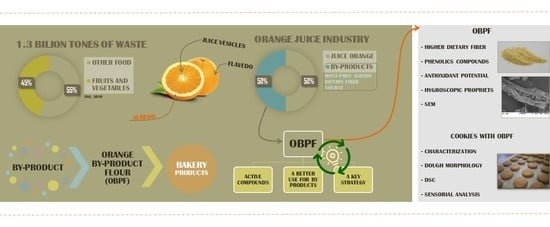
From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Orange By-Product Flour Production
2.2.2. Orange By-Product Flour Characterization
Chemical Composition
Flour Extraction
Total Phenolic Content
Antioxidant Potential
- FRAP (Ferric Reducing Antioxidant Power) Assay
- ORAC (Oxygen Radical Absorbance Capacity) Assay
Phenolic Profile
Hydration Properties
2.2.3. Cookie Production
2.2.4. Cookie Characterization
Physical Properties
Specific Volume
Hardness
Color Parameters
2.2.5. Morphology of the Dough and Cookies
2.2.6. Differential Scanning Calorimetry (DSC) Analysis of Cookies
2.2.7. Sensorial Analysis
2.2.8. Statistical Analyses
3. Results and Discussion
3.1. Orange By-Product Flour Characterization
3.1.1. Orange Juice and Flour Yield
3.1.2. Chemical Composition
3.1.3. Total Phenolic Content
3.1.4. Antioxidant Potential
3.1.5. Phenolic Profile
3.1.6. Hydration Properties
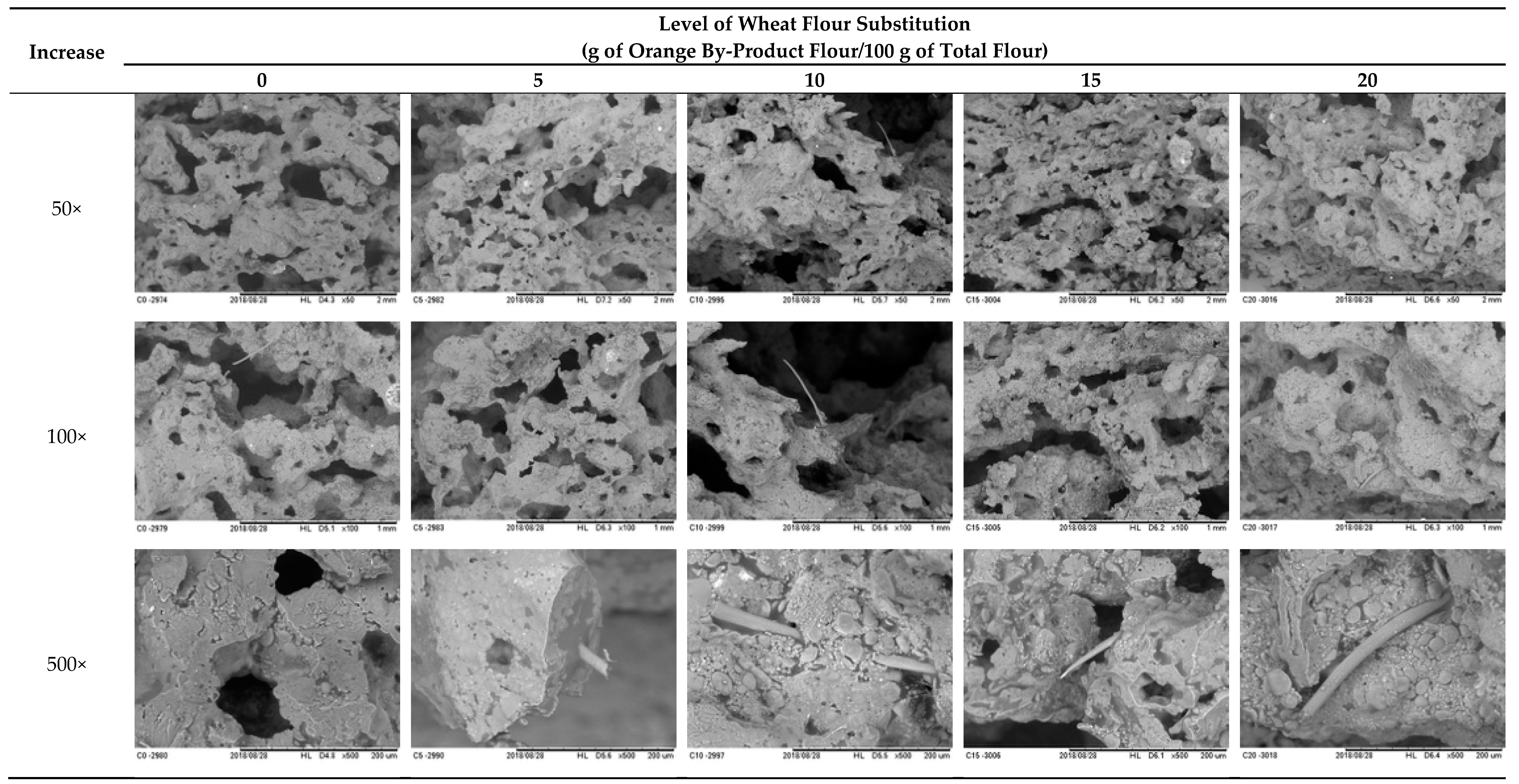
3.1.7. Microstructure Properties
3.2. Cookie Characterization
3.2.1. Physical Properties Specific Volume, Hardness, and Color Parameters
3.2.2. Microstructure
3.2.3. Differential Scanning Calorimetry (DSC)
3.2.4. Sensorial Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swaminathan, M.S. Food Losses and Food Waste. Combat. Hunger Achiev. Food Secur. 2015, 37–46. [Google Scholar] [CrossRef]
- FAO; UN SAVEFOOD. SAVE FOOD: Global Initiative on Food Loss and Waste Reduction. Retrieved March 2012, 12, 2018. [Google Scholar]
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; Van Holsteijn, F.; Sala, S. Quantification of food waste per product group along the food supply chain in the European Union: A mass flow analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef] [PubMed]
- FAO. Available online: http://www.fao.org/2019-ncov/q-and-a/impact-on-food-and-agriculture/en/ (accessed on 23 April 2020).
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 23 April 2020).
- USDA/FAS. Citrus: World Markets and Trade; United States Department Agriculture Foreign Agricultural Service: Washington, DC, USA, 2019; pp. 1–13.
- Arthey, D.; Ashurst, P.R. Fruit Processing; Springer Science & Business Media: Berlin, Germany, 1995. [Google Scholar]
- Berk, Z. (Ed.) Citrus Fruit Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 219–233. [Google Scholar]
