Improving the In Vitro Bioaccessibility of β-Carotene Using Pectin Added Nanoemulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Coarse Emulsion and Nanoemulsions Preparation
2.2.2. Particle Size
2.2.3. Electrical Charge
2.2.4. Stability
2.2.5. β-Carotene Extraction and Quantification
2.2.6. In Vitro Digestion
2.2.7. Bioaccessibility Determination
2.2.8. Optical Microscopy
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.1.1. Particle Size
3.1.2. Electrical Charge
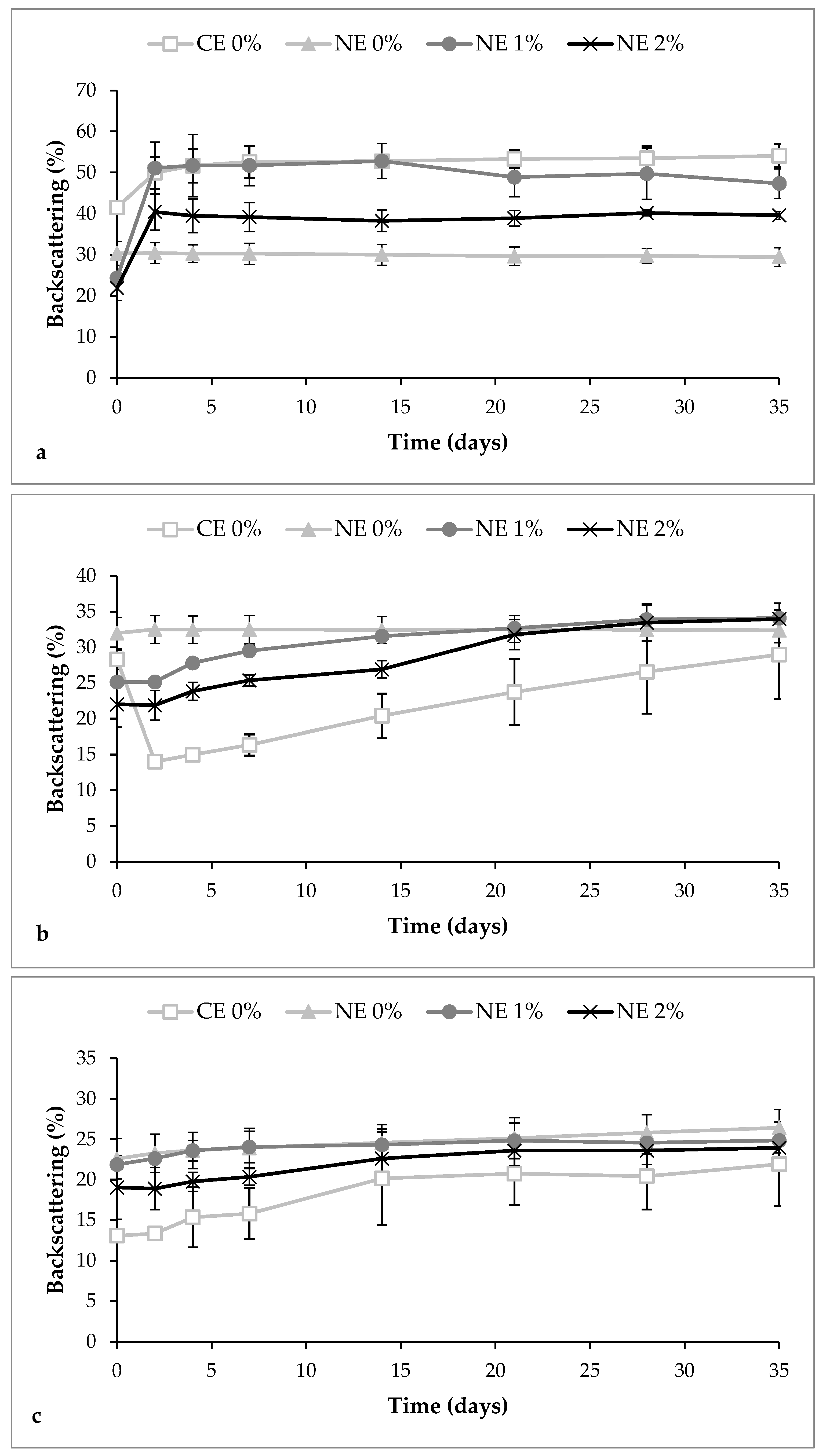
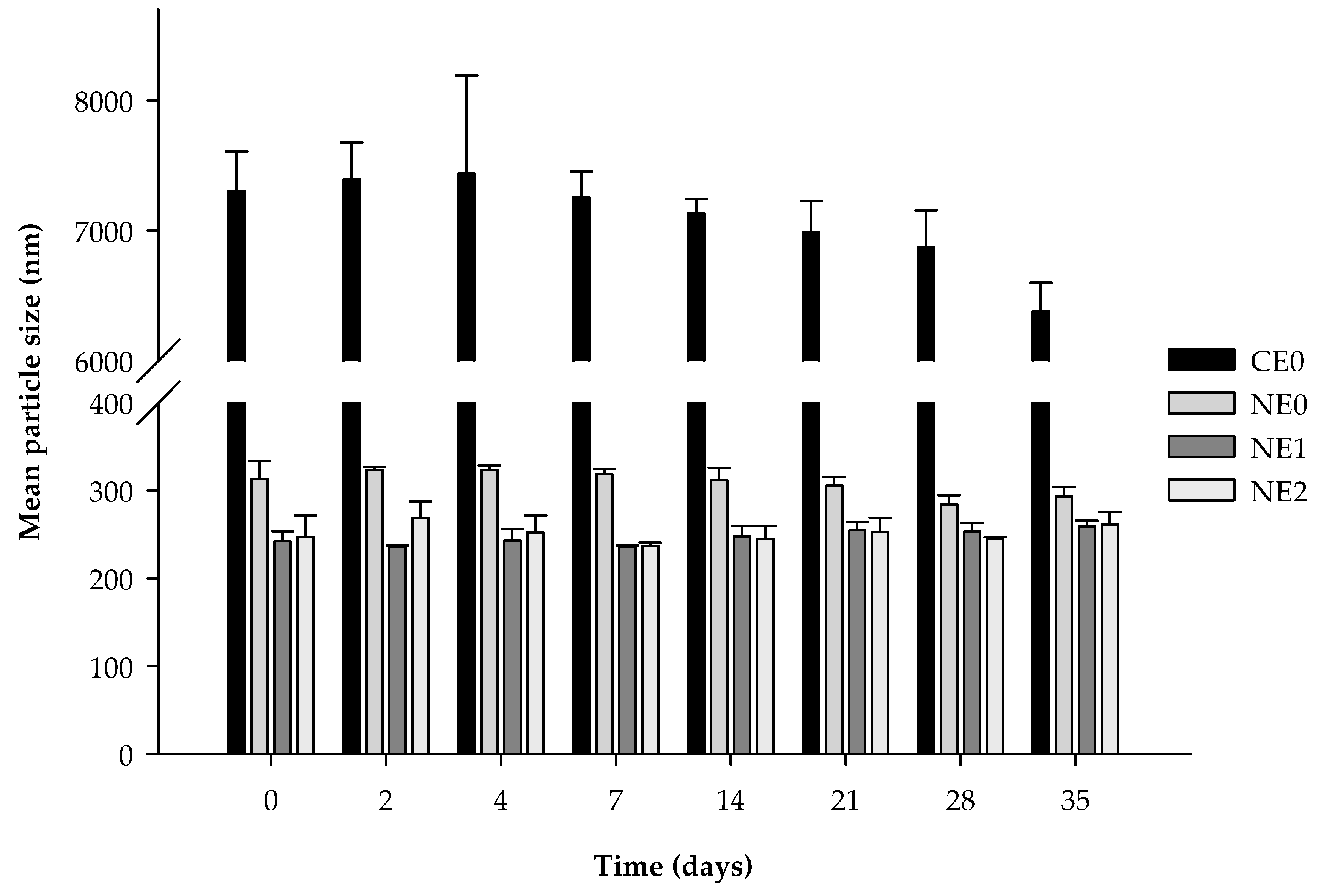
3.2. Stability
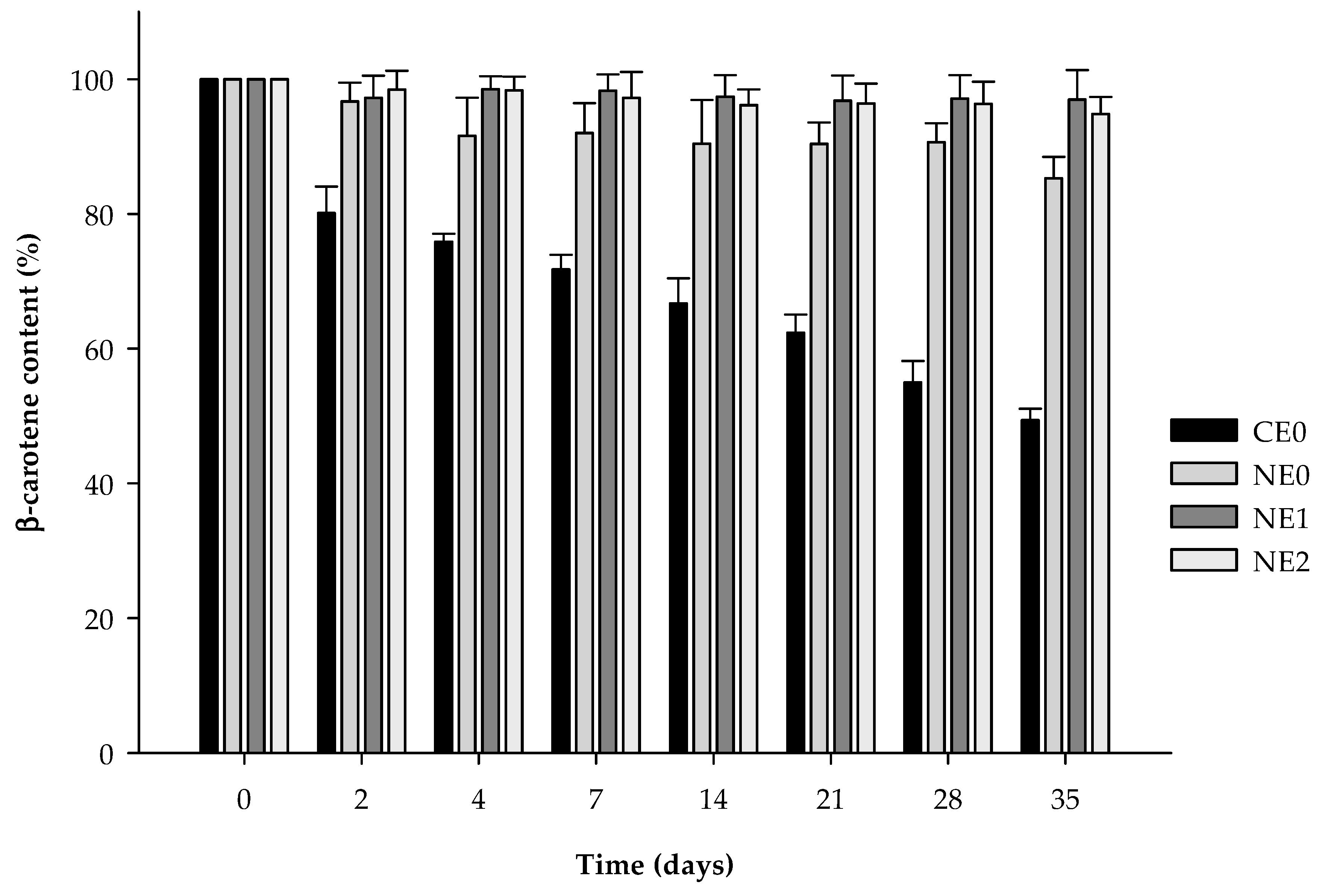
3.3. β-Carotene Degradation
3.4. Gastrointestinal Behaviour of the Emulsions
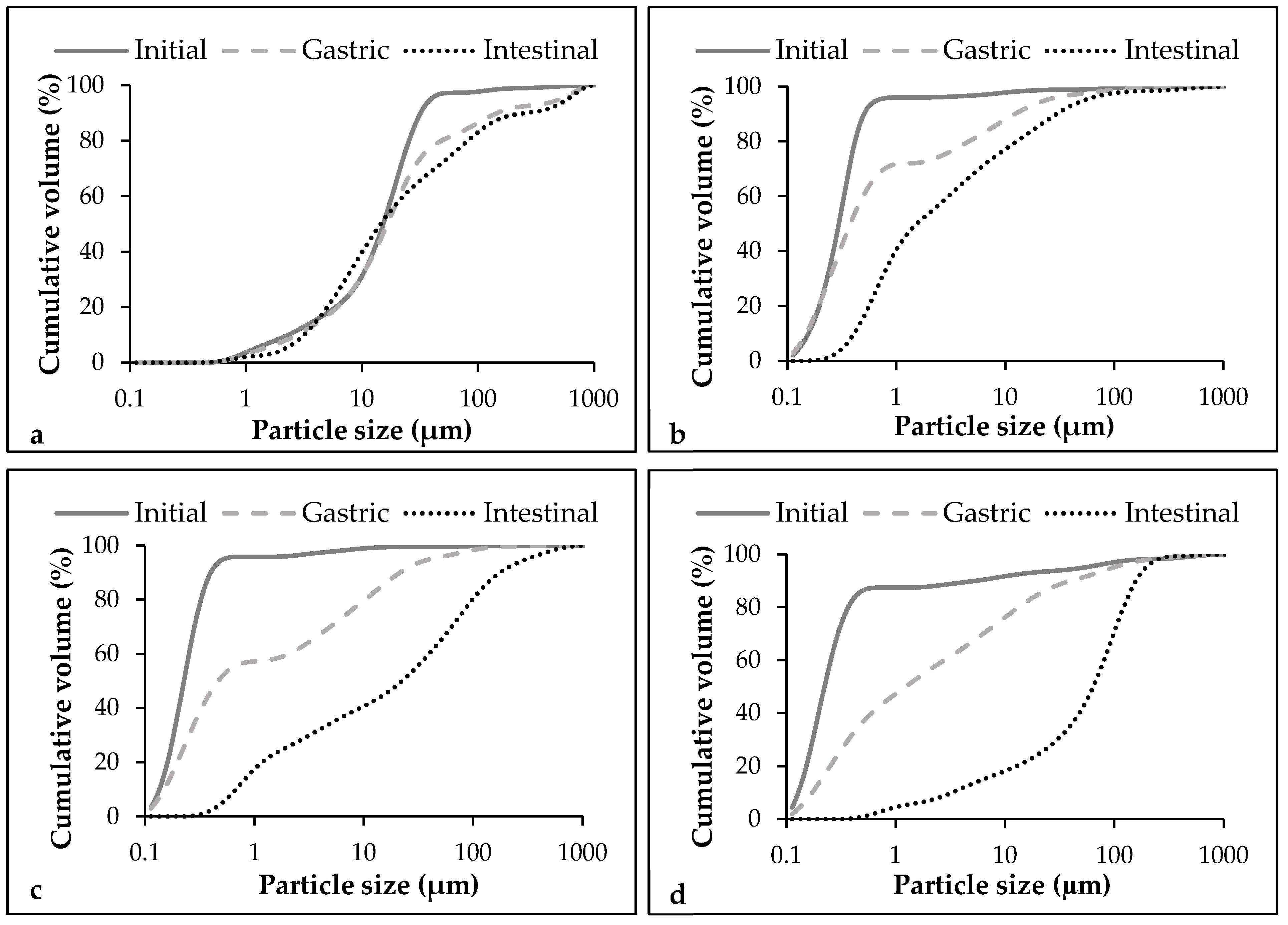
3.4.1. Particle Size
3.4.2. Electrical Charge
3.4.3. Oil Digestibility
3.4.4. β-Carotene Bioaccessibility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krinsky, N. Actions of Carotenoids in Biological Systems. Annu. Rev. Nutr. 1993, 13, 561–587. [Google Scholar] [CrossRef]
- Handelman, G.J. The evolving role of carotenoids in human biochemistry. Nutrition 2001, 17, 818–822. [Google Scholar] [CrossRef]
- Von Lintig, J. Colors with Functions: Elucidating the Biochemical and Molecular Basis of Carotenoid Metabolism. Annu. Rev. Nutr. 2010, 30, 35–56. [Google Scholar] [CrossRef]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.J.; Mei, X.Y.; Nakajima, M.; Yin, L.J. Preliminary study into the factors modulating β-carotene micelle formation in dispersions using an in vitro digestion model. Food Hydrocoll. 2012, 26, 427–433. