1. Introduction
The consumption of ready-to-eat (RTE) products has risen in recent years due to their easy availability and good quality [
1]. In this category meat products like rolled fillets of ham are frequently consumed. However, as during production, slicing and further handling the ham can be contaminated with bacteria, present in the food processing plant and household, and as the product is consumed without previous heating, RTE products might pose a risk for food-borne illnesses.
It is known that contaminated pig carcasses can transmit pathogens such as
Yersinia (
Y.)
enterocolitica or spoilage bacteria such as
Brochothrix (
B.)
thermosphacta from the slaughterhouse to meat processing plants, where they in turn can contaminate surfaces and equipment, e.g., slicing machines [
2,
3]. Thus, even hygienically safe meat products can be re-contaminated with these bacteria, e.g., during slicing.
Y. enterocolitica, which were found in RTE pork products, can cause human yersiniosis, a food-borne bacterial disease, often associated with diarrhea and bowel inflammation [
4]. Cases of yersiniosis are still high in some countries such as Germany. In the European Union a total of 6806 cases of yersiniosis were reported in 2018 [
5]. Pathogenic
Y. enterocolitica are most frequently found in pigs and therefore, pig meat products may pose a risk to the consumer. The highest bacterial counts of
Y. enterocolitica can be determined in the feces and tonsils of pigs and thus, in addition to fecal contamination, the handling of the head during slaughter may lead to a spreading of the microorganism [
6,
7].
B. thermosphacta is an important spoilage bacteria frequently found on meat and meat products [
8]. In addition to quality changes like off-odors and discoloration of the food, these bacteria can also lead to gastrointestinal disturbances, if the product is contaminated with high
B. thermosphacta concentrations [
9]. Growth of
Y. enterocolitica and
B. thermosphacta is influenced by numerous intrinsic or extrinsic factors like temperature, pH value, water activity, or the specific gas composition in modified atmosphere packages (MAP). The latter is important, as ham is frequently purchased in MAP [
10,
11].
Although there are already many measures during production to increase the hygienic safety of the product such as disinfection of food contact surfaces, maintenance of the cold chain and packaging under modified atmosphere, methods to reduce the contamination of RTE products like ham after production are of great interest for the food industry. Treatment with ultraviolet (UV) light has long gained in importance as a decontamination technique as it requires neither chemicals nor heat. UV-C light with wavelengths between 200 and 280 nm and especially the wavelength of 254 nm has proven to be the most effective within the UV spectrum (100–400 nm), as it generates pyrimidine dimers as well as pyrimidine-pyrimidone (6-4) photoproducts and denatures microbial DNA. These alterations may be lethal to bacteria or may reduce their reproduction abilities [
12]. However, photoreactivation is an efficient repair mechanism of bacteria to (partly) recover their viability and to minimize the effects of the UV treatment. The photolyase, an important enzyme of the photoreactivation process, is activated by visible light and catalyzes the repair of the damaged DNA without excising parts of the DNA strand [
13].
Inactivation studies using UV-C irradiation have already demonstrated effectiveness in lowering bacterial counts on meat without causing quality changes [
14,
15]. However, the effect is limited to surface decontamination, as UV-C light cannot penetrate deeper layers of the tissue [
16]. As the chemical composition of the product has a considerable influence on the effectiveness of the UV-C treatment [
17], the question arises, how this preservation method reduces the bacterial contamination of processed meat products such as rolled fillets of ham. Beside this, it is important to know how UV-C irradiation might not only influence bacterial numbers, but also other quality parameters of the meat products such as color values or antioxidant activities. Therefore, the aim of the present study was to analyze the effectiveness of UV-C treatment on
Y. enterocolitica and
B. thermosphacta counts and product quality parameters of rolled fillets of ham.
Y. enterocolitica was chosen in the present study, as, considering the above described information, higher risks for contamination of pork ham during production and packaging and therefore of infections of humans could be assumed in comparison to other important pathogens like
Listeria monocytogenes or
Staphyloccocus aureus. Even though
Y. enterocolitica does not find conditions for growth in some pork products such as ham, the inactivation of the pathogen on meat and meat products plays an important role and some approaches with this aim have already been published [
18]. Therefore, the focus of this study is on reducing bacterial contamination with the UV-C treatment and not on the influence of bacterial growth.