- USDA F. Citrus: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 15 April 2020).
- Topuz, A.; Topakci, M.; Canakci, M.; Akinci, I.; Ozdemir, F. Physical and nutritional properties of four orange varieties. J. Food Eng. 2005, 66, 519–523. [Google Scholar] [CrossRef]
- Bampidis, V.A.; Robinson, P.H. Citrus by-products as ruminant feeds: A review. Anim. Feed. Sci. Technol. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Sahraoui, N.; Abert, M.; El, M.; Boutekedjiret, C.; Chemat, F. Valorization of citrus by-products using Microwave Steam Distillation (MSD). Innov. Food Sci. Emerg. Technol. 2011, 12, 163–170. [Google Scholar] [CrossRef]
- Raeissi, S.; Diaz, S.; Espinosa, S.; Peters, C.J.; Brignole, E.A. Ethane as an alternative solvent for supercritical extraction of orange peel oils. J. Supercrit. Fluids 2008, 45, 306–313. [Google Scholar] [CrossRef]
- Aravantinos-Zafiris, G.; Oreopoulou, V.; Tzia, C.; Thomopoulos, C.D. Utilisation of orange by-products—Orange peel carotenoids. J. Sci. Food Agric. 1992, 59, 77–79. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martin-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; ĈížM, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- O’Shea, N.; Rößle, C.; Arendt, E.; Gallagher, E. Modelling the effects of orange pomace using response surface design for gluten-free bread baking. Food Chem. 2015, 166, 223–230. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, nutrition and the prevention of chronic diseases: Report of a joint WHO/FAO expert consultation, Geneva, 28 January–1 February 2002. WHO Tech. Rep. Ser. 2003, 2, 149. [Google Scholar]
- Girolfo, J.; Nascimento, M.; Ferreira, J. Estudo da aplicação de nisina para a inativação de Alicyclobacillus acidoterrestris. Blucher Chem. Eng. Proc 2014, 1, 572–576. [Google Scholar] [CrossRef] [Green Version]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Food Wastage Footprint. Available online: http://www.fao.org/3/i3347e/i3347e.pdf (accessed on 21 December 2019).
- Santana, M.d.F.S.d. Caracterização Físico-Química de Fibra Alimentar de Laranja e Maracujá. 2005. Available online: http://repositorio.unicamp.br/bitstream/REPOSIP/255947/1/Santana_MaristeladeFatimaSimpliciode_D.pdf (accessed on 15 April 2020).