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.D.J.; Failla, M.L.; Yahia, E.M.; Gardea-Bejar, A. Impact of the stage of ripening and dietary fat on in vitro bioaccessibility of β-carotene in “Ataulfo” mango. J. Agric. Food Chem. 2008, 56, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Role of iron and hydroperoxides in the degradation of lycopene in oil-in-water emulsions. J. Agric. Food Chem. 2009, 57, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. Development of polyphenol-protein-polysaccharide ternary complexes as emulsifiers for nutraceutical emulsions: Impact on formation, stability, and bioaccessibility of β-carotene emulsions. Food Hydrocoll. 2016, 61, 578–588. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Schuchmann, H.P.; Engel, R.; Walz, E.; Briviba, K. Encapsulation of Carotenoids. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V., Eds.; Springer: New York, NY, USA, 2010; pp. 211–252. ISBN 978-1-4419-1008-0. [Google Scholar]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z.; Lei, F.; Chang, Y.; Gao, Y. Investigation into the bioaccessibility and microstructure changes of β-carotene emulsions during in vitro digestion. Innov. Food Sci. Emerg. Technol. 2012, 15, 86–95. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, Y.; Lei, F.; Gao, Y. Investigation into the in vitro release properties of β-carotene in emulsions stabilized by different emulsifiers. LWT - Food Sci. Technol. 2014, 59, 867–873. [Google Scholar] [CrossRef]
