Effect of Diet on the Vitamin B Profile of Bovine Milk-Based Protein Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Protein Ingredient Manufacture
2.4. Liquid Chromatography–Mass Spectrometry (LC-MS/MS)
2.4.1. Sample Preparation
2.4.2. Operating Conditions
2.5. Nephelometry
2.6. Statistical Analysis
3. Results and Discussion
3.1. Overall Distribution
3.2. Vitamin Composition
3.2.1. Vitamin B1 (Thiamine)
3.2.2. Vitamin B2 (Riboflavin)
3.2.3. Vitamin B3 (Nicotinic Acid)
3.2.4. Vitamin B5 (Pantothenic Acid) and B6 (Pyridoxine)
3.2.5. Vitamin B7 (Biotin)
3.2.6. Vitamin B12 (Cobalamin)
3.3. Relationship between Skim Milk and Recommended Daily Allowances
3.4. Vitamin Content of Skim Milk for Use in Infant Milk Formula Manufacture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- German, J.B.; Gibson, R.A.; Krauss, R.M.; Nestel, P.; Lamarche, B.; van Staveren, W.A.; Steijns, J.M.; de Groot, L.C.P.G.M.; Lock, A.L.; Destaillats, F. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur. J. Nutr. 2009, 48, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Vijay, V.; Pimm, S.L.; Jenkins, C.N.; Smith, S.J. The impacts of oil palm on recent deforestation and biodiversity loss. PLoS ONE 2016, 11, e0159668. [Google Scholar] [CrossRef] [PubMed]
- Vermeir, I.; Verbeke, W. Sustainable food consumption: Exploring the consumer “attitude—Behavioral intention” gap. J. Agric. Environ. Ethic. 2006, 19, 169–194. [Google Scholar] [CrossRef]
- Park, Y.W. Recent trend in the dairy industry. J. Adv. Dairy Res. 2018, 6, 4. [Google Scholar] [CrossRef]
- Mobley, A.R.; Kraemer, D.; Nicholls, J. Putting the nutrient-rich foods index into practice. J. Am. Coll. Nutr. 2009, 28, 427–435. [Google Scholar] [CrossRef]
- Hewson, A.T.; Hart, D.J.; Dainty, J.R.; Bullock, N.R.; Finglas, P.M.; Turner, R.; Powers, H.J. Quantification of the bioavailability of riboflavin from foods by use of stable-isotope labels and kinetic modeling. Am. J. Clin. Nutr. 2007, 85, 1557–1564. [Google Scholar]
- Stokstad, E.L.R.; Tarr, J.B.; Tamura, T. Availability of vitamin B6 and pantothenate in an average American diet in man. Am. J. Clin. Nutr. 1981, 34, 1328–1337. [Google Scholar]
- Matte, J.J.; Guay, F.; Girard, C.L. Bioavailability of vitamin B12 in cows’ milk. Br. J. Nutr. 2011, 107, 61–66. [Google Scholar] [CrossRef]
- Walker, C.K.; Elliot, J.M. Lactational trends in vitamin B12 status on conventional and restricted-roughage rations. J. Dairy Sci. 1972, 55, 474–479. [Google Scholar] [CrossRef]
- Herbert, V. Vitamin B-12: Plant sources, requirements, and assay. Am. J. Clin.Nutr. 1988, 48, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. (Ed.) B—Water-soluble vitamins in bovine milk. In Handbook of Milk Composition; Academic Press: San Diego, CA, USA, 1995; pp. 688–692. [Google Scholar]
- Jensen, R.G. (Ed.) E—Fat-Soluble vitamins in bovine milk. In Handbook of Milk Composition; Academic Press: San Diego, CA, USA, 1995; pp. 718–725. [Google Scholar]
- Croissant, A.E.; Washburn, S.P.; Dean, L.L.; Drake, M.A. Chemical properties and consumer perception of fluid milk from conventional and pasture-based production systems. J. Dairy Sci. 2007, 90, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Pierce, K.M.; Berry, D.P.; Brennan, A.; Horan, B. The influence of genetic selection and feed system on the reproductive performance of spring-calving dairy cows within future pasture-based production systems. J. Dairy Sci. 2009, 92, 5258–5269. [Google Scholar] [CrossRef] [PubMed]
- Sabia, E.; Napolitano, F.; Claps, S.; De Rosa, G.; Barile, V.L.; Braghieri, A.; Pacelli, C. Environmental impact of dairy buffalo heifers kept on pasture or in confinement. Agric. Syst. 2018, 159, 42–49. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Butler, G.; Latif, M.A.; Leifert, C.; Davis, D.R. Organic production enhances milk nutritional quality by shifting fatty acid composition: A United States–Wide, 18-Month Study. PLoS ONE 2013, 8, e82429. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). Dairy 2014: Dairy Cattle Management Practices in the United States, 2014; USDA: Fort Collins, CO, USA, 2016.
