The Inhibition of Amylase and ACE Enzyme and the Reduction of Immunoreactivity of Sourdough Bread
Abstract
1. Introduction
2. Materials and Methods
Results Reading
3. Results and Discussion
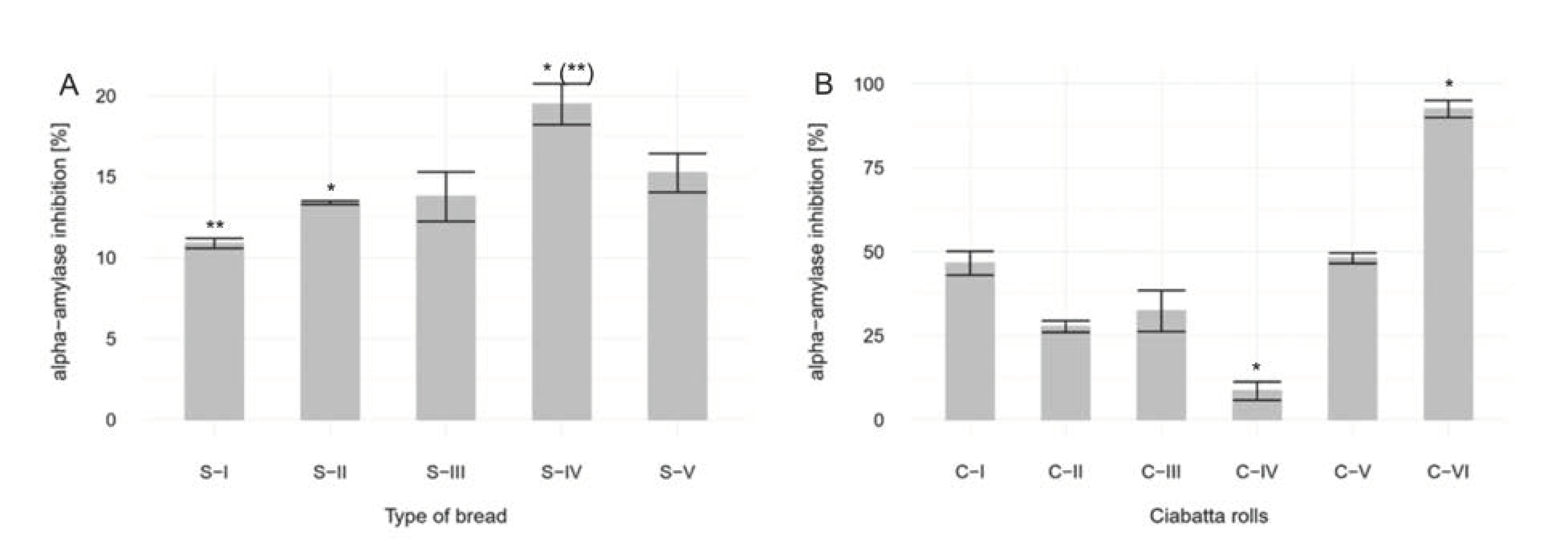
3.1. Analysis of Inhibitors of Alpha-amylase
3.1.1. Without in Vitro Digestion. Analysis of Sourdough Bread
3.1.2. With in Vitro digestion. Analysis of Ciabatta Rolls
3.2. Analysis of Angiotensin-Converting Enzyme
3.2.1. Without in Vitro Digestion. Analysis of Sourdough Bread
3.2.2. With in Vitro Digestion. Analysis of Ciabatta Rolls
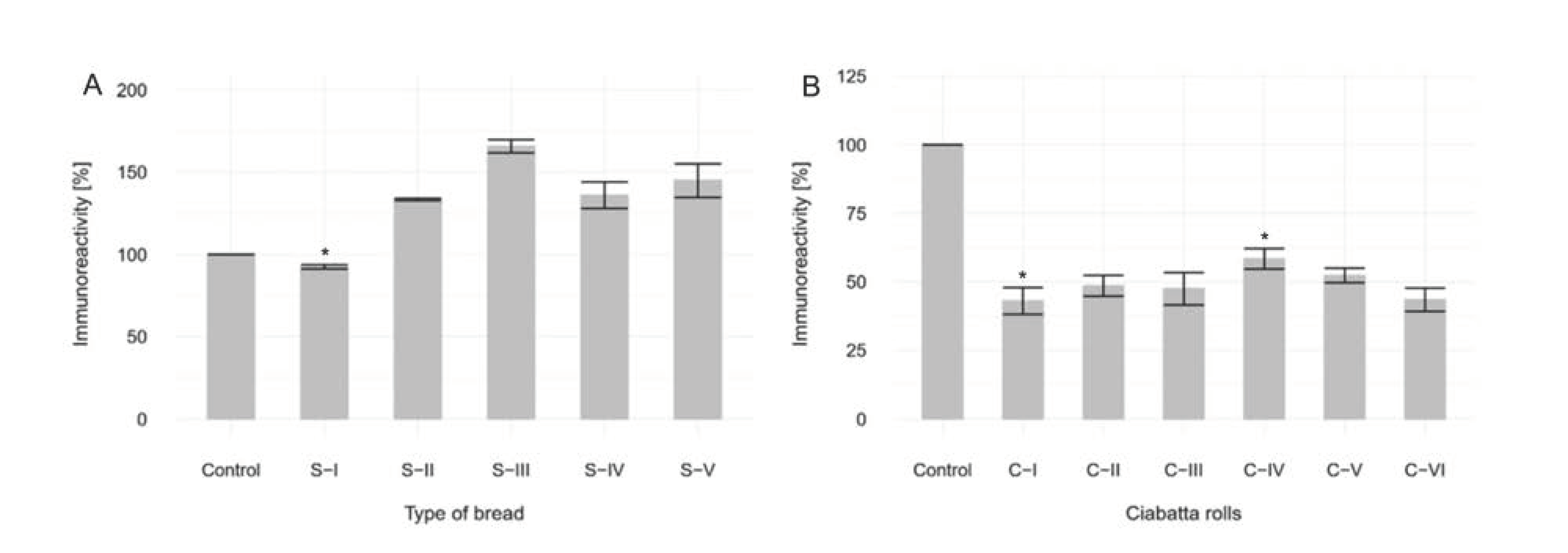
3.3. Elisa Immunoreactivity Assay
3.3.1. Without in Vitro Digestion. Analysis of Sourdough Bread
3.3.2. With in Vitro Digestion. Analysis of Ciabatta Rolls
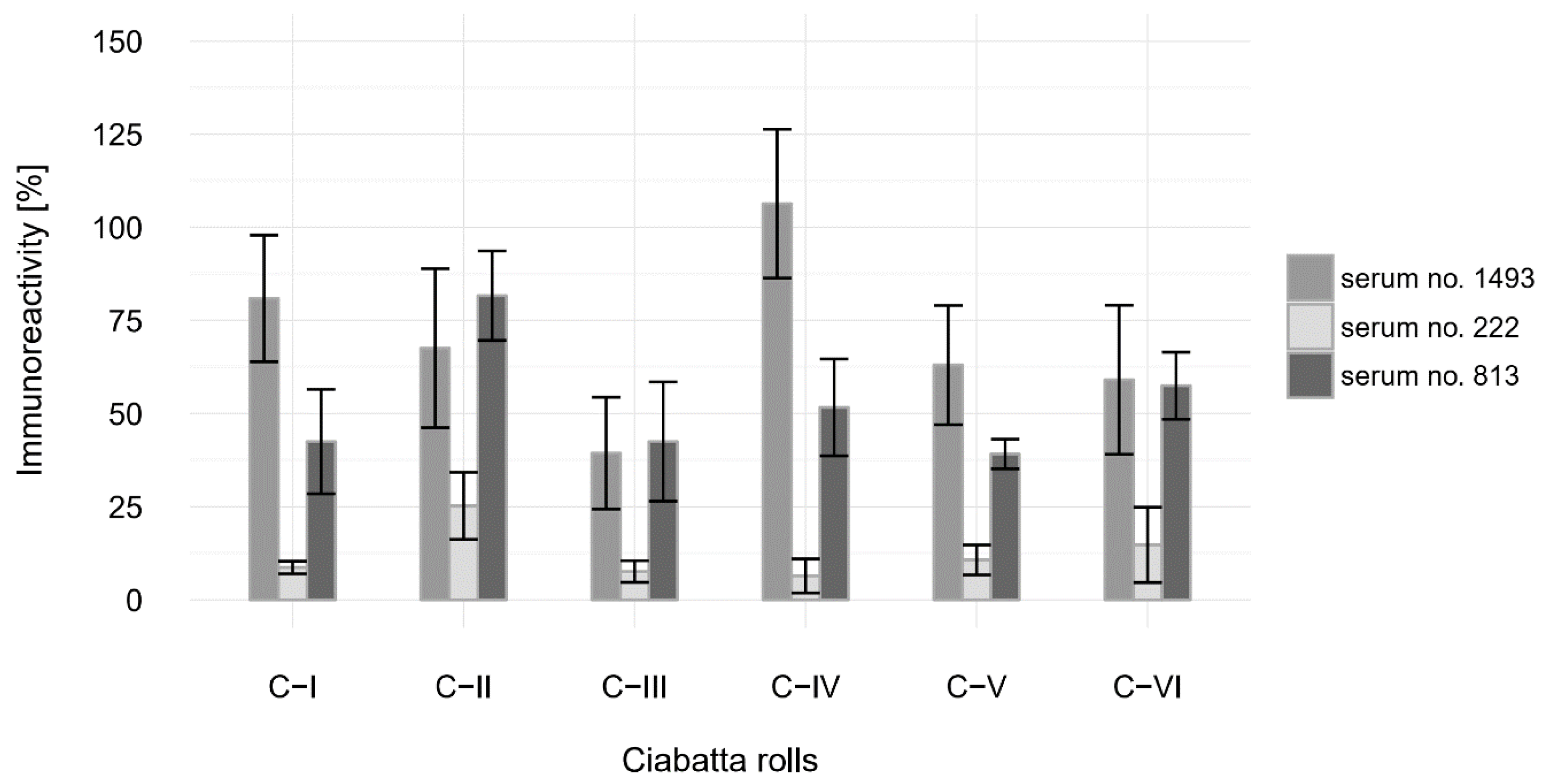
3.4. Immunoreactivity Analysis With Human Antibodies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diet, Nutrition and the Prevention of Chronic Diseases: Report of the Joint WHO/FAO Expert Consultation. Available online: http://www.who.int/dietphysicalactivity/publications/trs916/summary/en/ (accessed on 12 February 2020).
- Libman, K.; Freudenberg, N.; Sanders, D.; Puoane, T. The role of urban food policy in preventing diet-related non-communicable diseases in Cape Town and New York. Public Health 2015, 129, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.P.; Williams, K.; Narayan, K.M.V.; Leibson, C.; Haffner, S.M.; Stern, M.P. A Population Perspective on Diabetes Prevention: Whom should we target for preventing weight gain? Diabetes Care 2003, 26, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Bhutkar, M.A. In Vitro Studies on Alpha Amylase Inhibitory Activity of Some Indigenous Plants. Int. J. Chem. Sci. 2012, 10, 457–462. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Martín-Álvarez, P.J.; Pueyo, E. Assessment of the Spectrophotometric Method for Determination of Angiotensin-Converting-Enzyme Activity: Influence of the Inhibition Type. J. Agric. Food Chem. 2003. [Google Scholar] [CrossRef] [PubMed]