- Verrijssen, T.A.J.; Verkempinck, S.H.E.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. The effect of pectin on invitro β-carotene bioaccessibility and lipid digestion in low fat emulsions. Food Hydrocoll. 2015, 49, 73–81. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancement of carotenoid bioaccessibility from carrots using excipient emulsions: Influence of particle size of digestible lipid droplets. Food Funct. 2016, 7, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Miao, S. Structuring Food Emulsions to Improve Nutrient Delivery During Digestion. Food Eng. Rev. 2015, 7, 439–451. [Google Scholar] [CrossRef]
- Usón, N.; Garcia, M.J.; Solans, C. Formation of water-in-oil (W/O) nano-emulsions in a water/mixed non-ionic surfactant/oil systems prepared by a low-energy emulsification method. Colloids Surf. A Physicochem. Eng. Asp. 2004, 250, 415–421. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Ripening Stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef]
- McClements, D.J. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.H.E.; Zhang, X.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Comparative study on lipid digestion and carotenoid bioaccessibility of emulsions, nanoemulsions and vegetable-based in situ emulsions. Food Hydrocoll. 2019, 87, 119–128. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Troncoso, E.; Aguilera, J.M.; McClements, D.J. Influence of particle size on the in vitro digestibility of protein-coated lipid nanoparticles. J. Colloid Interface Sci. 2012, 382, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Impact of emulsifier nature and concentration on the stability of β-carotene enriched nanoemulsions during: In vitro digestion. Food Funct. 2019, 10, 713–722. [Google Scholar] [CrossRef]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Ochoa-Martínez, L.