- Weinrich, R.; Kühl, S.; Zühlsdorf, A.; Spiller, A. Consumer attitudes in germany towards different dairy housing systems and their implications for the marketing of pasture raised milk. Int. Food Agribus. Man. 2014, 17, 205–222. [Google Scholar]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef]
- Mitani, T.; Kobayashi, K.; Ueda, K.; Kondo, S. Discrimination of “grazing milk” using milk fatty acid profile in the grassland dairy area in Hokkaido. Anim. Sci. J. 2016, 87, 233–241. [Google Scholar] [CrossRef][Green Version]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef]
- Dufva, G.S.; Bartley, E.E.; Dayton, A.D.; Riddell, D.O. Effect of Niacin Supplementation on Milk Production and Ketosis of Dairy Cattle1, 2. J. Dairy Sci. 1983, 66, 2329–2336. [Google Scholar] [CrossRef]
- Zimmerly, C.A.; Weiss, W.P. Effects of supplemental dietary biotin on performance of Holstein cows during early lactation. J. Dairy Sci. 2001, 84, 498–506. [Google Scholar] [CrossRef]
- Midla, L.T.; Hoblet, K.H.; Weiss, W.P.; Moeschberger, M.L. Supplemental dietary biotin for prevention of lesions associated with aseptic subclinical laminitis (pododermatitisasepticadiffusa) in primiparous cows. Am. J. Vet. Res. 1998, 59, 733–738. [Google Scholar] [PubMed]
- Santschi, D.E.; Chiquette, J.; Berthiaume, R.; Martineau, R.; Matte, J.J.; Mustafa, A.F.; Girard, C.L. Effects of the forage to concentrate ratio on B-vitamin concentrations in different ruminal fractions of dairy cows. Can.J. Anim. Sci. 2005, 85, 389–399. [Google Scholar] [CrossRef]
- Nuernberg, K.; Dannenberger, D.; Nuernberg, G.; Ender, K.; Voigt, J.; Scollan, N.D.; Wood, J.D.; Nute, G.R.; Richardson, R.I. Effect of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid composition of longissimus muscle in different cattle breeds. Livest. Prod. Sci. 2005, 94, 137–147. [Google Scholar] [CrossRef]
- Magan, J.B.; O’Callaghan, T.F.; Zheng, J.; Zhang, L.; Mandal, R.; Hennessy, D.; Fenelon, M.A.; Wishart, D.S.; Kelly, A.L.; McCarthy, N.A. Impact of bovine diet on Metabolomic profile of skim milk and whey protein ingredients. Metabolites 2019, 9, 305. [Google Scholar] [CrossRef]