2. Materials and Methods
2.1. Ethics Statement
In the study, pig muscles were collected from a commercial pig slaughterhouse, UV-C treated and analyzed after processing to rolled fillets of ham. The slaughterhouse considered all European and German animal welfare regulations for transport, handling, and slaughter of the animals.
2.2. Test Organisms
The Y. enterocolitica isolate used for this study was obtained from the tonsils of a wild boar and was part of the strain collection of the Institute for Food Quality and Food Safety, Foundation University of Veterinary Medicine Hannover, Germany. The B. thermosphacta strain DSM 20171 (isolated from fresh pork sausage) was obtained from the German collection of microorganisms and cell cultures (DSMZ, Braunschweig, Germany). The isolates were stored at −80 °C in cryotubes until use. Both bacterial strains were used based on previous in vitro experiments and their porcine origin.
2.3. UV Equipment
The irradiation process was performed with the UV-Cabinet-H-NX-1/5 (Light Progress S.r.l., Anghiari, Italy). It has five 40 W low pressure mercury UV lamps which emit light with a wavelength of 253.7 nm. The UV-C intensity was determined with the Handheld HI 1 and the UV-Sensor SI 1 (UV-technik Meyer GmbH, Ortenberg, Germany). The UV-C intensity was 6.8 mW/cm2. As the UV-C dose (in mJ/cm2) is the product of the UV-C intensity (in mW/cm2) and the exposure time (in s), it was changed by altering the exposure time.
For each experiment the lamps were switched on at least 10 min before exposure, as previous experiments showed that this period was sufficient to guarantee a constant UV intensity output. The samples were irradiated at room temperature (ca. 22 °C).
2.4. Overview of the Experiments
Figure 1 gives an overview of the experiments. First, various UV-C doses were tested for their ability to reduce the bacterial count on rolled fillets of ham. In addition, different initial concentrations of bacteria were used to analyze in which way the reduction of bacteria was influenced by the amount of bacteria on the product.
For the storage study the ham was inoculated with either Y. enterocolitica, or B. thermosphacta, UV-C irradiated (408 or 4080 mJ/cm2) and packed under modified atmosphere. On days 0, 7, and 14 samples for microbiological and physicochemical (color and antioxidant activity) investigations were removed and analyzed.
In additional experiments the influence of photoreactivation after the UV-C irradiation of ham was tested.
2.5. Material
For the analysis of the dose dependent bacterial reduction, slices of rolled fillets of ham of the type of an air dried ham from five different batches of the same brand were purchased from a local supermarket. Additionally, three different batches of the ham were purchased to analyze whether photoreactivation occurs. Each package contained 100 g ham slices, packaged in polypropylene under modified atmosphere. The sales designation is low fat (maximum 2% fat) cured rolled fillet of ham which is produced from the Musculus longissimus thoracis et lumborum (LTL). The package was mostly transparent and partly painted. The ham contained the following ingredients: pork, iodized salt, potassium iodate, dried glucose syrup, dextrose, sugar, sodium acetate, sodium citrate, sodium ascorbate, sodium nitrite, spice extracts, beech wood smoke.
For the storage study, LTL from the left side of three different female pigs were collected from a local slaughterhouse 24 h after slaughter. Firstly, the LTL was freed from fat and tendons. For the dry curing process, the meat was evenly rubbed with 50 g nitrite curing salt (CS) per kg meat (meat: CS ratio: 20:1), vacuum packed, and stored at 4 °C. The CS consists of 99.5% sodium chloride (NaCl) and 0.5% sodium nitrite (NaNO2). After 14 days, the meat was unpacked and hung at 4 °C for a further 4 weeks. After this storage period the color of the ham, the water activity (aw), moisture, protein, fat, ash, NaCl, and NaNO2 content were analyzed. Furthermore, the total viable count (TVC) as well as the bacterial counts of Enterobacteriaceae, Yersinia spp., and Brochothrix spp. were determined. For further analyses the ham was portioned into slices (but not rolled) with a thickness of 1.5 mm and a mass of about 10 g.