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC, Association of Official Analytical Chemists. Total Dietary Fiber in Foods—Enzimatic-Gravimetric Method, 12th ed.; AOAC, Association of Official Analytical Chemists: Rockville, MD, USA, 1985; Volume 29. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Identification and characterisation of anthocyanins from wild mulberry (Morus Nigra L.) growing in Brazil. Food Sci. Technol. Int. 2007, 13, 17–25. [Google Scholar] [CrossRef]
- Sosulski, F.; Garratt, M.; Slimkard, A. Functional Properties of Ten Legume Flours. Can. Inst. Food Sci. Technol. J. 1976, 9, 66–69. [Google Scholar] [CrossRef]
- Pareyt, B.; Delcour, J.A. The role of wheat flour constituents, sugar, and fat in low moisture cereal based products: A review on sugar-snap cookies. Crit. Rev. Food Sci. Nutr. 2008, 48, 824–839. [Google Scholar] [CrossRef]
- AACC Intternational. Approved Methods of Analysis. In AACC Method 10-52.02 Baking Quality of Cookie Flour— Micro Method, 11th ed.; AACC Intternational: St. Paul, MN, USA, 2000. [Google Scholar]
- Vanin, F.M.; Michon, C.; Lucas, T. Effect of the drying rate on the complex viscosity of wheat flour dough transforming into crust and crumb during baking. J. Cereal Sci. 2013, 58, 290–297. [Google Scholar] [CrossRef]
- Chevallier, S.; Colonna, P.; Della Valle, G.; Lourdin, D. Contribution of Major Ingredients during Baking of Biscuit Dough Systems. J. Cereal Sci. 2000, 31, 241–252. [Google Scholar] [CrossRef]
- Larrea, M.A.; Chang, Y.K.; Martinez-Bustos, F. Effect of some operational extrusion parameters on the constituents of orange pulp. Food Chem. 2005, 89, 301–308. [Google Scholar] [CrossRef]
- Escobedo-Avellaneda, Z.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; Torres, J.A.; Welti-Chanes, J. Phytochemicals and antioxidant activity of juice, flavedo, albedo and comminuted orange. J. Funct. Foods 2014, 6, 470–481. [Google Scholar] [CrossRef]
- Danesi, E.D.G.; Granato, D.; Iwassa, I.J.; Pinzon, C.; Bolanho, B.C. Effects of industrial by-products from orange, peach palm and soybean on the quality traits and antioxidant activity of flours: A response surface approach. Int. Food Res. J. 2018, 25, 1219–1227. [Google Scholar]
- Infante, J.; Selani, M.M.; Maria, N.; de Toledo, N.M.V.; Silveira-Diniz, M.F.; Alencar, S.M.; Spoto, M. Agroindustriais De Frutas Tropicais. Braz. J. Food Nutr. (Araraquara) 2013, 24, 87–91. [Google Scholar]
- Djendoubi Mrad, N.; Boudhrioua, N.; Kechaou, N.; Courtois, F.; Bonazzi, C. Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod. Process. 2012, 90, 433–441. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Rößle, C.; Brunton, N. A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem. 2009, 116, 202–207. [Google Scholar] [CrossRef]
- Nieto-Calvache, J.E.; Pla, M.E.; Gerschenson, L.N. Dietary fibre concentrates produced from papaya by-products for agroindustrial waste valorisation. Int. J. Food Sci. Technol. 2018, 54, 1074–1080. [Google Scholar] [CrossRef]