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll. 2016, 52, 438–446. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids acting as emulsifying agents – How do they do it? Food Hydrocoll. 2018, 78, 2–14. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 2015, 45, 175–185. [Google Scholar] [CrossRef]
- Beysseriat, M.; Decker, E.A.; McClements, D.J. Preliminary study of the influence of dietary fiber on the properties of oil-in-water emulsions passing through an in vitro human digestion model. Food Hydrocoll. 2006, 20, 800–809. [Google Scholar] [CrossRef]
- Klinkesorn, U.; McClements, D.J. Influence of chitosan on stability and lipase digestibility of lecithin-stabilized tuna oil-in-water emulsions. Food Chem. 2009, 114, 1308–1315. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Xiao, H.; Du, Y.; Decker, E.A.; McClements, D.J. Controlling the functional performance of emulsion-based delivery systems using multi-component biopolymer coatings. Eur. J. Pharm. Biopharm. 2010, 76, 38–47. [Google Scholar] [CrossRef]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; McClements, D.J. Impact of dietary fibers [methyl cellulose, chitosan, and pectin] on digestion of lipids under simulated gastrointestinal conditions. Food Funct. 2014, 5, 3083–3095. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schmidt, K.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Pectins of different origin and their performance in forming andstabilizing oil-in-water-emulsions. Food Hydrocoll. 2015, 46, 59–66. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Influence of mandarin fiber addition on physico-chemical properties of nanoemulsions containing β-carotene under simulated gastrointestinal digestion conditions. LWT - Food Sci. Technol. 2017, 84, 331–337. [Google Scholar] [CrossRef]
