated vegetable oils achieved an oil separation <4% in RUTF on the benchtop and on an industrial scale.
- When RUTF is stored at 40 °C, instead of the typical storage at 30 °C, the oil separation is greater and follows a pattern that differs from the industrial RUTF that is in widescale use. We find no reason to recommend stability testing at 40 °C for the purposes of assessing oil separation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization; World Food Programme; UN Standing Nutrition Committee. UNICEF Community-Based Management of Severe Acute Malnutrition—A Joint Statement by the WHO, WFP, UNSSCN; UNICEF: New York, NY, USA, 2007.
- UNICEF. Technical Requirements of Ready-to-Use Therapeutic Foods, Request for Proposal; RFP-DAN-2013-501715; UNICEF: New York, NY, USA, 2013.
- Santini, A.; Novellino, E.; Armini, V.; Ritieni, A. State of the art of ready-to-use therapeutic food: A tool for nutraceuticals addition to foodstuff. Food Chem. 2013, 140, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Briend, A.; Akomo, P.; Bahwere, P.; De Pee, S.; Dibari, F.; Golden, M.H.; Manary, M.; Ryan, K. Developing food supplements for moderately malnourished children: Lessons learned from ready-to-use therapeutic foods. Food Nutr. Bull. 2015, 36 (Suppl. 1), S53–S58. [Google Scholar] [CrossRef] [PubMed]
- Maust, A.; Koroma, A.S.; Abla, C.; Molokwu, N.; Ryan, K.N.; Singh, L.; Manary, M.J. Severe and Moderate Acute Malnutrition Can Be Successfully Managed with an Integrated Protocol in Sierra Leone. J. Nutr. 2015, 145, 2604–2609. [Google Scholar] [CrossRef] [PubMed]
