Formation and Inhibition of Lipid Alkyl Radicals in Roasted Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Meat Sample
2.3. Preparation for Radical Determination by ESR
2.4. Radical Standard Curve of TEMPO
2.5. ESR Measurement
2.6. Total Antioxidant Capacity (T-AOC)
2.7. Statistical Analysis
3. Results and Discussion
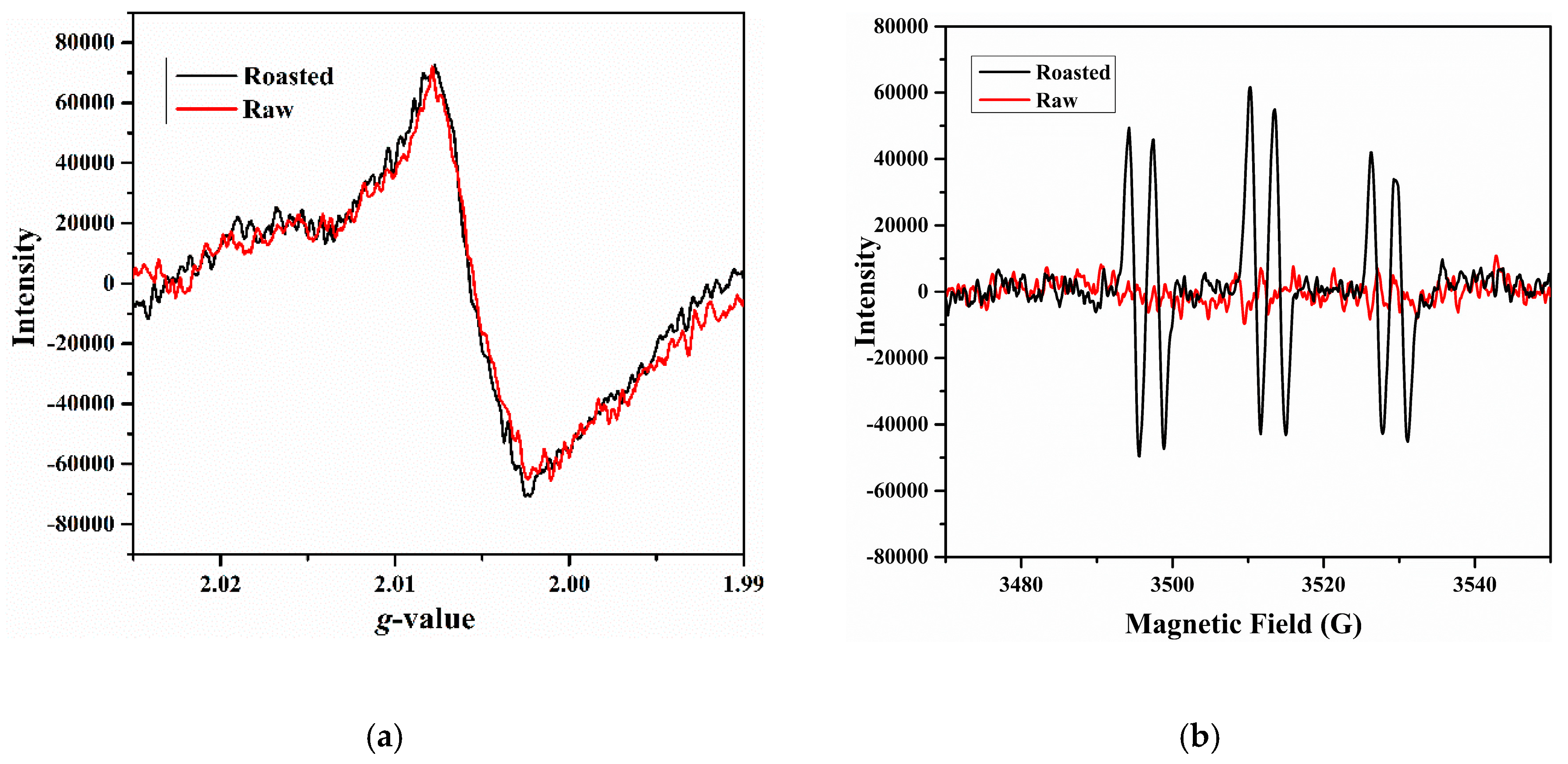
3.1. Characterization of Formed Radicals in Roasted Beef
3.2. Effects of the Heating Method and Time on Radical Formation
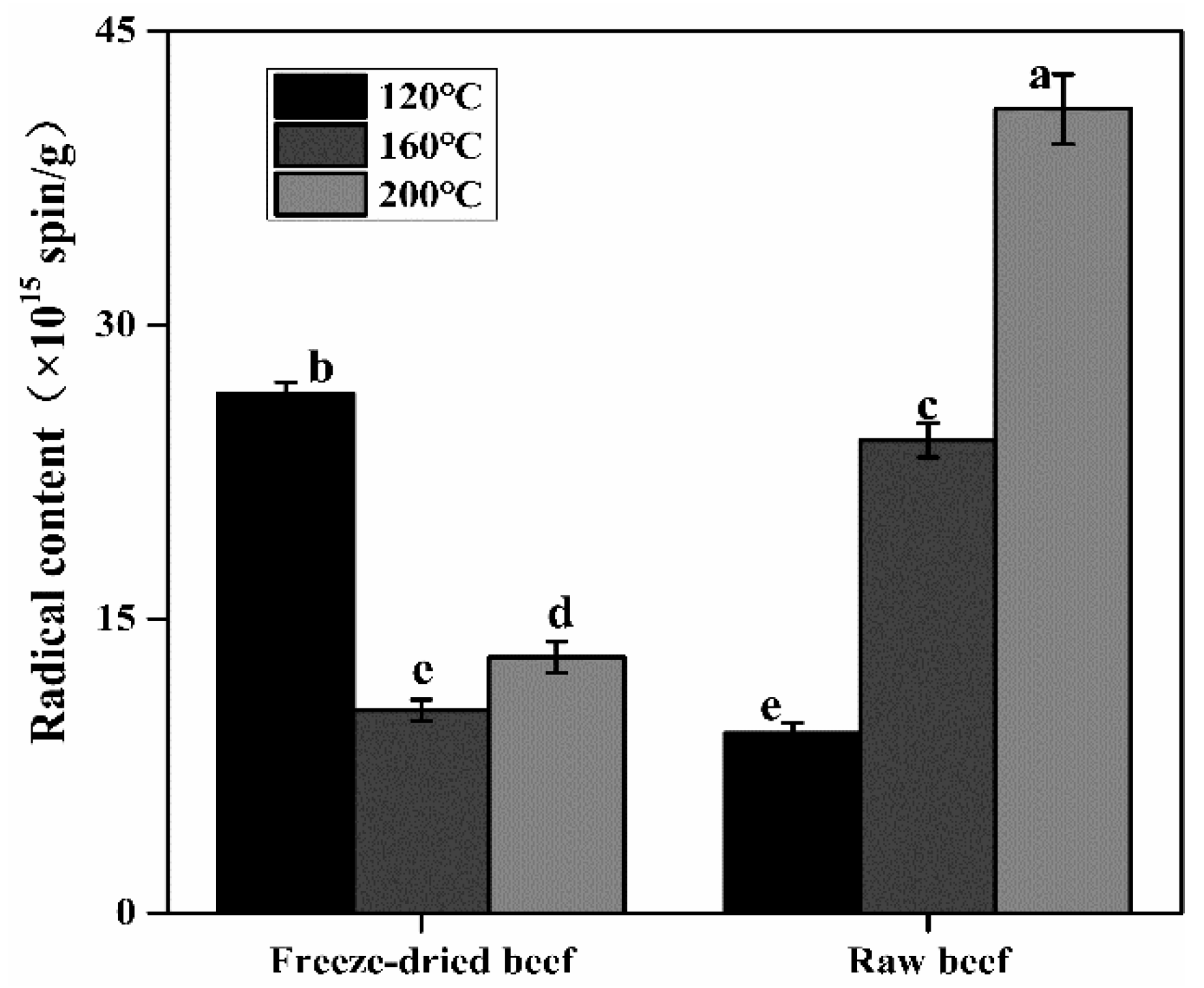
3.3. Effects of the Heating Temperature and Water Content on Radical Formation
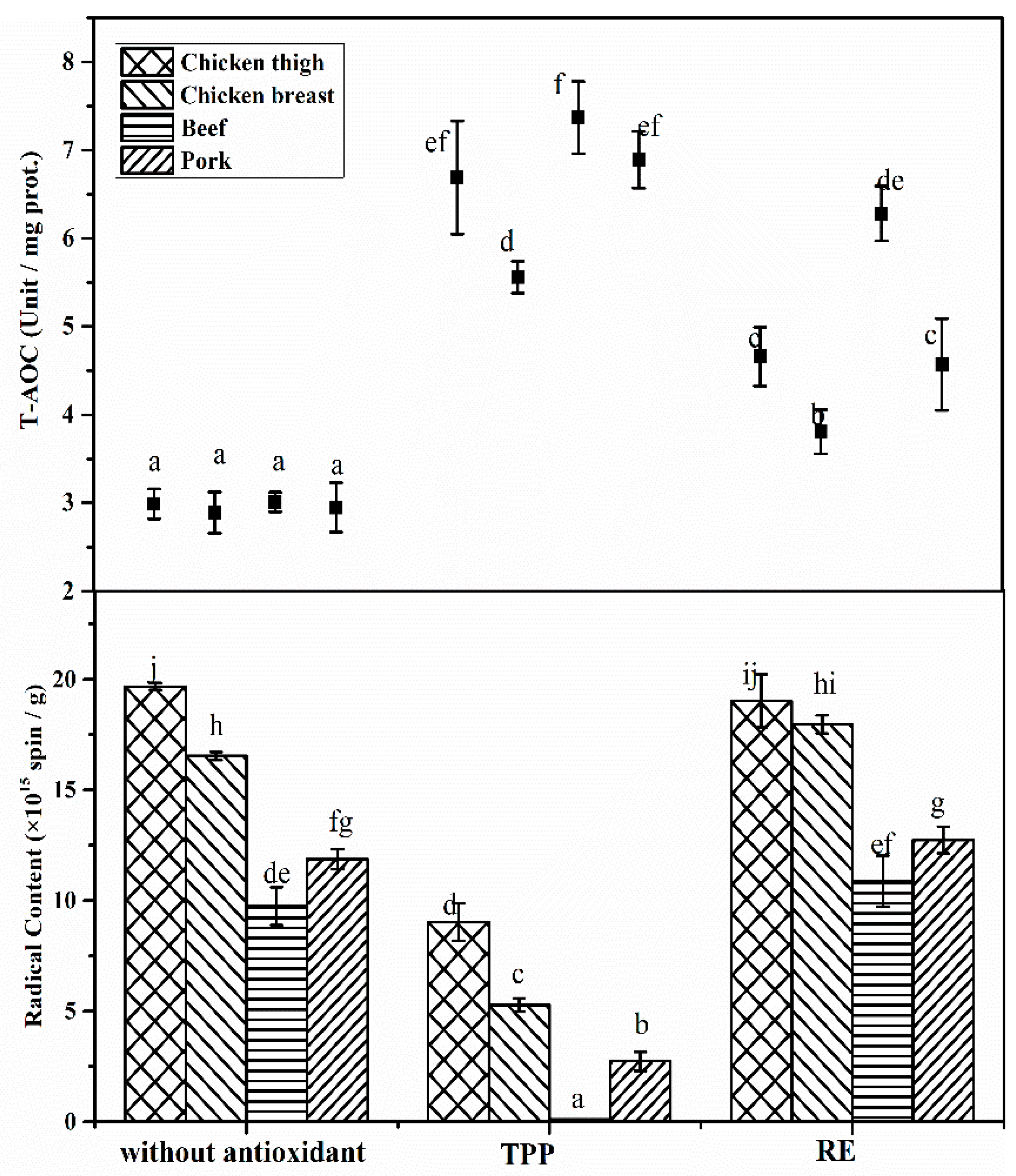
3.4. Effects of Antioxidants on Radical Formation among Meat Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miyakawa, H.; Mason, R.P.; Jiang, J.; Kadiiska, M.B. Lipid-derived free radical production in superantigen-induced interstitial pneumonia. Free Radic. Biol. Med. 2009, 47, 241–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ku, H.H.; Brunk, U.T.; Sohal, R.S. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic. Biol. Med. 1994, 15, 621–627. [Google Scholar] [CrossRef]
- Taub, I.A. Free radical reactions in food. J. Chem. Educ. 1984, 61, 313–324. [Google Scholar] [CrossRef]