- Feng, H.; Li, C.; Tan, C.P.; Fu, X.; Zhang, B.; Huang, Q. Physicochemical properties and in vitro bioaccessibility of lutein loaded emulsions stabilized by corn fiber gums. RSC Adv. 2017, 7, 38243–38250. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Modulating β-carotene bioaccessibility by controlling oil composition and concentration in edible nanoemulsions. Food Chem. 2013, 139, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food – an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef]
- Maa, Y.F.; Hsu, C.C. Performance of sonication and microfluidization for liquid-liquid emulsification. Pharm. Dev. Technol. 1999, 4, 233–240. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Denis, S.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Pectin influences the kinetics of in vitro lipid digestion in oil-in-water emulsions. Food Chem. 2018, 262, 150–161. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Mun, S.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Res. Int. 2007, 40, 770–781. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practice, and Techniques; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780849320231. [Google Scholar]
- Celus, M.; Salvia-Trujillo, L.; Kyomugasho, C.; Maes, I.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Structurally modified pectin for targeted lipid antioxidant capacity in linseed/sunflower oil-in-water emulsions. Food Chem. 2018, 241, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Burapapadh, K.; Kumpugdee-Vollrath, M.; Chantasart, D.; Sriamornsak, P. Fabrication of pectin-based nanoemulsions loaded with itraconazole for pharmaceutical application. Carbohydr. Polym. 