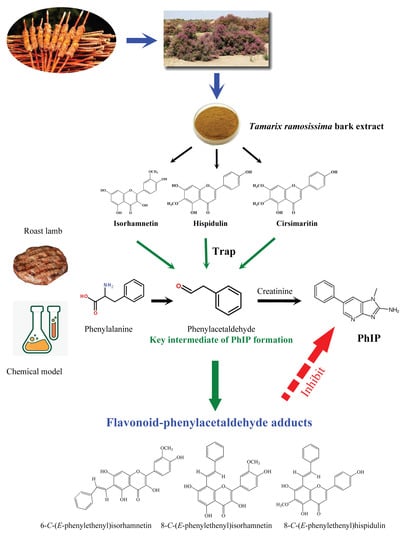
Isorhamnetin and Hispidulin from Tamarix ramosissima Inhibit 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) Formation by Trapping Phenylacetaldehyde as a Key Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Determination of Additive Levels in Lamb Patties and Model Systems
2.3. Preparation of Roast Lamb Patties
2.4. Analysis of the Effects of TRE and Flavonoids on PhIP Formation in Chemical Models
2.5. UPLC-MS Analysis of PhIP
2.6. Evaluation of the Phenylacetaldehyde-Scavenging Capabilities of TRE and the Flavonoids in a Model System Using GC-MS
2.7. Detection of Flavonoid-Phenylacetaldehyde Adducts in Roast Lamb Patties
2.8. Detection of Flavonoid-Phenylacetaldehyde Adducts in the Model System
2.9. UPLC-MS Analysis of Flavonoid-Phenylacetaldehyde Adducts
2.10. Statistical Analysis
3. Results and Discussion
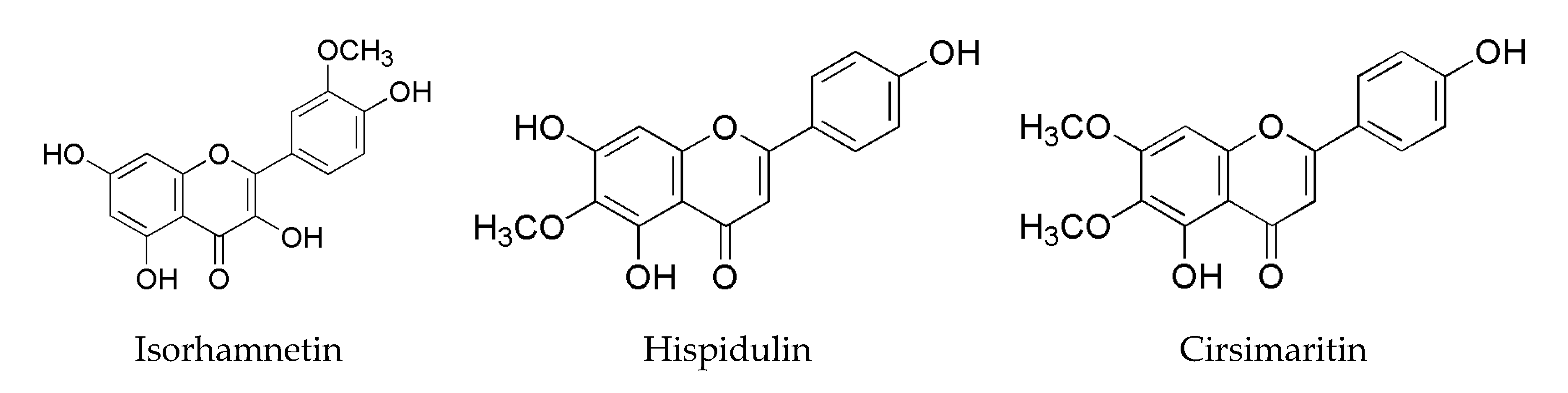
3.1. Effects of Additives on PhIP Formation in Lamb Patties and Model Systems
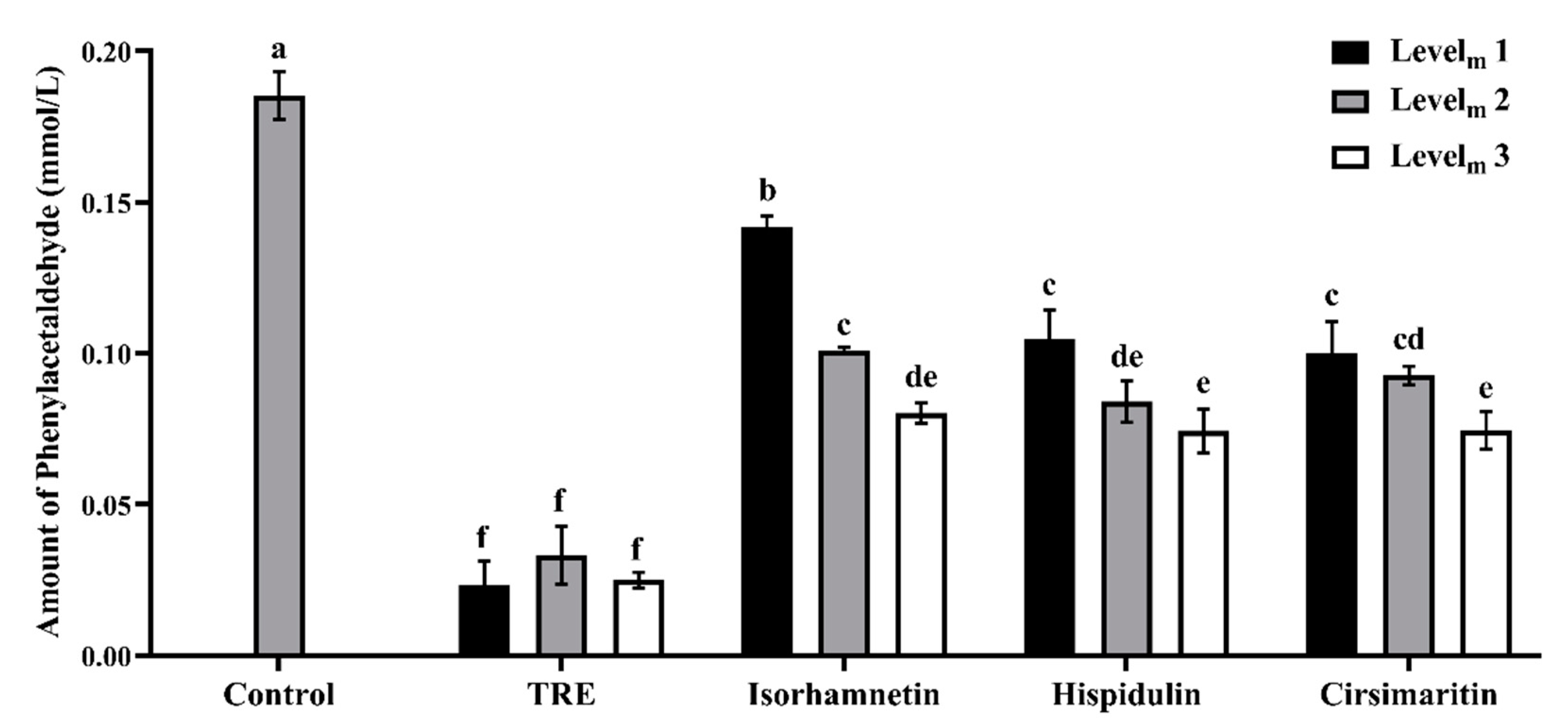
3.2. Phenylacetaldehyde-Scavenging Capabilities of TRE and Flavonoids in the Model System
3.3. Correlations between the Phenylacetaldehyde-Trapping and PhIP-Inhibiting Abilities of TRE and Flavonoids
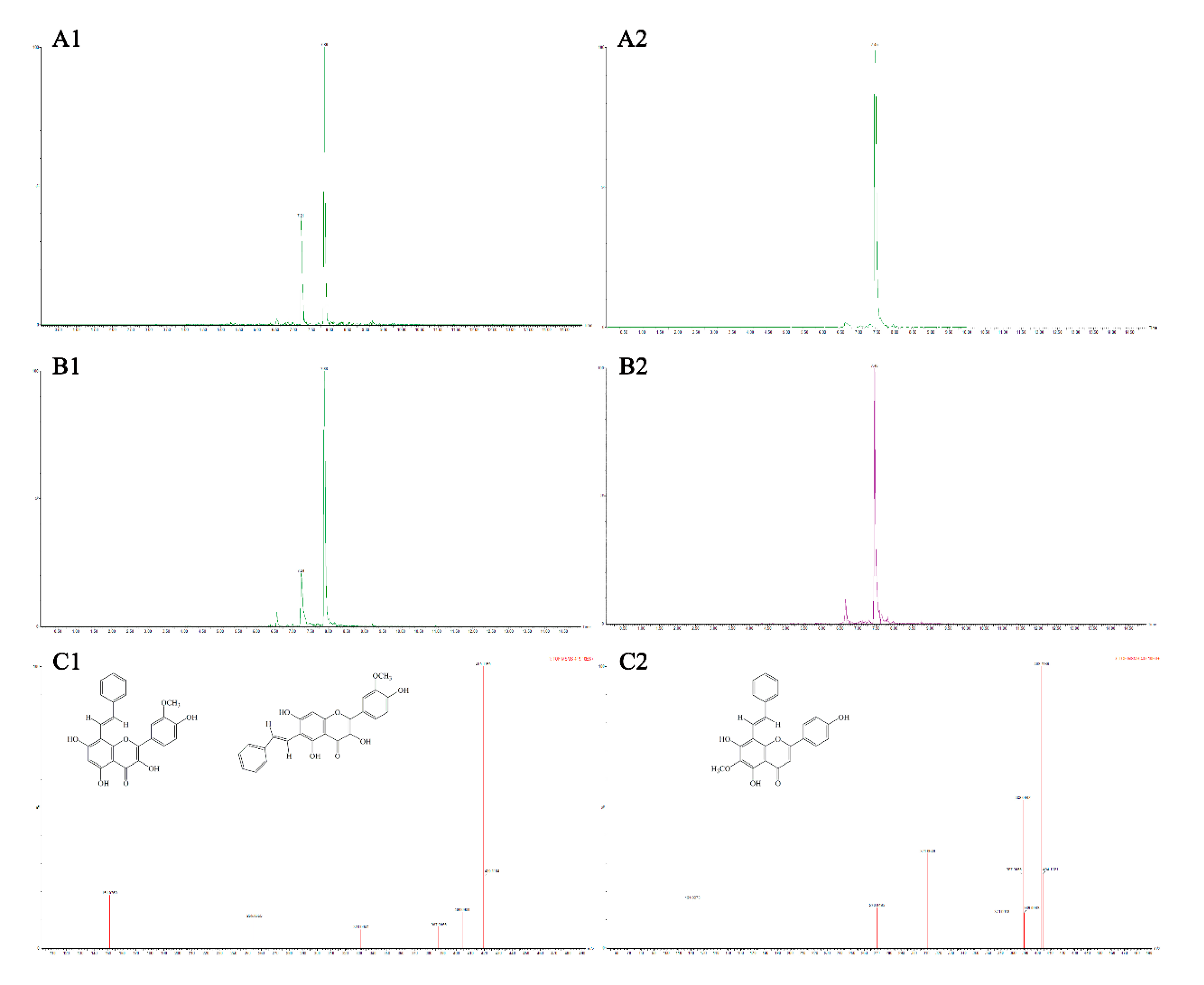
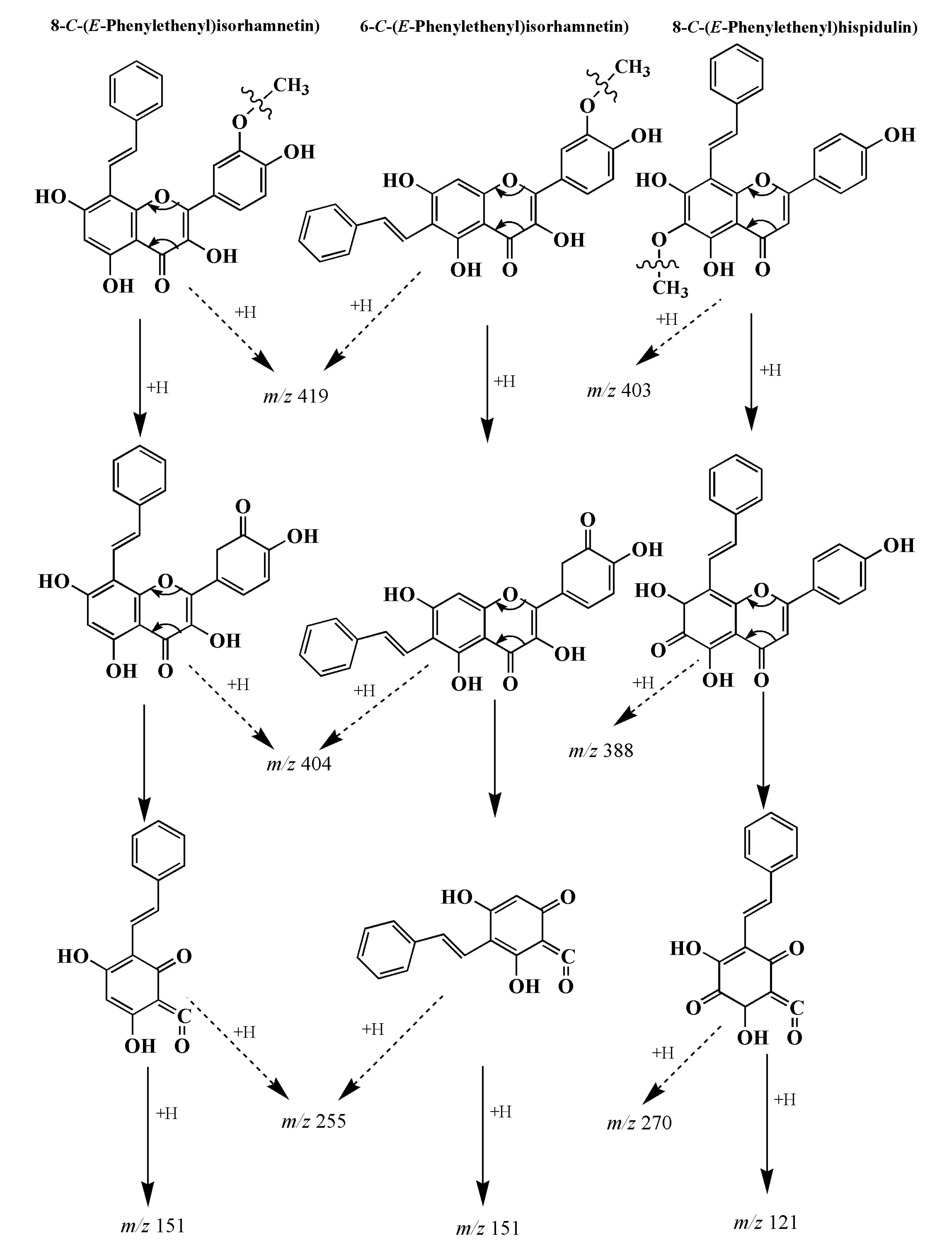