- Nogata, Y.; Nagamine, T.; Yanaka, M.; Ohta, H. Angiotensin I Converting Enzyme Inhibitory Peptides Produced by Autolysis Reactions from Wheat Bran. J. Agric. Food Chem. 2009, 57, 6618–6622. [Google Scholar] [CrossRef]
- Heap, G.A.; van Heel, D.A. Genetics and pathogenesis of coeliac disease. Semin. Immunol. 2009, 21, 346–354. [Google Scholar] [CrossRef]
- Onyango, C.; Mutungi, C.; Unbehend, G.; Lindhauer, M.G. Rheological and baking characteristics of batter and bread prepared from pregelatinised cassava starch and sorghum and modified using microbial transglutaminase. J. Food Eng. 2010, 97, 465–470. [Google Scholar] [CrossRef]
- Van Heel, D.A.; West, J. Recent advances in coeliac disease. Gut 2006, 55, 1037–1046. [Google Scholar] [CrossRef]
- Mäki, M.; Collin, P. Coeliac disease. Lancet 1997, 349, 1755–1759. [Google Scholar] [CrossRef]
- Moroni, A.V.; Dal, F.; Arendt, E.K. Sourdough in gluten-free bread-making: An ancient technology to solve a novel issue? Food Microbiol. 2009, 26, 676–684. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of morinda lucida benth leaf. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Trovato, C.; Bardella, M.T.; Forlani, F. IgA anti-gliadin antibody immunoreactivity to food proteins. Food Agric. Immunol. 2003, 15, 217–223. [Google Scholar] [CrossRef]
- Naveen, J.; Baskaran, V. Antidiabetic plant-derived nutraceuticals: A critical review. Eur. J. Nutr. 2018, 57, 1275–1299. [Google Scholar] [CrossRef]
- Tarling, C.A.; Woods, K.; Zhang, R.; Brastianos, H.C.; Brayer, G.D.; Andersen, R.J.; Withers, S.G. The Search for Novel Human Pancreatic α-Amylase Inhibitors: High-Throughput Screening of Terrestrial and Marine Natural Product Extracts. ChemBioChem 2008, 9, 433–438. [Google Scholar] [CrossRef]
- Slavin, J.L.; Jacobs, D.; Marquart, L. Grain Processing and Nutrition. Crit. Rev. Food Sci. Nutr. 2000, 40, 309–326. [Google Scholar] [CrossRef]
- Kariluoto, S.; Vahteristo, L.; Salovaara, H.; Katina, K.; Liukkonen, K.-H.; Piironen, V. Effect of Baking Method and Fermentation on Folate Content of Rye and Wheat Breads. Cereal Chem. J. 2004, 81, 134–139. [Google Scholar] [CrossRef]