2.6. Preparation and Treatment of Ham
B. thermosphacta was cultured on Columbia agar with sheep blood (Oxoid GmbH, Wesel, Germany) for 24 h at 25 °C and Y. enterocolitica was cultured on plate count agar (Oxoid) for 24 h at 30 °C. Colonies were removed from the plate and suspended in 4 mL sterile saline solution (0.85% NaCl). The bacterial suspension was adjusted to a 1.0 McFarland turbidity standard (approximately 108 colony forming units (cfu)/mL) with a densimat (BioMérieux SA France IDN 013615, Craponne, France). In further experiments, the bacterial suspension was adjusted to 106 cfu/mL to test if a lower initial bacterial concentration influences the reductions.
For the analysis of the dose dependent reduction and the photoreactivation the purchased ham slices, and for the storage studies the slices from the self-made hams, were evenly inoculated with 0.5 mL of the bacterial suspensions (either B. thermosphacta, or Y. enterocolitica). Twenty minutes after inoculation the slices were treated with UV-C. Therefore, the samples were placed at a distance of 10 cm under the UV lamp and either treated with doses of 408, 2040, 4080, or 6120 mJ/cm2 (dose dependent reduction) or with 408 or 4080 mJ/cm2 (storage study and photoreactivation study), respectively. The doses for the storage study were selected because in the initial reduction studies they either showed slight but not significant reductions (408 mJ/cm2) or clearly significant reductions (4080 mJ/cm2) in the bacterial counts.
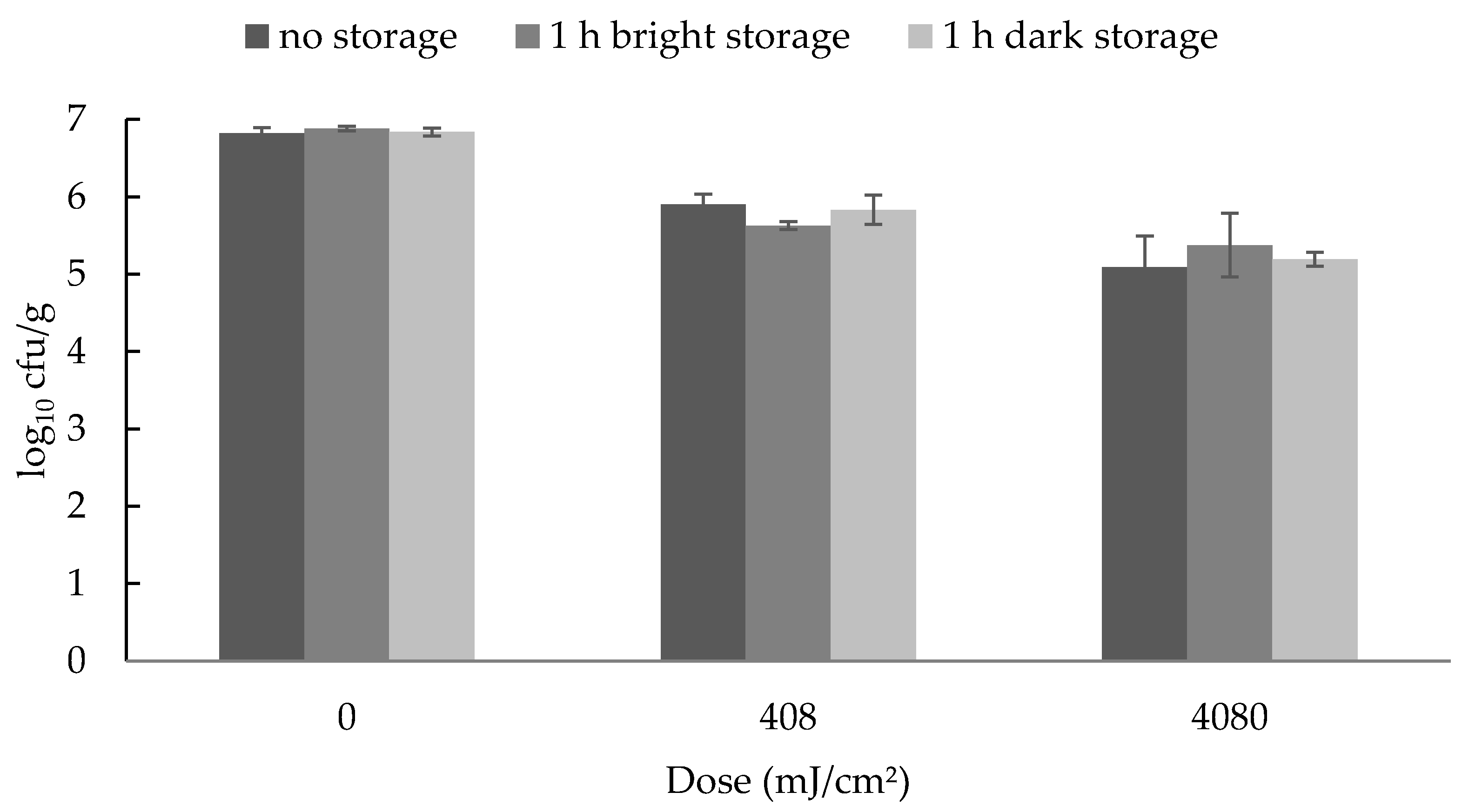
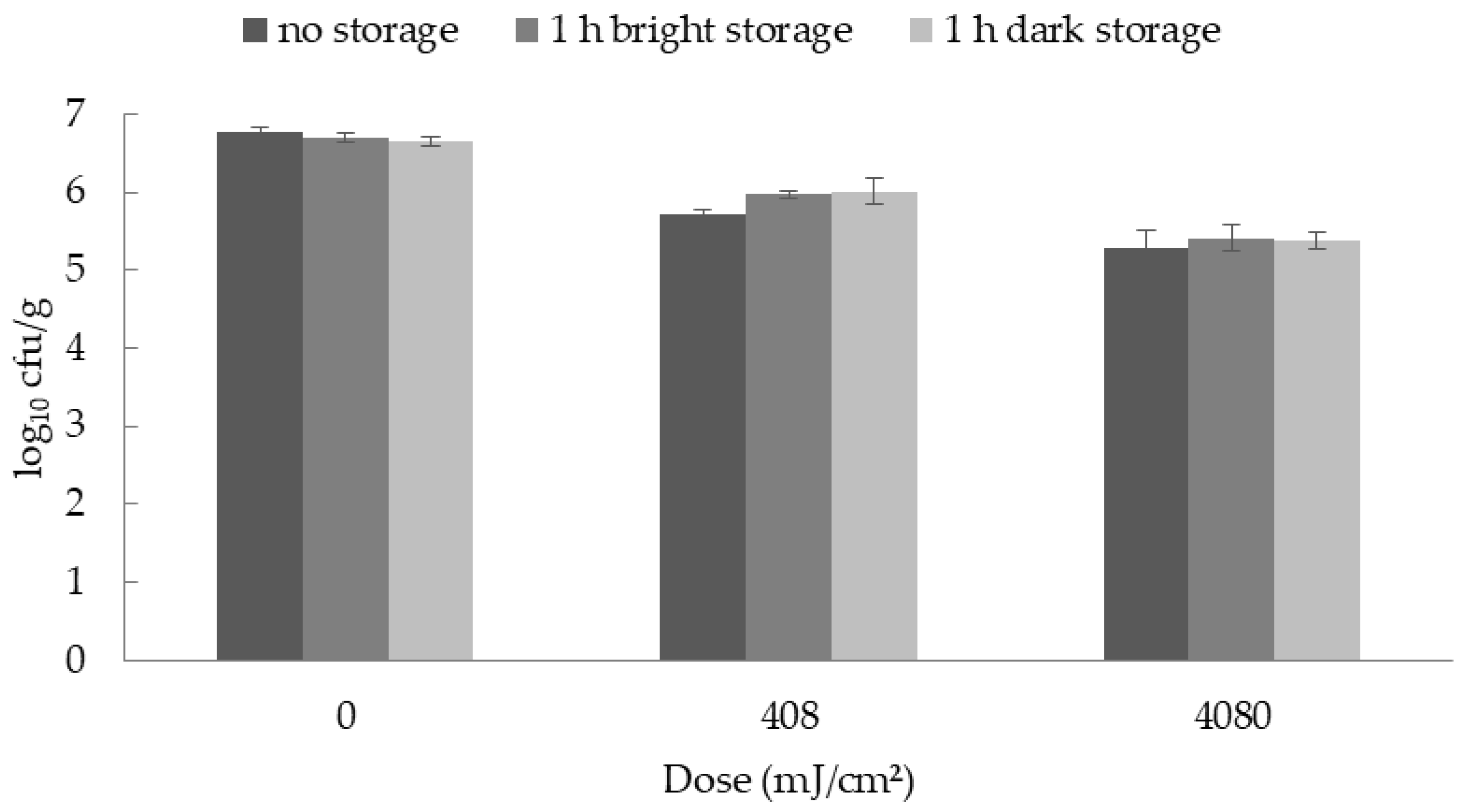