- Mallewa, J.; Szubert, A.J.; Mugyenyi, P.; Chidziva, E.; Thomason, M.J.; Chepkorir, P.; Abongomera, G.; Baleeta, K.; Etyang, A.; Warambwa, C.; et al. Effect of ready-to-use supplementary food on mortality in severely immunocompromised HIV-infected individuals in Africa initiating antiretroviral therapy (REALITY): An open-label, parallel-group, randomised controlled trial. Lancet HIV 2018, 5, e231–e240. [Google Scholar] [CrossRef]
- Ndekha, M.J.; Manary, M.J.; Ashorn, P.; Briend, A. Home-based therapy with ready-to-use therapeutic food is of benefit to malnourished, HIV-infected Malawian children. Acta Paediatr. 2005, 94, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Thakwalakwa, C.; Phiri, A.; Rollins, N.; Heikens, G.T.; Barnell, E.K.; Manary, M. Growth and HIV-free survival of HIV-exposed infants in Malawi: A randomized trial of two complementary feeding interventions in the context of maternal antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2014, 66, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sunguya, B.F.; Poudel, K.C.; Mlunde, L.B.; Otsuka, K.; Yasuoka, J.; Urassa, D.P.; Mkopi, N.P.; Jimba, M. Ready to Use Therapeutic Foods (RUTF) improves undernutrition among ART-treated, HIV-positive children in Dar es Salaam, Tanzania. Nutr. J. 