- Liukkonen, K.-H.; Katina, K.; Wilhelmsson, A.; Myllymaki, O.; Lampi, A.-M.; Kariluoto, S.; Piironen, V.; Heinonen, S.-M.; Nurmi, T.; Adlercreutz, H.; et al. Process-induced changes on bioactive compounds in whole grain rye. Proc. Nutr. Soc. 2003, 62, 117–122. [Google Scholar] [CrossRef]
- Ternes, W.; Freund, W. Effects of different doughmaking techniques on thiamin content of bread. Getreide Mehl und Brot 1988, 42, 293–297. [Google Scholar]
- Freitas, D.; Le Feunteun, S.; Panouillé, M.; Souchon, I. The important role of salivary α-amylase in the gastric digestion of wheat bread starch. Food Funct. 2018, 9, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xie, Y.; Peng, C.; Liu, Y.; Wang, H. A Novel Antidiabetic Food Produced via Solid-State Fermentation of Tartary Buckwheat using L. plantarum TK9 and L. paracasei TK1501. Food Technol. Biotechnol. 2018, 56, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Liu, Y.; Liu, Y.; Fan, L.; Ma, Y.; Shi, Y. Isolation and Characterization of Angiotensin I Converting Enzyme (ACE) Inhibitory Peptides from Rice Bran Proteins and Evaluation of Activity and Stability. Pak. J. Zool. 2020, 52, 1383–1391. [Google Scholar] [CrossRef]
- Petrat-Melin, B.; Le, T.T.; Møller, H.S.; Larsen, L.B.; Young, J.F. Short communication: Inhibition of angiotensin 1-converting enzyme by peptides derived from variants of bovine β-casein upon apical exposure to a Caco-2 cell monolayer. J. Dairy Sci. 2017, 100, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Kilara, A.; Panyam, D. Peptides From Milk Proteins and Their Properties. Crit. Rev. Food Sci. Nutr. 2003, 43, 607–633. [Google Scholar] [CrossRef]
- Hu, Y.; Stromeck, A.; Loponen, J.; Lopes-Lutz, D.; Schieber, A.; Gänzle, M.G. LC-MS/MS Quantification of Bioactive Angiotensin I-Converting Enzyme Inhibitory Peptides in Rye Malt Sourdoughs. J. Agric. Food Chem. 2011, 59, 11983–11989. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Thiele, C.; Gänzle, M.G.; Vogel, R.F. Contribution of Sourdough Lactobacilli, Yeast, and Cereal Enzymes to the Generation of Amino Acids in Dough Relevant for Bread Flavor. Cereal Chem. J. 2002, 79, 45–51. [Google Scholar] [CrossRef]