- Egan, M.; Lynch, M.B.; Hennessy, D. Including white clover in nitrogen fertilized perennial ryegrass swards: Effects on dry matter intake and milk production of spring calving dairy cows. J. Agric. Sci. 2016, 155, 657–668. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Faulkner, H.; McAuliffe, S.; O’Sullivan, M.G.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; Stanton, C.; Ross, R.P. Quality characteristics, chemical composition, and sensory properties of butter from cows on pasture versus indoor feeding systems. J. Dairy Sci. 2016, 99, 9441–9460. [Google Scholar] [CrossRef]
- AOAC. Cobalamin (vitamin Bu activity) in vitamin preparations. Microbiological methods. Final action 1960. In AOAC Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- Xia, J.; Wishart, D.S. Using metaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Vázquez-Fresno, R.; Serra-Cayuela, A.; Dong, E.; Mandal, R.; Hennessy, D.; McAuliffe, S.; Dillon, P.; Wishart, D.S.; Stanton, C.; et al. Pasture feeding changes the bovine rumen and milk metabolome. Metabolites 2018, 8, 27. [Google Scholar] [CrossRef]
- Marks, J. A Guide to the Vitamins. Their Role in Health and Disease; Medical and Technical Publishing Co. Ltd.: Lancaster, UK, 1975. [Google Scholar]
- Pan, X.; Nan, X.; Yang, L.; Jiang, L.; Xiong, B. Thiamine status, metabolism and application in dairy cows: A review. Br. J. Nutr. 2018, 120, 491–499. [Google Scholar] [CrossRef]
- Duckett, S.K.; Neel, J.P.S.; Clapham, W.M.; Fontenot, J.P. Effects of winter stocker growth rate and finishing system on: III. Tissue proximate, fatty acid, vitamin, and cholesterol content1. J. Anim. Sci. 2009, 87, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Shingfield, K.; Salo-Väänänen, P.; Pahkala, E.; Toivonen, V.; Jaakkola, S.; Piironen, V.; Huhtanen, P. Effect of forage conservation method, concentrate level and propylene glycol on the fatty acid composition and vitamin content of cows’ milk. J. Dairy Res. 2005, 72, 349–361. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R. Vitamins in Animal and Human Nutrition, 2nd ed.; Iowa State University Press: Ames, IA, USA, 2000. [Google Scholar]