2012, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Glosz, C.M.; Schaffner, A.A.; Reaves, S.K.; Manary, M.J.; Papathakis, P.C. Effect of Nutritional Interventions on Micronutrient Status in Pregnant Malawian Women with Moderate Malnutrition: A Randomized, Controlled Trial. Nutrients 2018, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Hendrixson, D.T.; Koroma, A.S.; Callaghan-Gillespie, M.; Weber, J.; Papathakis, P.; Manary, M.J. Use of a novel supplementary food and measures to control inflammation in malnourished pregnant women in Sierra Leone to improve birth outcomes: Study protocol for a prospective, randomized, controlled clinical effectiveness trial. BMC Nutr. 2018, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Ordiz, M.I.; Ryan, K.N.; Cimo, E.D.; Stoner, M.E.; Loehnig, M.E.; Manary, M.J. Effect of emulsifier and viscosity on oil separation in ready-to-use therapeutic food. Int. J. Food Sci. Nutr. 2015, 66, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Manary, M.J. Local production and provision of ready-to-use therapeutic food (RUTF) spread for the treatment of severe childhood malnutrition. Food Nutr. Bull. 2006, 27 (Suppl. 3), S83–S89. [Google Scholar] [CrossRef] [PubMed]
- Irena, A.H.; Bahwere, P.; Owino, V.O.; Diop, E.I.; Bachmann, M.O.; Mbwili-Muleya, C.; Dibari, F.; Sadler, K.; Collins, S. Comparison of the effectiveness of a milk-free soy-maize-sorghum based ready-to-use therapeutic food with 25% milk in nutrition management of severely