- Cämmerer, B.; Kroh, L.W. Shelf life of linseeds and peanuts in relation to roasting. LWT-Food Sci. Technol. 2009, 42, 545–549. [Google Scholar] [CrossRef]
- Yeretzian, C.; Pascual, E.C.; Goodman, B.A. Effect of roasting conditions and grinding on free radical contents of coffee beans stored in air. Food Chem. 2012, 131, 811–816. [Google Scholar] [CrossRef]
- Szöcs, F. Free radicals in wheat seeds studied by electron spin resonance. J. Food Sci. 2002, 67, 2079–2082. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Min, S.; Patra, J.K.; Shin, H.S. Factors influencing inhibition of eight polycyclic aromatic hydrocarbons in heated meat model system. Food Chem. 2018, 239, 993–1000. [Google Scholar] [CrossRef]
- Yu, C.; Shao, Z.; Liu, B.; Zhang, Y.; Wang, S. Inhibition of 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) formation by alkoxy radical scavenging of flavonoids and their quantitative structure-activity relationship in a model system. J. Food Sci. 2016, 81, C1908–C1913. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Wang, S. Effects of rosemary extract, grape seed extract and green tea polyphenol on the formation of N-nitrosamines and quality of western-style smoked sausage. J. Food Process. Preserv. 