3.4. Analysis of Flavonoid-Phenylacetaldehyde Adducts in Roast Lamb Patties and Chemical Reaction Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Mhamdi, B.; Chaieb, K.; Bakrouf, A.; Magne, C.; Abdelly, C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 2009, 47, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Sultanova, N.A.; Makhmoor, T.; Abilov, Z.A.; Parween, Z.; Omurkamzinova, V.B.; Urrahman, A.; Choudhary, M.I. Antioxidant and antimicrobial activities of Tamarix ramosissima. J. Ethnopharmacol. 2001, 78, 201–205. [Google Scholar] [CrossRef]
- Sultanova, N.A.; Makhmoor, T.; Yasin, A.; Abilov, Z.A.; Omurkamzinova, V.B.; Attaurrahman, N.; Choudhary, M.I. Isotamarixen: A new antioxidant and prolyl endopeptidase-inhibiting triterpenoid from Tamarix hispida. Planta Med. 2004, 70, 65–67. [Google Scholar] [PubMed]
- Umbetova, A.K.; Choudhary, M.I.; Sultanova, N.A.; Burasheva, G.S.; Abilov, Z.A. Flavonoids of plants from the genus Tamarix. Chem. Nat. Compd. 2004, 40, 728–729. [Google Scholar] [CrossRef]
- Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, hispidulin, and cirsimaritin identified in Tamarix ramosissima barks from southern Xinjiang and their antioxidant and antimicrobial activities. Molecules 2019, 24, 390. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahimtallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Skog, K.; Johansson, M.A.E.; Jagerstad, M. Carcinogenic heterocyclic amines in model systems and cooked foods: A review on formation, occurrence and intake. Food Chem. Toxicol. 1998, 36, 879–896. [Google Scholar] [CrossRef]