For the photoreactivation study, the samples were divided into three different treatment groups. The first group was microbiologically analyzed immediately after the UV-C irradiation. The second group was exposed to visible light for 1 h after irradiation and then analyzed, whereas the third group of samples was stored without previous light exposure.
The analyses of the dose dependent reductions were performed with three (B. thermosphacta) or five (Y. enterocolitica) different batches of rolled fillets of ham, purchased from a supermarket and the analyses of the photoreactivation were performed with three different batches. The storage study was performed with three different batches of rolled fillets of ham, made from the LTL of three pigs (n = 3).
2.7. Storage
The UV-C treated and untreated (control) samples were packed under modified atmosphere (70% O2 and 30% N) in plastic trays (ES-Plastic GmbH, Hutthurm, Germany) and were stored at 7 °C until further analysis. On the treatment day (designated as day 0) and the days 7 and 14 samples were collected for microbiological and physicochemical analyses. The used samples were excluded from further experiments.
2.8. Microbiological Parameters
The samples were homogenized in bags (Stomacher 400 Strainer Bags, Seward limited, Worthing, UK) with a Stomacher (Stomacher 400 Circulator, Seward) in a dilution of 1:10 with sterile saline solution with peptone (0.85% NaCl and 0.1% peptone) for 2 min at 230 rpm. After homogenization, the samples were serially diluted.