2020, in press. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Guerra, M.; Sanches-Silva, A. Evaluation of the oxidative status of salami packaged with an active whey protein film. Foods 2019, 8, 387. [Google Scholar] [CrossRef]
- Embuscado, M.E. Herbs and spices as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 251–283. [Google Scholar]
- Orak, H.; Yagar, H.; Isbilir, S.; Demirci, A.; Gumus, T. Antioxidant and antimicrobial activities of white, green and black tea extracts. Acta Aliment. 2013, 42, 379–389. [Google Scholar] [CrossRef]
- Martinez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and antimicrobial activity of rosemary, pomegranate and olive extracts in fish patties. Antioxidant 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Belles, M.; Alonso, V.; Roncales, P.; Beltran, J.A. Effect of borage and green tea aqueous extracts on the quality of lamb leg chops displayed under retail conditions. Meat Sci. 2017, 129, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bragagnolo, N.; Danielsen, B.; Skibsted, L.H. Rosemary as antioxidant in pressure processed chicken during subsequent cooking as evaluated by electron spin resonance spectroscopy. Innov. Food Sci. Emerg. Technol. 2007, 8, 24–29. [Google Scholar] [CrossRef]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 109, 21–30. [Google Scholar] [CrossRef]
- Escudero, R.; Valhondo, M.; Ordoñez, J.A.; Hoz, L.D.L.; Cabeza, M.C.; Velasco, R.; Camberoa, M.I. Electron spin resonance (ESR) spectroscopy study of dry-cured ham treated with electron-beam. Food Chem. 2012, 133, 1530–1537. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, S.; Li, M.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Evaluation on the formation of lipid free radicals in the oxidation process of peanut oil. LWT-Food Sci. Technol. 2019, 104, 24–29. [Google Scholar] [CrossRef]
- Zang, S.; Tian, S.; Jiang, J.; Han, D.; Yu, X.; Wang, K.; Li, D.; Lu, D.; Yu, A.; Zhang, Z. Determination of antioxidant capacity of diverse fruits by electron spin resonance (ESR) and UV–vis spectrometries. Food Chem. 2017, 221, 1221–1225. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission of the People’s Republic of China. National Food Safety Standard—Determination of Moisture in Foods; GB 5009.3-2016; Ministry of Agriculture: Beijing, China, 2016.