- Wong, K.; Su, J.; Knize, M.G.; Koh, W.; Seow, A. Dietary exposure to heterocyclic amines in a Chinese population. Nutr. Cancer 2005, 52, 147–155. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans. World Health Organ. 1993, 56, 163–242. [Google Scholar]
- National Toxicology Program (NTP). Selected heterocyclic amines. In NTP 11th Report on Carcinogens; Public Health Service; NTP: Research Triangle Park, NC, USA, 2004; Volume 11, pp. 135–138. [Google Scholar]
- Alaejos, M.S.; Afonso, A.M. Factors that affect the content of heterocyclic aromatic amines in foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 52–108. [Google Scholar] [CrossRef]
- Jinap, S.; Iqbal, S.Z.; Talib, N.H.; Hasnol, N.D.S. Heterocyclic aromatic amines in deep fried lamb meat: The influence of spices marination and sensory quality. J. Food Sci. Technol. 2016, 53, 1411–1417. [Google Scholar]
- Viegas, O.; Amaro, L.F.; Ferreira, I.M.; Olivia Pinho, O. Inhibitory effect of antioxidant-rich marinades on the formation of heterocyclic aromatic amines in pan-fried beef. J. Agric. Food Chem. 2012, 60, 6235–6240. [Google Scholar] [CrossRef] [PubMed]
- Garcialomillo, J.; Viegas, O.; Gonzalezsanjose, M.L.; Ferreira, I.M. Influence of red wine pomace seasoning and high-oxygen atmosphere storage on carcinogens formation in barbecued beef patties. Meat Sci. 2017, 125, 10–15. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, S.; Wang, M.; Chen, J.; Zheng, Z. Inhibitory effects of selected dietary flavonoids on the formation of total heterocyclic amines and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in roast beef patties and in chemical models. Food Funct. 