2010, 82, 384–393. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- McClements, D.J. Comments on viscosity enhancement and depletion flocculation by polysaccharides. Food Hydrocoll. 2000, 14, 173–177. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Van Loey, A.M.; Kyomugasho, C.; Grauwet, T.; Hendrickx, M.E.; Bourgeois, M.; Denis, S. Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
- Tan, C.P.; Nakajima, M. β-Carotene nanodispersions: Preparation, characterization and stability evaluation. Food Chem. 2005, 92, 661–671. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Xu, D.; Wang, X.; Jiang, J.; Yuan, F.; Gao, Y. Impact of whey protein - Beet pectin conjugation on the physicochemical stability of β-carotene emulsions. Food Hydrocoll. 2012, 28, 258–266. [Google Scholar] [CrossRef]
- Chen, B.; Mcclements, D.J.; Decker, E.A. Role of continuous phase anionic polysaccharides on the oxidative stability of menhaden oil-in-water emulsions. J. Agric. Food Chem. 2010, 58, 3779–3784. [Google Scholar] [CrossRef] [PubMed]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Charleer, L.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion stability during gastrointestinal conditions effects lipid digestion kinetics. Food Chem. 2018, 246, 179–191. [Google Scholar] [CrossRef] [PubMed]
- van Aken, G.A.; Bomhof, E.; Zoet, F.D.; Verbeek, M.; Oosterveld, A. Differences in in vitro gastric behaviour between homogenized milk and emulsions stabilised by Tween 80, whey protein, or whey protein and caseinate. Food Hydrocoll. 2011, 25, 781–788. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Potential biological fate of ingested nanoemulsions: Influence of particle characteristics. Food Funct. 2012, 3, 202–220. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- Simo, O.K.; Mao, Y.; Tokle, T.; Decker, E.A.; McClements, D.J. Novel strategies for fabricating reduced fat foods: Heteroaggregation of lipid droplets with polysaccharides. Food Res. Int. 2012, 48, 337–345. [Google Scholar] [CrossRef]
- Rao, J.; Decker, E.A.; Xiao, H.; Mcclements, D.J. Nutraceutical nanoemulsions: Influence of carrier oil composition (digestible versus indigestible oil) on β-carotene bioavailability. J. Sci. Food Agric. 2013, 93, 3175–3183. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; McClements, D.J. Factors affecting lipase digestibility of emulsified lipids using an in vitro digestion model: Proposal for a standardised pH-stat method. Food Chem. 2011, 126, 498–505. [Google Scholar] [CrossRef]
- Yi, J.; Li, Y.; Zhong, F.; Yokoyama, W. The physicochemical stability and invitro bioaccessibility of beta-carotene in oil-in-water sodium caseinate emulsions. Food Hydrocoll. 2014, 35, 19–27. [Google Scholar] [CrossRef]
- Verrijssen, T.A.J.; Christiaens, S.; Verkempinck, S.H.E.; Boeve, J.; Grauwet, T.; Van Loey, A.M.; Salvia-Trujillo, L.; Hendrickx, M.E. In vitro β-Carotene Bioaccessibility and Lipid Digestion in Emulsions: Influence of Pectin Type and Degree of Methyl-Esterification. J. Food Sci. 2016, 81, C2327–C2336. [Google Scholar] [CrossRef] [PubMed]