- Frank, O.; Luisada-Opfer, A.F.; Feingold, S.; Baker, H. Vitamin-binding by human and some animal plasma proteins. Nutr. Rep. Int. 1970, 6, 161–168. [Google Scholar]
- Hunt, C.H.; Kick, C.H.; Burroughs, E.W.; Bethke, R.M.; Schalk, A.F.; Gerlaugh, P. Studies on riboflavin and thiamin in the rumen content of cattle. J. Nutr. 1941, 21, 85–92. [Google Scholar] [CrossRef]
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Mannion, D.T.; Hennessy, D.; McAuliffe, S.; O’Sullivan, M.G.; Leeuwendaal, N.; Beresford, T.P.; Dillon, P.; Kilcawley, K.N.; Sheehan, J.J.; et al. Effect of pasture versus indoor feeding systems on quality characteristics, nutritional composition, and sensory and volatile properties of full-fat Cheddar cheese. J. Dairy Sci. 2017, 100, 6053–6073. [Google Scholar] [CrossRef]
- Edelman, M.; Colt, M. Nutrient Value of Leaf vs. Seed. Front. Chem. 2016, 4, 32. [Google Scholar] [CrossRef]
- Hayes, B.W.; Mitchell, J.G.E.; Little, C.O.; Bradley, N.W. Concentrations of B-vitamins in ruminal fluid of steers fed different levels and physical forms of hay and grain. J. Anim. Sci. 1966, 25, 539–542. [Google Scholar] [CrossRef]
- Poulsen, N.A.; Rybicka, I.; Poulsen, H.D.; Larsen, L.B.; Andersen, K.K.; Larsen, M.K. Seasonal variation in content of riboflavin and major minerals in bulk milk from three Danish dairies. Int. Dairy J. 2015, 42, 6–11. [Google Scholar] [CrossRef]
- Mavropoulou, I.P.; Kosikowski, F.V. Composition, solubility, and stability of whey powders. J. Dairy Sci. 1973, 56, 1128–1134. [Google Scholar] [CrossRef]
- Glass, L.; Hedrick, T.I. Nutritional composition of sweet- and acid-type dry wheys. II. Vitamin, Mineral and calorie contents. J. Dairy Sci. 1977, 60, 190–196. [Google Scholar] [CrossRef]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Ann. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Panda, N.; Panigrahy, K.K.; Gupta, S.K.; Mishra, S.P.; Laishram, M. Role of niacin supplementation in dairy cattle: A review. Asian J. Dairy Food Res. 2017, 36, 93–99. [Google Scholar] [CrossRef]
- Erickson, P.S.; Murphy, M.R.; McSweeney, C.S.; Trusk, A.M. Niacin absorption from the Rumen1. J. Dairy Sci. 1991, 74, 3492–3495. [Google Scholar] [CrossRef]