- Chen, X.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- López, G.; Ros, G.; Rincón, F.; Periago, M.J.; Martínez, M.C.; Ortuño, J. Relationship between Physical and Hydration Properties of Soluble and Insoluble Fiber of Artichoke. J. Agric. Food Chem. 1996, 44, 2773–2778. [Google Scholar] [CrossRef]
- Saeid, A.; Hoque, S.; Kumar, U.; Das, M.; Muhammad, N.; Rahman, M.; Ahmed, M. Comparative studies on nutritional quality of commercial wheat flour in Comparative studies on nutritional quality of commercial wheat flour in Bangladesh. Bangladesh J. Sci. Ind. Res. 2015, 50, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Larrea, M.A.; Chang, Y.K.; Martinez-Bustos, F. Some functional properties of extruded orange pulp and its effect on the quality of cookies. LWT 2005, 38, 213–220. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičacute ová, J.; Jurasová, M.; Kukurová, K. Application of citrus dietary fibre preparations in biscuit production. J. Food Nutr. Res. 2011, 50, 182–190. [Google Scholar]
- Baumgartner, B.; Özkaya, B.; Saka, I.; Özkaya, H. Functional and physical properties of cookies enriched with dephytinized oat bran. J. Cereal Sci. 2018, 80, 24–30. [Google Scholar] [CrossRef]
- Blanco Canalis, M.S.; León, A.E.; Ribotta, P.D. Incorporation of dietary fiber on the cookie dough. Effects on thermal properties and water availability. Food Chem. 2019, 271, 309–317. [Google Scholar] [CrossRef]
- Kulp, K.; Olewnik, M.; Lorenz, K.; Collins, F. Starch Functionality in Cookie Systems. Starch Stärke 1991, 43, 53–57. [Google Scholar] [CrossRef]

| Ingredients (g) | Level of Wheat Flour Substitution (g of Orange By-Product Flour/100 g of Total Flour) | ||||
|---|---|---|---|---|---|
| Control | 5 | 10 | 15 | 20 | |
| Wheat flour | 238.50 | 226.57 | 214.65 | 202.73 | 190.80 |
| OBPF | 0 | 11.93 | 23.85 | 35.77 | 47.70 |
| Margarine | 43.5 | 43.5 | 43.5 | 43.5 | 43.5 |
| Brown sugar | 86 | 86 | 86 | 86 | 86 |
| Sugar | 86 | 86 | 86 | 86 | 86 |
| Water | 8 | 8 | 8 | 8 | 8 |
| Chemical leavener | 5 | 5 | 5 | 5 | 5 |
| Analysis/Component | Value | |
|---|---|---|
| Chemical composition | Water content (g/100 g of DM *) | 10.38 ± 0.36 |
| Protein (g/100 g of DM) | 5.94 ± 0.1 | |
| Fat (g/100 g of DM) | 0.33 ± 0.1 | |
| Ash (g/100 g of DM) | 2.72 ± 0.02 | |
| Dietary fiber (g/100 g of DM) | 73.61 | |
| Glycidic fraction (g/100 g of DM) | 80.63 | |
| Antioxidant properties | Phenolic compound (mg GAE/100g of DM) ** | 534 ± 30 |
| FRAP (µmolTEq/100 g of DM) *** | 93 ± 5 | |
| ORAC (µmolTEq/100 g of DM) *** | 11,728 ± 541 | |
| Functional properties | WAI (g of water/g of DM) | 13.28 ± 0.50 |
| OAI (g of oil/g of DM) | 2.78 ± 0.02 | |
| WSI (%) | 9.94 | |
| Microstructure | SEM image at 500× magnification | 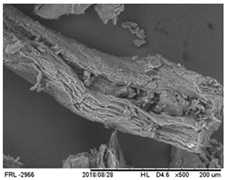 |
| N° | RT | [M-H]-(m/z) | MS2 (m/z) | Compound |
|---|---|---|---|---|
| 1 | 6.8 | 609.1467 | 519/489/429/399/369.0601 | Luteolin 6,8-di-C-glucoside |
| 2 | 7.6 | 593.1517 | 473/353/383/353/325.0666/297 | Apigenin 6,8-di-C-glycopyranoside (Vicenin-2) |
| 3 | 7.8 | 623.1613 | 503/413/383/355/312.0646 | Chrysoeriol 6,8-di-C-glucoside (Stellarin 2) |
| 4 | 8.2 | 623.1635 | 503/413/383/355/312.0659 | Chrysoeriol 6,8-di-C-glucoside (Stellarin 2) |
| 5 | 9.6 | 563.1427 | 413/293.0459 | - |