Yersinia spp. counts were determined using cefsulodin irgasan novobiocin agar (Oxoid) at 30 °C for 24 h (ISO 10273:2017).
Brochothrix spp. were quantified using streptomycin-inosit-neutral-red agar plates (Oxoid) at 25 °C for 48 h according to Hechelmann [
19].
Enterobacteriaceae were grown on violet red bile glucose agar plates (Oxoid) at 37 °C for 48 h (ISO 21528:2017) and TVC were determined on plate count agar (Oxoid) at 30 °C for 72 h (ISO 4833-1:2013).
The detection limits for TVC and Enterobacteriaceae were 1.0 log10 cfu/g and for Y. enterocolitica and B. thermosphacta 2.0 log10 cfu/g. If no colonies were determined on the agar plates with the initial dilution, the half detection limit (0.7 log10 or 1.7 log10 cfu/g meat) was used for further calculations.
2.9. Physical and Chemical Parameters
A Minolta CR 400 colorimeter (Konica-Minolta GmbH, Langenhagen, Germany; 2° standard observer, D65 illuminant, 8 mm measuring field) was used to analyze the instrumental color parameters. In advance, it was calibrated with a standard white plate (Konica-Minolta GmbH). The results are expressed as CIE L* (lightness), a* (redness), and b* (yellowness). The color measurements took place immediately after the package was opened and the ham was placed on a white background. Each value was an average of five measurements. Additionally, the total color difference delta (Δ) E was determined between the treatment groups using the following formula:
ΔE shows an objective difference between two colors and described the difference of L*a*b*-measurements before and after the UV-C treatment.