- Niehoff, I.; Hüther, L.; Lebzien, P. Niacin for dairy cattle: A review. Br. J. Nutr. 2008, 101, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, G.A. Niacin-Tryptophan Relationships in Man and Niacin Requirement. Am. J. Clin. Nutr. 1958, 6, 479–486. [Google Scholar] [CrossRef]
- Yao, K.; Fang, J.; Yin, Y.; Feng, Z.; Tang, Z.; Wu, G. Tryptophan metabolism in animals: Important roles in nutrition and health. Front. Biosci. 2011, 3, 286–297. [Google Scholar]
- Fukuwatari, T.; Shibata, K. Nutritional aspect of tryptophan metabolism. Int. J. Tryptophan Res. IJTR 2013, 6, 3–8. [Google Scholar] [CrossRef]
- Ragaller, V.; Lebzien, P.; Südekum, K.-H.; Hüther, L.; Flachowsky, G. Pantothenic acid in ruminant nutrition: A review. J. Anim. Physiol. Anim. Nutr. 2011, 95, 6–16. [Google Scholar] [CrossRef]
- McDowell, L.R. (Ed.) 9—Vitamin B6. In Vitamins in Animal Nutrition; Academic Press: San Diego, CA, USA, 1989; pp. 236–255. [Google Scholar]
- McElroy, L.W.; Goss, H. A quantitative study of vitamins in the rumen contents of sheep and cows fed vitamin-low diets. II. Vitamin B6 (pyridoxine). J. Nutr. 1940, 20, 541–550. [Google Scholar] [CrossRef]
- Yagi, T.; Iwamoto, S.; Mizuseki, R.; Furuya, M.; Nakayama, K. Contents of All Forms of Vitamin B6, Pyridoxine-&beta-Glucoside and 4-Pyridoxic Acid in Mature Milk of Japanese Women According to 4-Pyridoxolactone-Conversion High Performance Liquid Chromatography. J. Nutr. Sci. Vitaminol. 2013, 59, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Schreiner, M.G.; Mayer, H.K. Rapid determination of the various native forms of vitamin B6 and B2 in cow’s milk using ultra-high performance liquid chromatography. J. Chromatogr. A 2017, 1500, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Graulet, B. Ruminant milk: A source of vitamins in human nutrition. Anim. Front. 2014, 4, 24–30. [Google Scholar] [CrossRef][Green Version]
- Alm, L. Effect of Fermentation on B-vitamin content of milk in Sweden. J. Dairy Sci. 1982, 65, 353–359. [Google Scholar] [CrossRef]