| 6 | 10.0 | 609.1488 | 300.0288/151 | Quercetin 3-O-rutinoside (Rutina) |
| 7 | 11.7 | 579.1741 | 271.0613/151 | Naringenin-7-hesperidoside (Naringin) |
| 8 | 13.0 | 609.1831 | 301.0717/164 | Hesperetin 7-O-rutinoside (Hesperidin) |
| Parameter | Level of Wheat Flour Substitution (g of Orange By-Product Flour/100 g of Total Flour) | ||||
| 0 | 5 | 10 | 15 | 20 | |
| Diameter (mm) | 54.05 ± 0.7 b | 53.14 ± 1 ab | 53.07 ± 1 ab | 53.58 ± 0.8 ab | 52.55 ± 0.8 a |
| Height (mm) | 12.63 ± 0.8 b | 12.39 ± 0.4 ab | 12.15 ± 0.5 ab | 11.78 ± 0.6 a | 11.82 ± 0.7 a |
| Spread factor (SF) | 4.96 ± 0.3 a | 4.29 ± 0.2 a | 4.38 ± 0.3 a | 4.56 ± 0.3 a | 4.46 ± 0.2 a |
| Specific volume (mL/g) | 1.56 ± 0.04 c | 1.50 ± 0.1 b | 1.55 ± 0.04 c | 1.48 ± 0.03 b | 1.38 ± 0.03 a |
| Hardness (N) | 101.01 ± 22 a | 114.0 ± 20 a | 117.0 ± 15 a | 176.9 ± 45 b | 275.6 ± 37 c |
| L* | 64.19 ± 8 b | 61.68 ± 5 ab | 60.82 ± 7 ab | 57.71 ± 3 a | 56.20 ± 4 a |
| Chroma a* | 8.94 ± 0.7 a | 10.01 ± 2 a | 10.19 ± 2 a | 8.93 ± 1 a | 9.60 ± 0.9 a |
| Chroma b* | 28.98 ± 2 a | 29.63 ± 4 a | 29.45 ± 4 a | 26.83 ± 0.9 a | 27.22 ± 2 a |
| Sensorial attribute * | 0 | 5 | 10 | 15 | 20 |
| Color | 6.42 ± 1.5 ab | 6.68 ± 1.6 bc | 7.05 ± 1.4 c | 6.00 ± 1.6 a | 6.65 ± 1.5 bc |
| Aroma | 6.53 ± 1.5 ab | 6.82 ± 1.4 bc | 6.98 ± 1.4 c | 6.14 ± 1.4 a | 6.55 ± 1.5 ab |
| Texture | 6.41 ± 1.9 c | 6.27 ± 1.8 c | 6.48 ± 1.9 c | 4.71 ± 2.1a | 5.5 ± 2.2 b |
| Flavor | 6.62 ± 1.7 bc | 7.04 ± 1.4 cd | 7.16 ± 1.5 d | 5.81 ± 1.8 a | 6.52 ± 1.6 b |
| Overall acceptability | 6.66 ± 1.5bc | 6.81 ± 1.4 c | 7.07 ± 1.3 c | 5.52 ± 1.6 a | 6.35 ± 1.5 b |
| Level of Wheat Flour Substitution (g of Orange By-Product Flour/100 g of Total Flour) | Temperature (°C) | ΔH (J/g) | |||
|---|---|---|---|---|---|
| Ts | To | Tm | Tstop | ||
| 0 | 105.16 ± 5.62 a | 108.9 ± 7.01 a | 131.01 ± 5.29 a | 156.71 ± 1.18 a | 4.30 ± 0.38 a |
| 5 | 111.46 ± 6.95 a | 118.06 ± 3.03 b | 131.81± 3.49 a | 158.00 ± 2.56 a | 4.18 ± 0.17 a |
| 10 | 108.23 ± 4.44 a | 112.59 ± 3.26 ab | 128.14 ± 2.18 a | 157.39 ± 2.75 a | 5.67 ± 0.25 b |
| 15 | 110.11 ± 4.57 a | 113.92 ± 5.28 ab | 130.32 ± 0.67 a | 156.14 ± 0.25 a | 4.40 ± 1.16 a |
| 20 | 111.59 ± 0.46 a | 116.70 ± 0.55 ab | 131.90 ± 0.73 a | 157.39 ± 1.78 a | 4.11 ± 0.55 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, L.A.d.; Lizi, J.M.; Chagas, E.G.L.d.; Carvalho, R.A.d.; Vanin, F.M. From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy. Foods 2020, 9, 593. https://doi.org/10.3390/foods9050593
Castro LAd, Lizi JM, Chagas EGLd, Carvalho RAd, Vanin FM. From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy. Foods. 2020; 9(5):593. https://doi.org/10.3390/foods9050593
Chicago/Turabian StyleCastro, Larissa Alves de, Jaqueline Miranda Lizi, Eduardo Galvão Leite das Chagas, Rosemary Aparecida de Carvalho, and Fernanda Maria Vanin. 2020. "From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy" Foods 9, no. 5: 593. https://doi.org/10.3390/foods9050593
APA StyleCastro, L. A. d., Lizi, J. M., Chagas, E. G. L. d., Carvalho, R. A. d., & Vanin, F. M. (2020). From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy. Foods, 9(5), 593. https://doi.org/10.3390/foods9050593