- Carlsen, C.U.; Andersen, M.L.; Skibsted, L.H. Oxidative stability of processed pork. assay based on esr-detection of radicals. Eur. Food Res. Technol. 2001, 213, 170–173. [Google Scholar] [CrossRef]
- Bolumar, T.; Skibsted, L.H.; Vibeke, O. Kinetics of the formation of radicals in meat during high pressure processing. Food Chem. 2012, 134, 2114–2120. [Google Scholar] [CrossRef]
- Abbas, K.; Babić, N.; Peyrot, F. Use of spin traps to detect superoxide production in living cells by electron paramagnetic resonance (EPR) spectroscopy. Methods 2016, 109, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Dodd, N.J.; Swartz, H.M. The nature of the ESR signal in lyophilized tissue and its relevance to malignancy. Br. J. Cancer 1984, 49, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Zhao, B.L.; Guo, Y.J.; Xin, W.J. Evidence for L·against LOO being spin-trapped by 4-POBN during the reaction of Fe2+-induced lipid peroxidation. Appl. Magn. Reson. 1991, 2, 521–531. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Cao, P.; Liu, Y. Effect of temperature on thermal oxidation of palmitic acid studied by combination of EPR spin trapping technique and SPME-GC–MS/MS. Food Chem. 2017, 234, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Labuza, T.P.; Dugan, L.R. Kinetics of lipid oxidation in foods. CRC Crit. Rev. Food Technol. 1971, 2, 355–405. [Google Scholar] [CrossRef]
- Min, B.; Nam, K.C.; Cordray, J.; Ahn, D.U. Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. J. Food Sci. 2008, 73, C439–C446. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.A.; Buettner, G.R.; Burns, C.P. Free radical-mediated lipid peroxidation in cells: Oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry 1993, 15, 4449–4453. [Google Scholar] [CrossRef]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Nissen, L.R.; Månsson, L.; Bertelsen, G.; Huynh-Ba, T.; Skibsted, L.H. Protection of dehydrated chicken meat by natural antioxidants as evaluated by electron spin resonance spectrometry. J. Agric. Food Chem. 2000, 48, 5548–5556. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Mechanisms of radical formation in beef and chicken meat during high pressure processing evaluated by electron spin resonance detection and the addition of antioxidants. Food Chem. 2014, 150, 422–428. [Google Scholar] [CrossRef]
- Beltran, E.; Pla, R.; Yuste, J.; Mor-Mur, M. Use of antioxidants to minimize rancidity in pressurized and cooked chicken slurries. Meat Sci. 2004, 66, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Delgado, L.H.; Mateo, J.; Caro, I.; Leal Ramos, M.Y.; Mendez, N.G.; Cansino, R.G.; González-Mondragón, E.G. Natural antioxidants in fresh and processed meat. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 207–236. [Google Scholar]
| Cooking Methods | Time (min) | PT | PM | PT × PM | |||
|---|---|---|---|---|---|---|---|
| 10 | 15 | 20 | 25 | ||||
| Roast | 10.54 ± 1.04 b | 34.62 ± 1.56 g | 41.01 ± 1.78 i | 29.99 ± 1.64 e,f | *** | *** | *** |
| Grill | 26.83 ± 1.57 d | 38.08 ± 1.69 h | 46.53 ± 1.83 j | 39.30 ± 1.72 h,i | |||
| Barbecue | 7.64 ± 0.96 a | 18.01 ± 1.44 c | 32.25 ± 1.52 f,g | 28.30 ± 1.61 d,e | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Zhu, Y.; Ren, X.; Zhang, Y.; Peng, Z.; Zhou, G. Formation and Inhibition of Lipid Alkyl Radicals in Roasted Meat. Foods 2020, 9, 572. https://doi.org/10.3390/foods9050572
Bao Y, Zhu Y, Ren X, Zhang Y, Peng Z, Zhou G. Formation and Inhibition of Lipid Alkyl Radicals in Roasted Meat. Foods. 2020; 9(5):572. https://doi.org/10.3390/foods9050572
Chicago/Turabian StyleBao, Yingjie, Yuxia Zhu, Xiaopu Ren, Yawei Zhang, Zengqi Peng, and Guanghong Zhou. 2020. "Formation and Inhibition of Lipid Alkyl Radicals in Roasted Meat" Foods 9, no. 5: 572. https://doi.org/10.3390/foods9050572
APA StyleBao, Y., Zhu, Y., Ren, X., Zhang, Y., Peng, Z., & Zhou, G. (2020). Formation and Inhibition of Lipid Alkyl Radicals in Roasted Meat. Foods, 9(5), 572. https://doi.org/10.3390/foods9050572