- Woollard, D.C.; Indyk, H.E. CHAPTER 24 Biotin Analysis in Dairy Products. In B Vitamins and Folate: Chemistry, Analysis, Function and Effects; The Royal Society of Chemistry: London, UK, 2012; pp. 377–395. [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press (US): Washington, DC, USA, 1998. [Google Scholar]
- Miller, B.L.; Meiske, J.C.; Goodrich, R.D. Effects of grain source and concentrate level on B-Vitamin production and absorption in steers. J. Anim. Sci. 1986, 62, 473–483. [Google Scholar] [CrossRef]
- Frigg, M.; Hartmann, D.; Straub, O.C. Biotin kinetics in serum of cattle after intravenous and oral dosing. Int. J. Vitam. Nutr. Res. 1994, 64, 36–40. [Google Scholar]
- Schwab, E.C.; Shaver, R.D. B-Vitamin nutrition in dairy cows. J. Nutr. 2006, 35, 1326–1336. [Google Scholar]
- Briggs, M.H.; Heard, T.W.; Whitcroft, A.; Hogg, M.L. Studies on urea-fed cattle rumen levels of B vitamins and related coenzymes. Life Sci. 1964, 3, 11–14. [Google Scholar] [CrossRef]
- Abel, H.J.; Immig, I.; Gomez, C.; Steinberg, W. Research note: Effect of increasing dietary concentrate levels on microbial biotin metabolism in the artificial rumen simulation system (RUSITEC). ArchivfürTierernährung 2001, 55, 371–376. [Google Scholar]
- Rosendo, O.; Bates, D.B.; McDowell, L.R.; Staples, C.R.; McMahon, R.; Wilkinson, N.S. Availability and ability of biotin for promoting forage fiber in vitro ruminal digestibility. J. Anim. Vet. Adv. 2003, 2, 350–357. [Google Scholar]
- Andrews, E.D. Cobalt concentrations in some new zealand fodder plants grown on cobalt-sufficient and cobalt-deficient soils. N.Z.J. Agric. Res. 1966, 9, 829–838. [Google Scholar] [CrossRef]
- Lowe, R.H.; Evans, H.J. Cobalt requirement for the growth of rhizobia. J. Bacteriol. 1962, 83, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Huwait, E.A.; Kumosani, T.A.; Moselhy, S.S.; Mosaoa, R.M.; Yaghmoor, S.S. Relationship between soil cobalt and vitamin B12 levels in the liver of livestock in Saudi Arabia: Role of competing elements in soils. Afr. Health Sci. 2015, 15, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Codex Alimentarius Commission. Codex Alimentarius: Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants; Codex standard 72; Food and Agriculture Organization of the United Nations, World Health Organization: Rome, Italy, 1981.