- Escudero, E.; Sentandreu, M.A.; Toldrá, F. Characterization of Peptides Released by in Vitro Digestion of Pork Meat. J. Agric. Food Chem. 2010, 58, 5160–5165. [Google Scholar] [CrossRef]
- Hwang, J. Impact of processing on stability of angiotensin I-converting enzyme (ACE) inhibitory peptides obtained from tuna cooking juice. Food Res. Int. 2010, 43, 902–906. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Baraniak, B. Angiotensin I Converting Enzyme Inhibitory Peptides Obtained after In Vitro Hydrolysis of Pea (Pisum sativum var. Bajka) Globulins. Biomed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.; Xue, J.; Yang, J.; Zhang, Q.; Zhang, H.; Chen, Y. Fermentation characteristics and angiotensin I-converting enzyme-inhibitory activity of Lactobacillus helveticus isolate H9 in cow milk, soy milk, and mare milk. J. Dairy Sci. 2015, 98, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Vermeirssen, V.; Van Der Bent, A.; Van Camp, J.; Van Amerongen, A.; Verstraete, W. A quantitative in silico analysis calculates the angiotensin I converting enzyme (ACE) inhibitory activity in pea and whey protein digests. Biochimie 2004, 86, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Claudia, T.; Simone, G.; Gänzle*, M. Gluten Hydrolysis and Depolymerization during Sourdough Fermentation. J. Agric. Food Chem. 2004. [Google Scholar] [CrossRef]
- Leszczyńska, J.; Diowksz, A.; Łącka, A.; Wolska, K.; Bartos, A. Evaluation of immunore activity of wheat bread made from fermented wheat flour. Czech J. Food Sci. 2012, 30, 336–342. [Google Scholar] [CrossRef]