The water activity of the ham was measured with an aw-Kryometer (AWK-40, NAGY Messsysteme GmbH, Gäufelden, Germany). Before each experiment the kryometer was calibrated with a 10% NaCl solution. The final aw-value for statistical analysis was the mean of three measurements.
For the determination of the moisture content, 3 g of the homogenized sample was mixed with sea sand and dried in a drying oven (Binder GmbH, Tuttlingen, Germany) at 103 °C for 4 h (ISO 1442:1997).
To analyze the ash concentration, 5 g of the homogenized sample were burned in a muffle furnace (Carbolite®, LAT GmbH, Garbsen, Germany) for 1–2 h at 600 °C (ISO 936:1998).
The protein concentration was calculated by analysis of nitrogen concentration, using the Kjeldahl method (Vapodest 50s®, Gerhardt Laboratory Systems GmbH, Koenigswinter, Germany) (ISO 937:1978).
Fat was determined after acid hydrolysis and extraction in Soxhlet equipment (LAT GmbH, Garbsen, Germany) (ISO 1443:1973).
The nitrite content was measured according to ISO 2916:1975. The sample was extracted with hot water and the proteins were precipitated. After filtration, sulfanilamide and naphthylethylenediamine hydrochloride were added. The presence of nitrite was shown by the formation of red complexes which was detected photometrically at 538 nm (Evolution 201-UV-VIS-Spectrophotometer, Thermo Scientific, Langenselbold, Germany).
The sodium chloride content was analyzed in accordance with ISO 1841:1996. The sample was extracted with hot water and clarified. The amount of chlorides was then measured by potentiometric titration using a 0.1 mol/L AgNO3 soluted in 0.3 mol/L HNO3.
To measure the antioxidant capacity, an ABTS [2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid)] radical cation solution was created as described by Re et al. [
20]. ABTS was dissolved in water to a 7 mM concentration. To radicalize the solution 2.45 mM potassium persulfate was added. Before use, the ABTS
● radical solution was stored for 12–16 h in the dark at room temperature. The preparation of the samples and the measurement were performed according to Sacchetti et al. [
21] with slight modifications. Approximately 1 g of the frozen meat sample added with 6 mL distilled water was transferred to a 50 mL tube and homogenized for 1 min at 30,000 rpm on ice with a Polytron PT 2500 homogenizer (Kinematica GmbH, Luzern, Switzerland). Afterwards, the tube was wrapped in an aluminum sheet and extracted in a shaker at 7 °C for 1 h. The homogenate was centrifuged for 15 min at 2340
g (Hermle Z383 K, Hermle Labortechnik, Wehingen, Germany). The bleaching rate of ABTS in the presence of the sample was monitored photometrically at 734 nm (Evolution 201-UV-VIS-Spectrophotometer, Thermo Scientific). To start the reaction, 2.50 mL ABTS
● radical solution was mixed with 20 µL supernatant of the centrifuged sample and 500 µL distilled water. The discoloration after 7 min was used to determine the antioxidant activity in µmol Trolox eq. per g of sample. For this, standard solutions with Trolox, the hydrophilic homologue of tocopherol, were prepared (0, 2.5, 5.0, 7.5, 10.0, 15.0 µM) and analyzed with the ABTS
● radical solution at 734 nm. For further analysis only calibration curves with correlation coefficients of at least 0.99 were used.
2.10. Statistical Analysis
The data of all experiments were statistically analyzed with SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA) using the SAS PROC MIXED procedure considering the following model:
where Y
ij = observation value; µ = overall mean, D
i = fixed effect of UV-dose; B
j = random effect of batch; ε
ij = random error.
It was followed by the TUKEY multiple comparison test. All values were presented as means ± standard error (SE). Means were marked significant if the p value was lower than 0.05. All experiments were independently replicated at least three times.