acutely malnourished Zambian children: An equivalence non-blinded cluster randomized controlled trial. Matern. Child Nutr. 2015, 11, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Bahwere, P.; Balaluka, B.; Wells, J.C.; Mbiribindi, C.N.; Sadler, K.; Akomo, P.; Dramaix-Wilmet, M.; Collins, S. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk- and peanut-based formulation for treating severe acute malnutrition: A noninferiority, individually randomized controlled efficiacy clinical trial. Am. J. Clin. Nutr. 2016, 103, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Hendrixson, D.T.; Godbout, C.; Los, A.; Callaghan-Gillespie, M.; Mui, M.; Wegner, D.; Bryant, T.; Koroma, A.; Manary, M.J. Treatment of Severe Acute Malnutrition With Oat or Standard Ready-To-Use Therapeutic Food: A Triple-Blind, Randomised Controlled Clinical Trial. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- Csáki, K.F. Synthetic surfactant food additives can cause intestinal barrier dysfunction. Med. Hypotheses 2011, 76, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Koren, O.; Goodrich, J.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
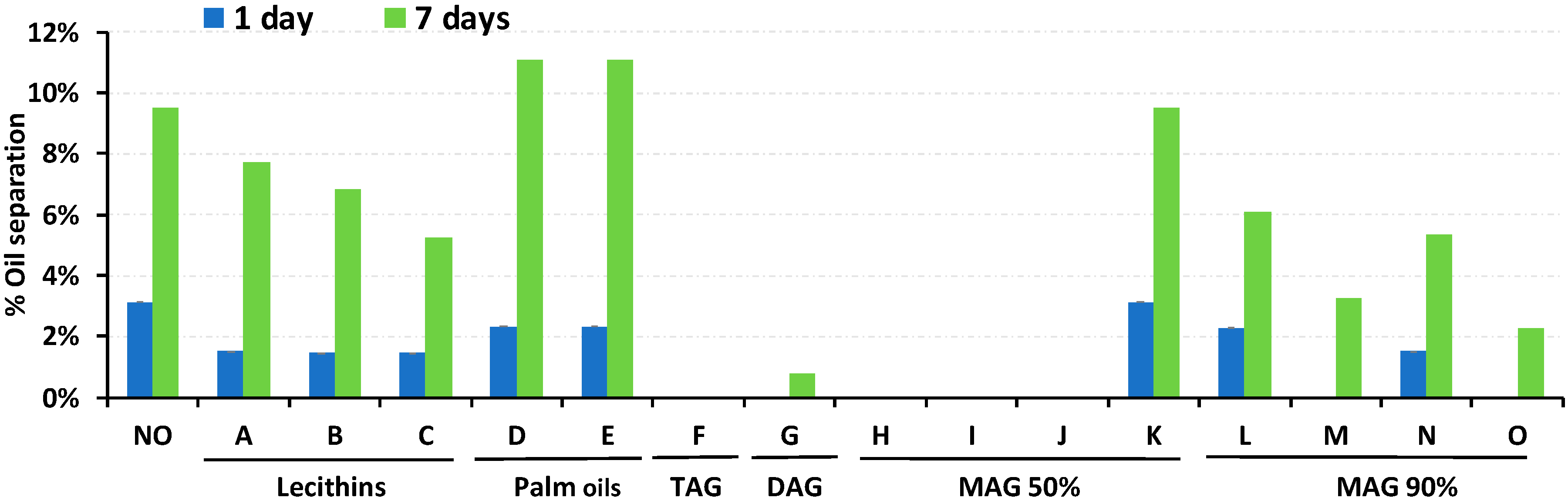
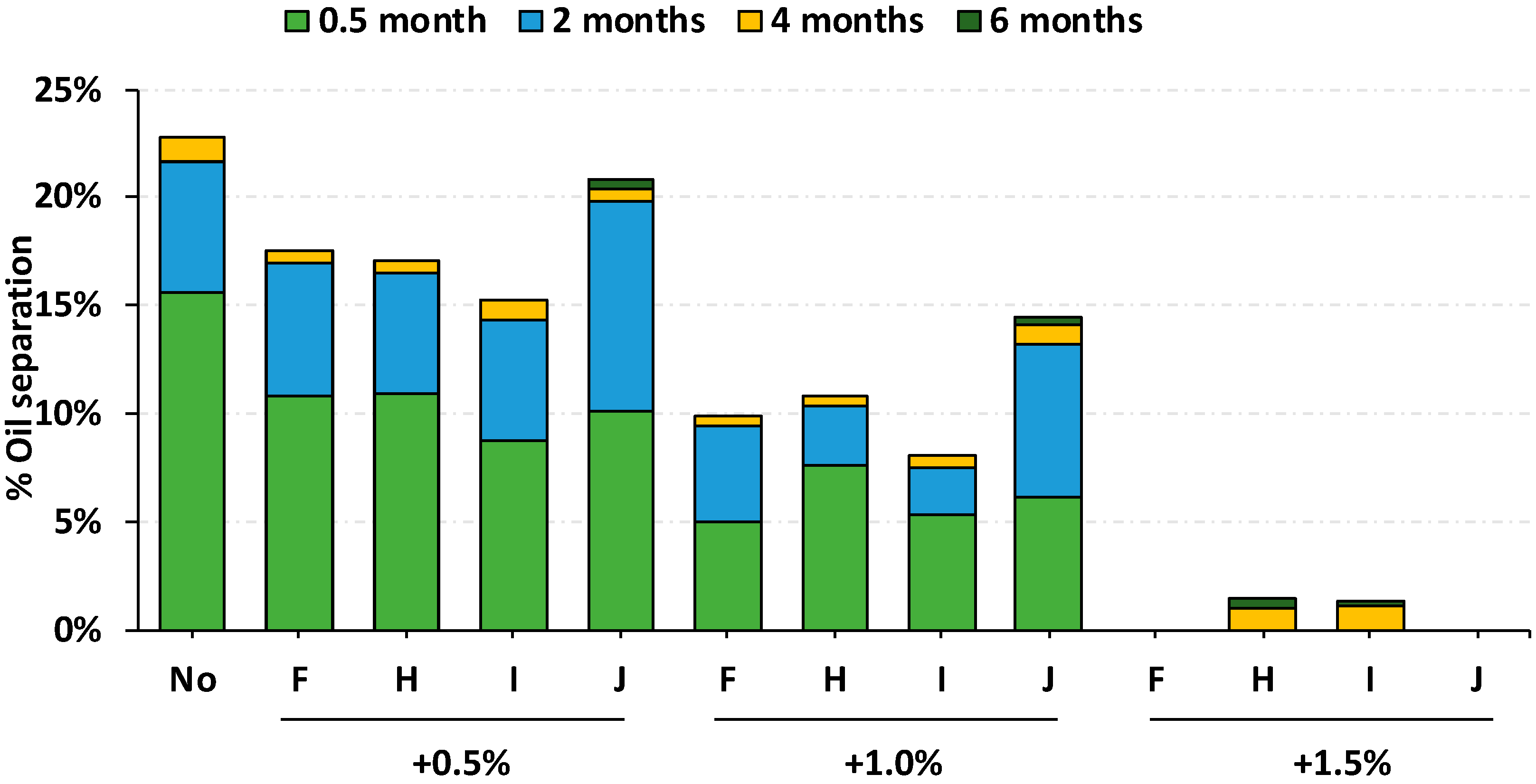
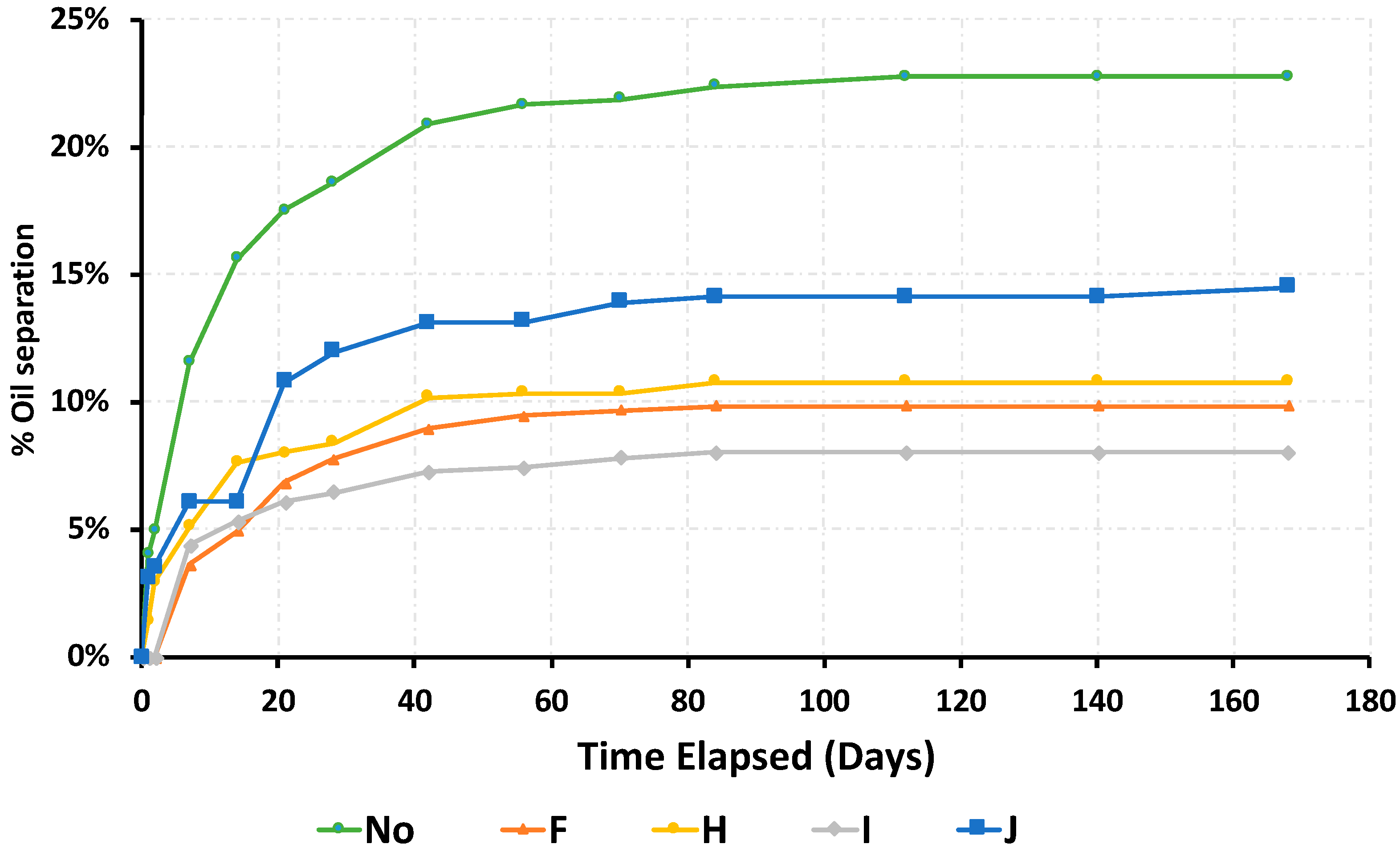
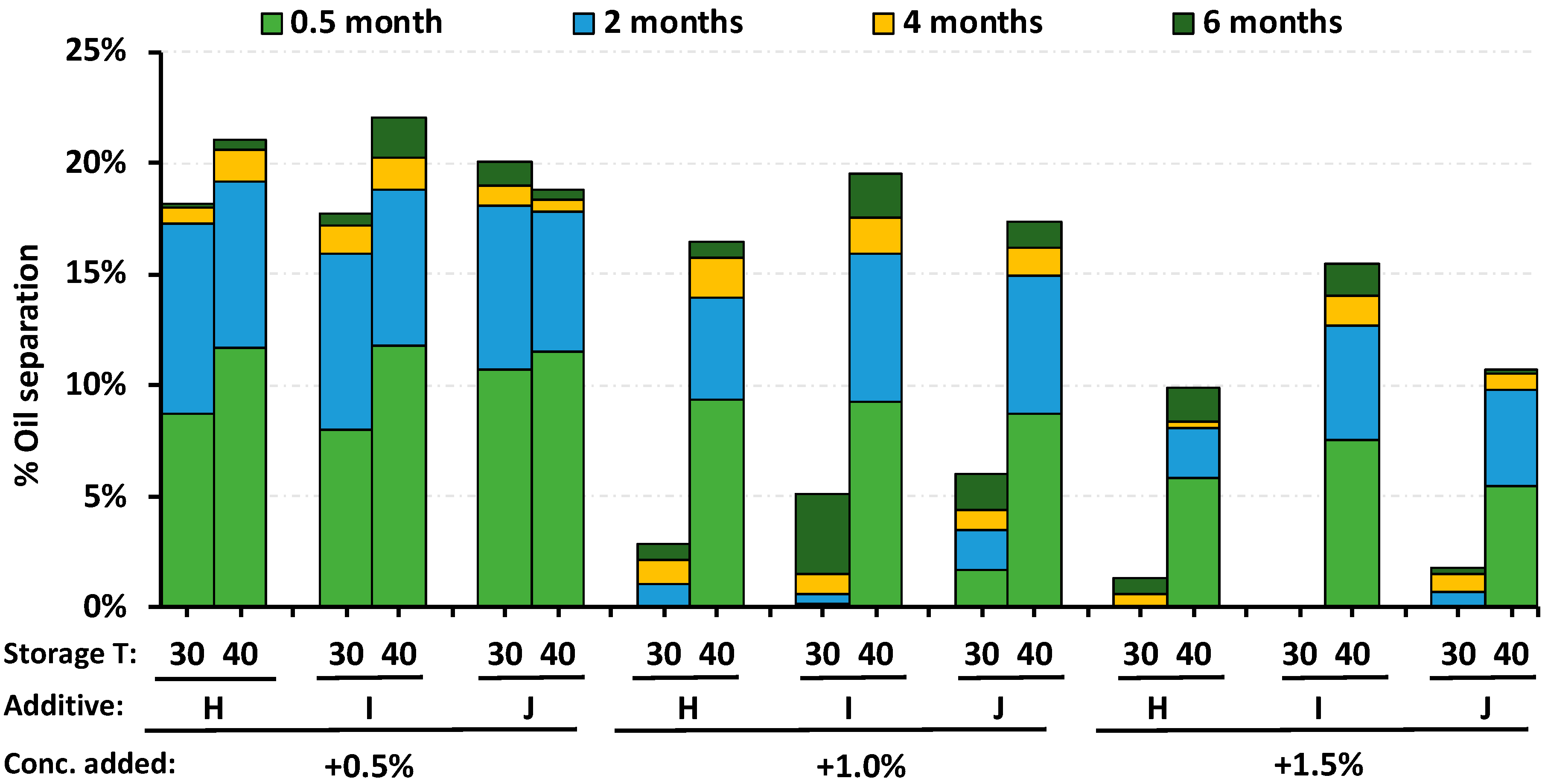
| Additive Type | Mono-Glyceride Content | Free Fatty Acid (max) | Free Glycerin (max) | Iodine Value | Ingredient Form | Melting/Dropping Point | Trans Fat | Additive Product Name |