- De Angelis, M.; Rizzello, C.G.; Fasano, A.; Clemente, M.G.; De Simone, C.; Silano, M.; De Vincenzi, M.; Losito, I.; Gobbetti, M. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue probiotics and gluten intolerance. Biochim. Biophys. Acta-Mol. Basis Dis. 2006, 1762, 80–93. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Lavermicocca, P.; De Vincenzi, M.; Giovannini, C.; Faccia, M.; Gobbetti, M. Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef]
- Leszczyńska, J.; Diowksz, A.; Łącka, A.; Bryszewska, M.; Wolska, K.; Ambroziak, W. Decrease of wheat flour allergenicity via lactic acid fermentation. Food Agric. Immunol. 2009, 20, 139–145. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Aldo, B.; Aldo, B.; Aldo, B.; Giuseppe, C.; Maria, R.; Coda, R.; Gobbetti, M. Use of selected sourdough lactic acid bacteria to hydrolyze wheat and rye proteins responsible for cereal allergy. Eur. Food Res. Technol. 2006. [Google Scholar] [CrossRef]
- Rollan, G.; De Angelis, M.; Gobbetti, M.; de Valdez, G.F. Proteolytic activity and reduction of gliadin-like fractions by sourdough lactobacilli. J. Appl. Microbiol. 2005, 99, 1495–1502. [Google Scholar] [CrossRef]
- Mickowska, B.; Romanová, K.; Urminská, D. Reduction of immunoreactivity of rye and wheat prolamins by lactobacilli and Flavourzyme proteolysis during sourdough fermentation—A way to obtain low-gluten bread. J. Food Nutr. Res. 2019, 58, 153–166. [Google Scholar]
- Di Cagno, R.; De Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; De Vincenzi, M.; Giovannini, C.; D’Archivio, M.; Landolfo, F.; Parrilli, G.; et al. Sourdough Bread Made from Wheat and Nontoxic Flours and Started with Selected Lactobacilli Is Tolerated in Celiac Sprue Patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar] [CrossRef]
- Davis, P.J.; Smales, C.M.; James, D.C. How can thermal processing modify the antigenicity of proteins? Allergy 2001, 56, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Simonato, B.; Pasini, G.; Giannattasio, M.; Peruffo, A.D.B.; De Lazzari, F.; Curioni, A. Food Allergy to Wheat Products: The Effect of Bread Baking and in Vitro Digestion on Wheat Allergenic Proteins. A Study with Bread Dough, Crumb, and Crust. J. Agric. Food Chem. 2001, 49, 5668–5673. [Google Scholar] [CrossRef] [PubMed]