- Clayton, P.T. B6-responsive disorders: A model of vitamin dependency. J. Inherit. Metab. Dis. 2006, 29, 317–326. [Google Scholar] [CrossRef]
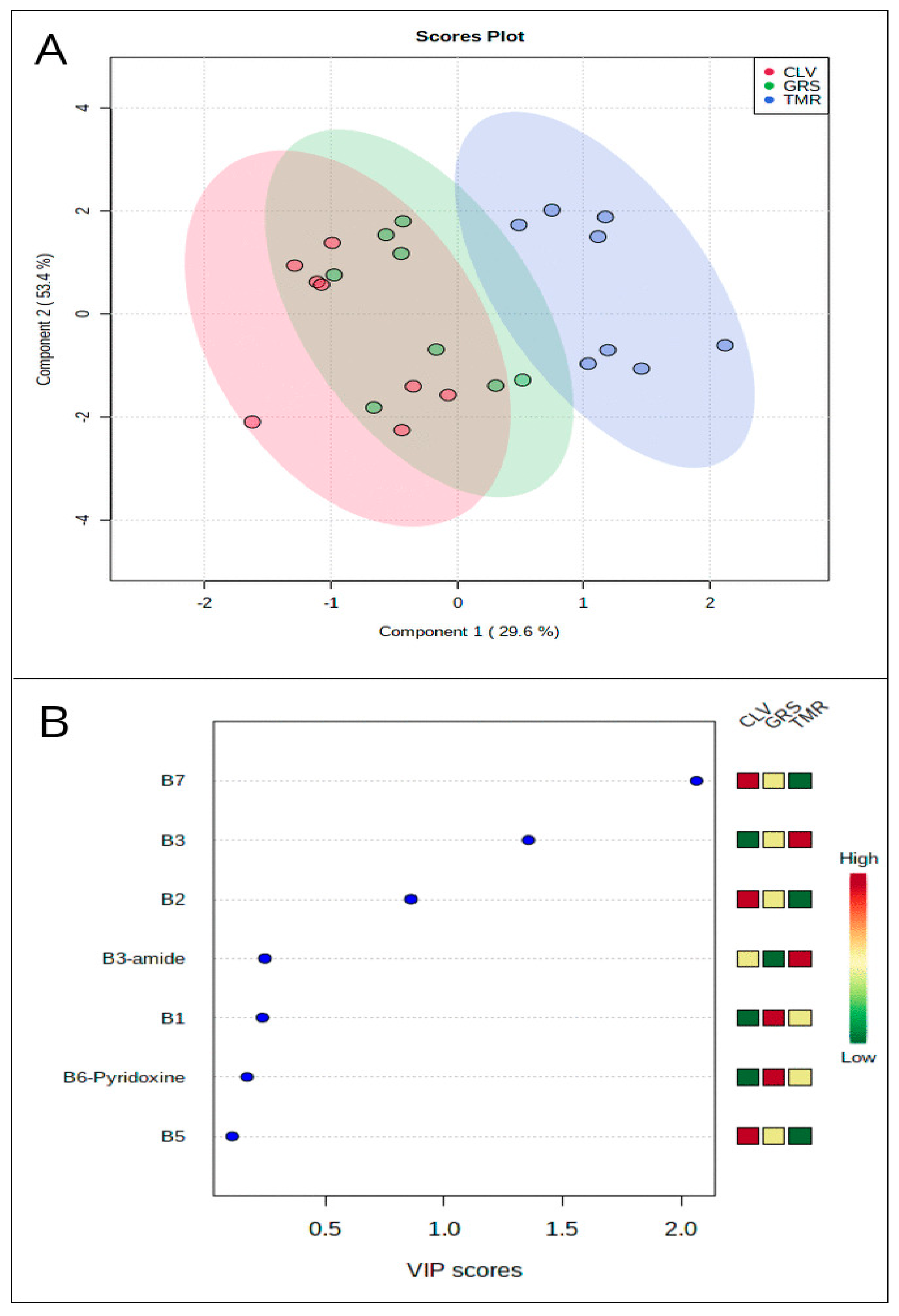
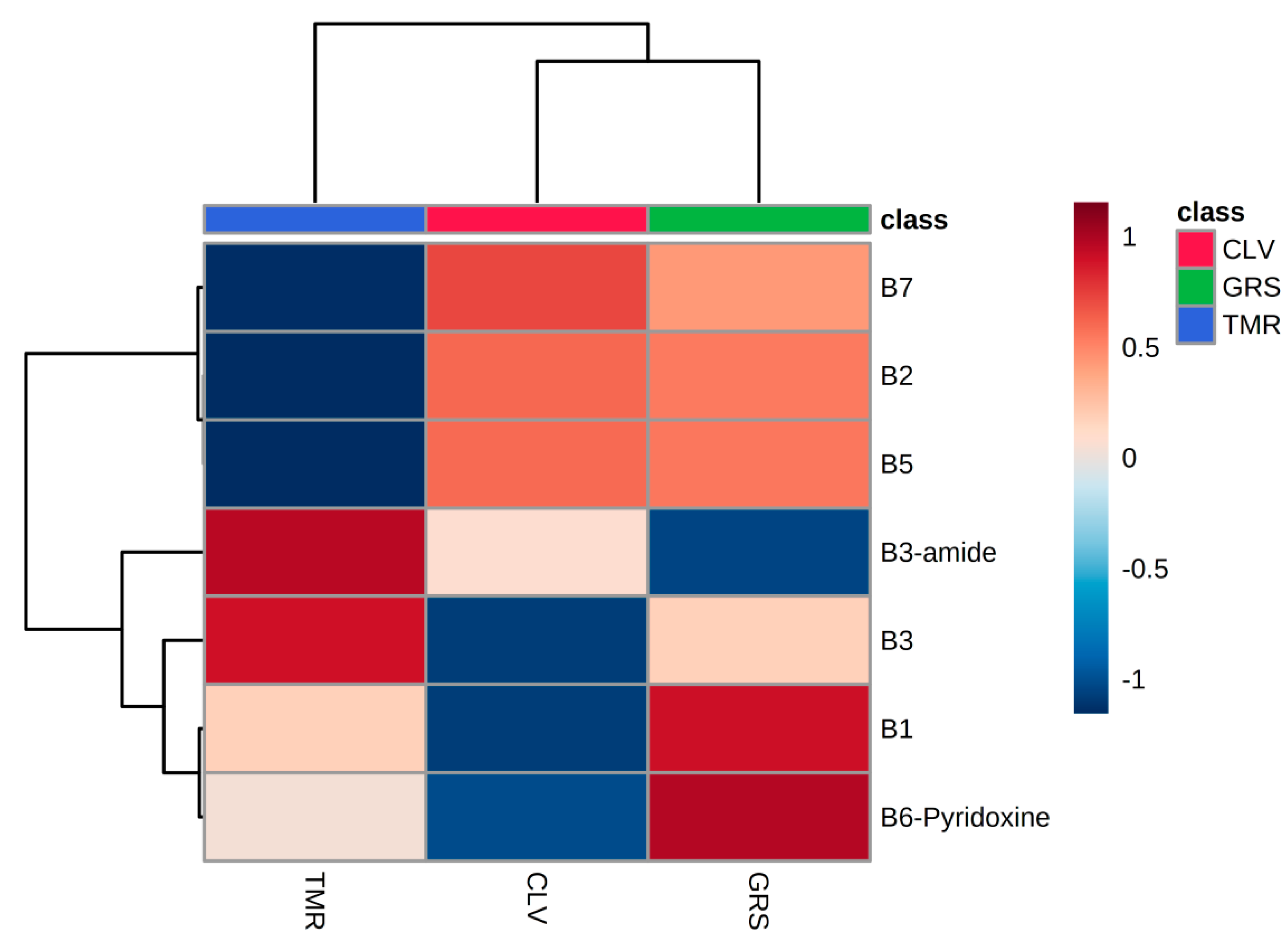
| Sample Type | Water-Soluble Vitamin (μg/g Protein) | GRS | CLV | TMR |
|---|---|---|---|---|
| Skim milk powder | B1 | 5.47 b | 5.44 b | 4.31 a |
| B2 | 422 b | 432 b | 250 a | |
| B3 | 0.86 | 0.63 | 0.94 | |
| B3-amide | 15.4 | 15.4 | 18.0 | |
| B5 | 112 | 117 | 125 | |
| B6-Pyridoxine | 0.13 | 0.11 | 0.13 | |
| B7 | 0.46 | 0.53 | 0.25 | |
| Sweet whey powder | B1 | 29.5 | 27.9 | 36.3 |
| B2 | 1489 b | 1400 b | 636 a | |
| B3 | 4.60 a | 3.37 a | 5.41 b | |
| B3-amide | 74.9 | 82.0 | 81.9 | |
| B5 | 738 | 695 | 675 | |
| B6-Pyridoxine | 0.71 | 0.77 | 0.77 | |
| B7 | 3.04 a,b | 3.12 b | 1.23 a | |
| Micellar casein whey powder | B1 | 23.2 | 22.1 | 20.9 |
| B2 | 191 b | 232 b | 85.2 a | |
| B3 | 4.48 | 4.51 | 4.91 | |
| B3-amide | 117 | 150 | 137 | |
| B5 | 869 | 1067 | 811 | |
| B6-Pyridoxine | 0.77 | 0.86 | 0.74 | |
| B7 | 3.97 b | 5.65 b | 1.77 a | |
| Acid whey powder | B1 | 30.9 | 22.4 | 29.4 |
| B2 | 81.7 | 83.8 | 49.6 | |
| B3 | 5.89 | 4.03 | 8.03 | |
| B3-amide | 110 a | 115 a,b | 129 b | |
| B5 | 929 | 843 | 933 | |
| B6-Pyridoxine | 1.03 | 0.77 | 0.80 | |
| B7 | 3.55 b | 3.75 b | 1.95 a |
| Water-Soluble Vitamin (μg/g Protein) | Sweet Whey Powder | Micellar Casein Whey Powder | Acid Whey Powder |
|---|---|---|---|
| B1 | 31.2 b | 22.1 a | 27.6 a |
| B2 | 1175 b | 170 a | 71.7 a |
| B3 | 4.46 | 4.64 | 5.98 |
| B3-amide | 79.6 a | 135 b | 118 b |
| B5 | 703 | 916 | 902 |
| B6-Pyridoxine | 0.75 | 0.79 | 0.87 |
| B7 | 2.46 | 3.80 | 3.08 |
| Water- Soluble Vitamin | Recommended Daily Allowance (mg) | Mass (mg) in 200 mL Skim Milk | % of Adult Human RDA | Mass (mg) in 200 mL Skim Milk | % of Adult Human RDA | Mass (mg) in 200 mL Skim Milk | % of Adult Human RDA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | GRS | Female | Male | CLV | Female | Male | TMR | Female | Male | |