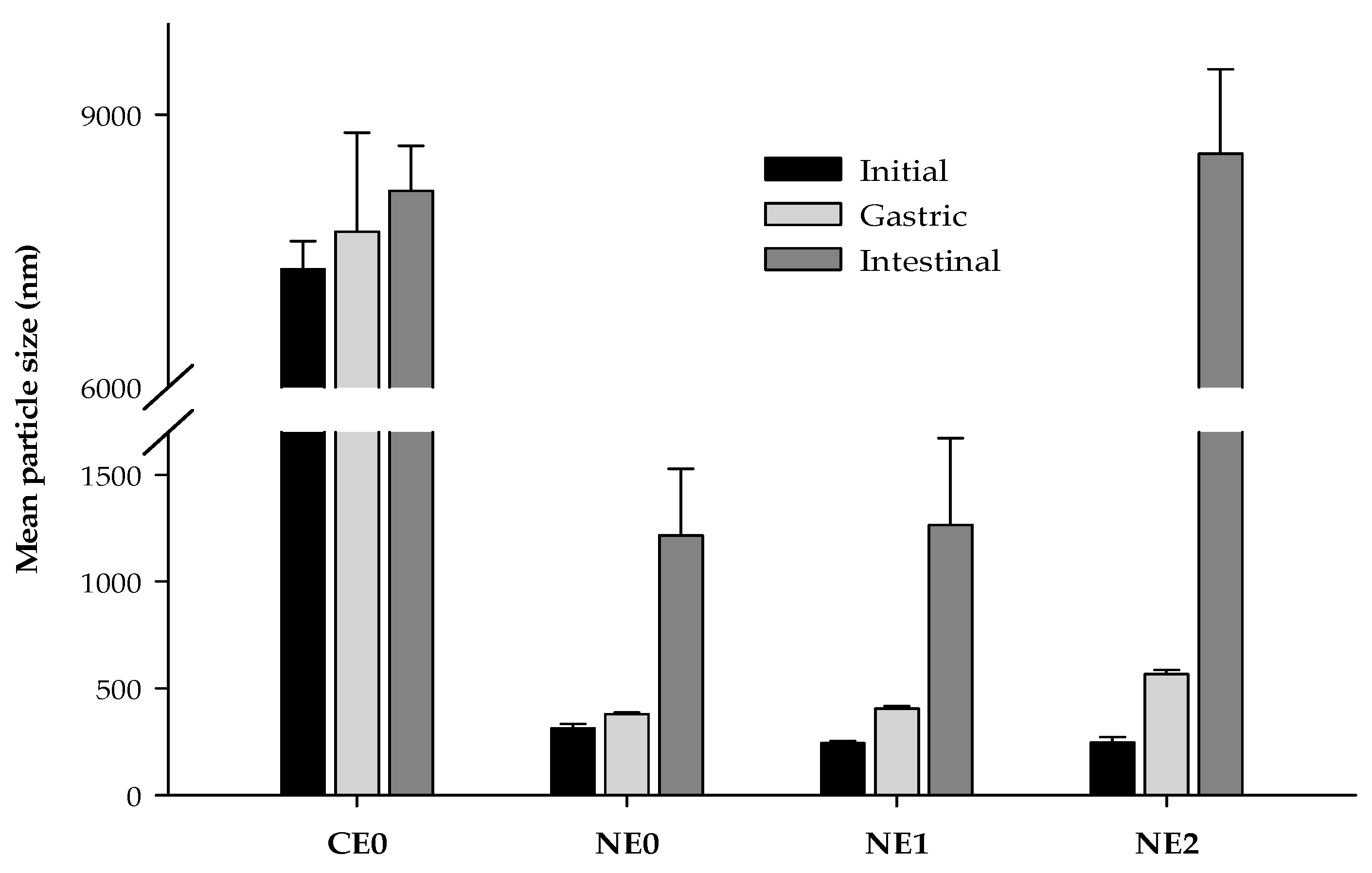
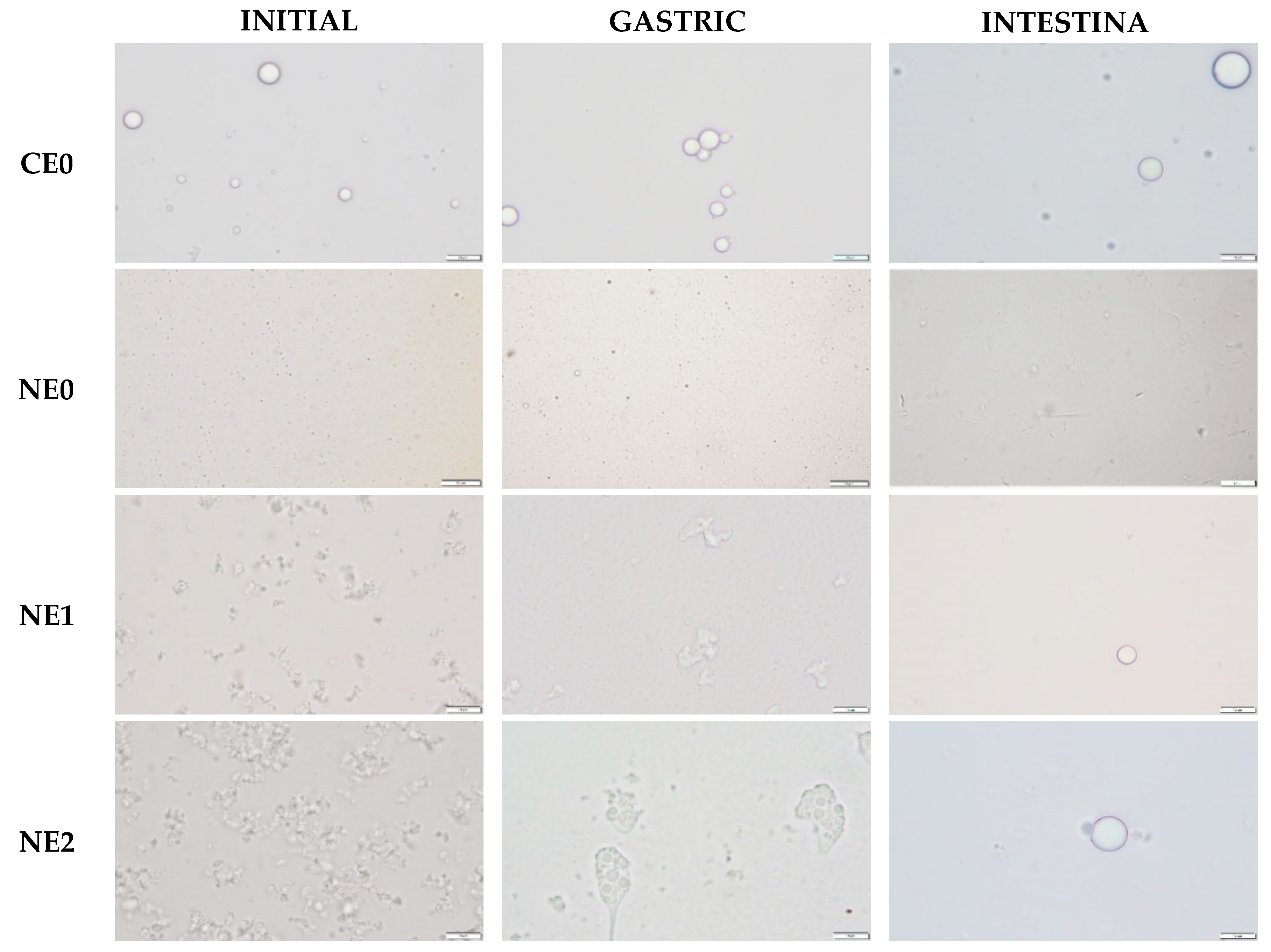


| CE0 1 | NE0 2 | NE1 3 | NE2 4 | |
|---|---|---|---|---|
| Particle diameter d32 (nm) | 7300 ± 307 B | 313.4 ± 20.2 Ab | 242.6 ± 10.9 a | 247.1 ± 24.6 a |
| Particle diameter d90 (nm) | 25464 ± 1401 B | 540.7 ± 59.7 Ab | 448.4 ± 21.4 a | 439.3 ± 23.3 a |
| Viscosity (mPa·s) | 1.31 ± 0.02 A | 1.23 ± 0.05 Aa | 6.51 ± 0.10 b | 19.77 ± 0.26 c |
| ζ-potential (mV) | −32.1 ± 1.9 A | −20.9 ± 2.1 Ba | −7.6 ± 0.2 b | −7.4 ± 0.4 b |
| β-carotene bioaccessibility (%) | 20.9 ± 1.4 A | 25.0 ± 2.4 Ba | 29.5 ± 1.7 b | 36.9 ± 2.2 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixé-Roig, J.; Oms-Oliu, G.; Ballesté-Muñoz, S.; Odriozola-Serrano, I.; Martín-Belloso, O. Improving the In Vitro Bioaccessibility of β-Carotene Using Pectin Added Nanoemulsions. Foods 2020, 9, 447. https://doi.org/10.3390/foods9040447
Teixé-Roig J, Oms-Oliu G, Ballesté-Muñoz S, Odriozola-Serrano I, Martín-Belloso O. Improving the In Vitro Bioaccessibility of β-Carotene Using Pectin Added Nanoemulsions. Foods. 2020; 9(4):447. https://doi.org/10.3390/foods9040447
Chicago/Turabian StyleTeixé-Roig, Júlia, Gemma Oms-Oliu, Sara Ballesté-Muñoz, Isabel Odriozola-Serrano, and Olga Martín-Belloso. 2020. "Improving the In Vitro Bioaccessibility of β-Carotene Using Pectin Added Nanoemulsions" Foods 9, no. 4: 447. https://doi.org/10.3390/foods9040447
APA StyleTeixé-Roig, J., Oms-Oliu, G., Ballesté-Muñoz, S., Odriozola-Serrano, I., & Martín-Belloso, O. (2020). Improving the In Vitro Bioaccessibility of β-Carotene Using Pectin Added Nanoemulsions. Foods, 9(4), 447. https://doi.org/10.3390/foods9040447