| S-I | Wheat sourdough bread | Wheat flour (0.75% ash content), wheat sourdough, water, barley malt, baker’s yeast, salt |
| S-II | Wheat-rye sourdough bread with flax and sunflower seeds | Wheat flour (0.75% ash content), rye flour (0.72% ash content), wheat sourdough, water, baker’s yeast, flax and sunflower seeds, plant oil, salt |
| S-III | Wheat-rye sourdough bread fermented spontaneously with wholegrain rye flour | Wheat flour (0.75% ash content), rye flour (2.00% ash content), wheat sourdough, water, barley malt, baker’s yeast, salt |
| S-IV | Ciabatta type 1 | Wheat flour (0.55% ash content), wheat sourdough, water, baker’s yeast, salt |
| S-V | Ciabatta type 2 | Wheat flour (0.55% ash content), wheat sourdough, water, baker’s yeast, salt |
| Control | [prolonged fermentation] | Wheat flour (0.55% ash content), water, baker’s yeast, salt |
| Sample | Fermentation Time (h) | Sourdough (%) | Yeast (%) |
|---|---|---|---|
| C-I | 22 | 9 | 1 |
| C-II | 22 | 15 | 0 |
| C-III | 3 | 15 | 4 |
| C-IV | 22 | 30 | 0 |
| C-V | 3 | 30 | 4 |
| C-VI | 22 | 50 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diowksz, A.; Malik, A.; Jaśniewska, A.; Leszczyńska, J. The Inhibition of Amylase and ACE Enzyme and the Reduction of Immunoreactivity of Sourdough Bread. Foods 2020, 9, 656. https://doi.org/10.3390/foods9050656
Diowksz A, Malik A, Jaśniewska A, Leszczyńska J. The Inhibition of Amylase and ACE Enzyme and the Reduction of Immunoreactivity of Sourdough Bread. Foods. 2020; 9(5):656. https://doi.org/10.3390/foods9050656
Chicago/Turabian StyleDiowksz, Anna, Alicja Malik, Agnieszka Jaśniewska, and Joanna Leszczyńska. 2020. "The Inhibition of Amylase and ACE Enzyme and the Reduction of Immunoreactivity of Sourdough Bread" Foods 9, no. 5: 656. https://doi.org/10.3390/foods9050656
APA StyleDiowksz, A., Malik, A., Jaśniewska, A., & Leszczyńska, J. (2020). The Inhibition of Amylase and ACE Enzyme and the Reduction of Immunoreactivity of Sourdough Bread. Foods, 9(5), 656. https://doi.org/10.3390/foods9050656