| B1 | 1.1 | 1.2 | 0.038 | 3.5 | 3.2 | 0.038 | 3.5 | 3.2 | 0.029 | 2.6 | 2.4 |
| B2 | 1.1 | 1.3 | 2.964 | 269 | 228 | 3.049 | 277 | 235 | 1.697 | 154 | 131 |
| B3 complex | 14 | 16 | 0.114 | 0.8 | 0.7 | 0.113 | 0.8 | 0.7 | 0.128 | 0.9 | 0.8 |
| B5 | 5.0 * | 5.0 * | 0.789 | 16 | 16 | 0.829 | 17 | 17 | 0.846 | 17 | 17 |
| B7 | 0.03 * | 0.03 * | 0.0033 | 11 | 11 | 0.0037 | 12 | 12 | 0.0017 | 5.7 | 5.7 |
| B12 | 0.0024 | 0.0024 | 0.0006 | 27 | 27 | 0.0007 | 30 | 30 | 0.0007 | 29 | 29 |
| Water-Soluble Vitamin | Mass (mg/100 mL) | |||
|---|---|---|---|---|
| GRS | CLV | TMR | Minimum Requirement (CODEX STAN 72, 1981) | |
| B1 | 0.004 | 0.004 | 0.003 | 0.039 |
| B2 | 0.279 | 0.287 | 0.160 | 0.052 |
| B3 complex | 0.011 | 0.011 | 0.012 | 0.195 |
| B5 | 0.074 | 0.078 | 0.080 | 0.260 |
| B6-Pyridoxine | 0.000088 | 0.000075 | 0.000086 | 0.023 |
| B7 | 0.0003 | 0.0003 | 0.0002 | 0.001 |
| B12 | 0.000060 | 0.000068 | 0.000065 | 0.000065 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magan, J.B.; O’Callaghan, T.F.; Zheng, J.; Zhang, L.; Mandal, R.; Hennessy, D.; Fenelon, M.A.; Wishart, D.S.; Kelly, A.L.; McCarthy, N.A. Effect of Diet on the Vitamin B Profile of Bovine Milk-Based Protein Ingredients. Foods 2020, 9, 578. https://doi.org/10.3390/foods9050578
Magan JB, O’Callaghan TF, Zheng J, Zhang L, Mandal R, Hennessy D, Fenelon MA, Wishart DS, Kelly AL, McCarthy NA. Effect of Diet on the Vitamin B Profile of Bovine Milk-Based Protein Ingredients. Foods. 2020; 9(5):578. https://doi.org/10.3390/foods9050578
Chicago/Turabian StyleMagan, Jonathan B., Tom F. O’Callaghan, Jiamin Zheng, Lun Zhang, Rupasri Mandal, Deirdre Hennessy, Mark A. Fenelon, David S. Wishart, Alan L. Kelly, and Noel A. McCarthy. 2020. "Effect of Diet on the Vitamin B Profile of Bovine Milk-Based Protein Ingredients" Foods 9, no. 5: 578. https://doi.org/10.3390/foods9050578
APA StyleMagan, J. B., O’Callaghan, T. F., Zheng, J., Zhang, L., Mandal, R., Hennessy, D., Fenelon, M. A., Wishart, D. S., Kelly, A. L., & McCarthy, N. A. (2020). Effect of Diet on the Vitamin B Profile of Bovine Milk-Based Protein Ingredients. Foods, 9(5), 578. https://doi.org/10.3390/foods9050578