|---|---|---|---|---|---|---|---|---|
| Lecithins | ||||||||
| A. Soy Lecithin | Not stated | Not stated | Not stated | Not stated | Liquid | N/A | Not stated | Generic Soy lecithin |
| B. Deoiled soy lecithin | Not stated | Not stated | Not stated | Not stated | Powder | N/A | Not stated | Lecigran™ 1000 P |
| C. Soy lecithin | Not stated | Not stated | Not stated | Not stated | Powder | N/A | Not stated | Metarin®® P |
| Palm oils | ||||||||
| D. Refined, bleached and dried palm oil | <3% | <0.1% | Not stated | 58 | Liquid-solid | 22 °C | Not stated | Generic Palm Olein |
| E. Non-hydrogenated, modified, liquid palm oil | <3% | 0.05% | Not stated | Not stated | Liquid | 20 °C | <1% | Durkex™NT 100-MB |
| Triglycerides | ||||||||
| F. Fully hydrogenated rapeseed oil | 3%–8% | 0.50% | Not stated | 3 g I2/100 g | Pellets | 61 °C | Not stated | Palsgaard®® 6111 pellets |
| Diglycerides | ||||||||
| G. Soy lecithin | 3%–8% | 1% | 1.50% | 1.5 | Bead | 70 °C | <0.1% | Trancendim®® 180 |
| Monoglyceride composition about 50% | ||||||||
| H. Fully hydrogenated soybean oil | ≥42% | 1% | 1% | 3 | Beads | 60–65 °C | Not stated | BFP®® 74K |
| I. Refined fully hydrogenated vegetable fat blend | 40%–60% | 1.5% | 2% | 2 | Beads | 64 °C | <0.5% | Grindsted®® Mono-Di HV 52 K-A |
| J. Hydrogenated soybean oil | ≥52% | Not stated | 2% | Not stated | Beads | 57–62 °C | <0.1% | Aldo™ HMS KFG |
| K. Hydrogenated vegetable oil | 46% | Not stated | 1.5% | Not stated | Liquid-solid | 19–23 °C | Not Detected | Aldo™ MO KFG |
| Monoglyceride composition about 90% | ||||||||
| L. Fully hydrogenated soybean oil | 90% | 1.50% | 1% | 1 | Beads | 72 °C | < 0.5% | Dimodan®® HS K-A |
| M. Fully hydrogenated palm-based oil | 90% | 1.50% | 1% | 1 | Beads | 69 °C | <1% | Dimodan®® HP US MB |
| N. Hydrogenated soybean oil | 90% | 1.50% | 1.20% | 1.2 | Bead | 72 °C | Not stated | Alphadim®® 90 SBK |
| O. Rapeseed | 90% | Not stated | 1% | 1 | Powder | 80 °C | <1% | Grindsted®® Crystallizer 110 R Kosher |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuzarte, A.; Mui, M.; Ordiz, M.I.; Weber, J.; Ryan, K.; Manary, M.J. Reducing Oil Separation in Ready-to-Use Therapeutic Food. Foods 2020, 9, 706. https://doi.org/10.3390/foods9060706
Zuzarte A, Mui M, Ordiz MI, Weber J, Ryan K, Manary MJ. Reducing Oil Separation in Ready-to-Use Therapeutic Food. Foods. 2020; 9(6):706. https://doi.org/10.3390/foods9060706
Chicago/Turabian StyleZuzarte, Andrea, Melody Mui, Maria Isabel Ordiz, Jacklyn Weber, Kelsey Ryan, and Mark J. Manary. 2020. "Reducing Oil Separation in Ready-to-Use Therapeutic Food" Foods 9, no. 6: 706. https://doi.org/10.3390/foods9060706
APA StyleZuzarte, A., Mui, M., Ordiz, M. I., Weber, J., Ryan, K., & Manary, M. J. (2020). Reducing Oil Separation in Ready-to-Use Therapeutic Food. Foods, 9(6), 706. https://doi.org/10.3390/foods9060706