2016, 7, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yan, Y.; Xia, J.; Zhang, S.; Wang, M.; Chen, J.; Xu, Y. A phenylacetaldehyde-flavonoid adduct, 8-C-(E-phenylethenyl)-norartocarpetin, exhibits intrinsic apoptosis and MAPK pathways-related anticancer potential on HepG2, SMMC-7721 and QGY-7703. Food Chem. 2016, 197, 1085–1092. [Google Scholar] [CrossRef]
- Cheng, K.; Wong, C.; Cho, C.; Chu, I.; Sze, K.; Lo, C.; Chen, F.; Wang, M. Trapping of phenylacetaldehyde as a key mechanism responsible for naringenin’s inhibitory activity in mutagenic 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine formation. Chem. Res. Toxicol. 2008, 21, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Li, Y.; He, Z.; Qin, F.; Chen, J. Effect of phenolic compounds from spices consumed in China on heterocyclic amine profiles in roast beef patties by UPLC-MS/MS and multivariate analysis. Meat Sci. 2016, 116, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Alcon, E.; Hidalgo, F.J. Ammonia and formaldehyde participate in the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in addition to creati(ni)ne and phenylacetaldehyde. Food Chem. 2014, 155, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, F.; Yong, W.; Chen, S.; Yang, M.; Ling, Y.; Chu, X.; Lin, J. Potential sources of carcinogenic heterocyclic amines in Chinese mutton shashlik. Food Chem. 2010, 123, 647–652. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Z.; Shao, Z.; Yu, C.; Wang, S. Effects of antioxidants of bamboo leaves and flavonoids on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) formation in chemical model systems. J. Agric. Food Chem. 2014, 62, 4798–4802. [Google Scholar] [CrossRef]
- Yu, C.; Shao, Z.; Liu, B.; Zhang, Y.; Wang, S. Inhibition of 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) formation by alkoxy radical scavenging of flavonoids and their quantitative structure-activity relationship in a model system. J. Food Sci. 2016, 81, C1908–C1913. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Wu, Q.; Zheng, Z.; Peng, X.; Simon, J.; Chen, F.; Wang, M. Inhibitory effect of fruit extracts on the formation of heterocyclic amines. J. Agric. Food Chem. 2007, 55, 10359–10365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Chen, F.; Wang, M. Inhibitory activities of dietary phenolic compounds on heterocyclic amine formation in both chemical model system and beef patties. Mol. Nutr. Food Res. 2007, 51, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Li, L.; Zhang, N.; Zhao, Y.; Cheng, K.; Yan, B.; Wang, Q.; Zhao, J.; Wang, M.; Zhang, H. A comparison of mutagenic PhIP and beneficial 8-C-(E-phenylethenyl)quercetin and 6-C-(E-phenylethenyl)quercetin formation under microwave and conventional heating. Food Funct. 2018, 9, 3853–3859. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Shin, H. Inhibition of mutagenic 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) formation using various food ingredients in a model systems. Food Sci. Biotechnol. 2013, 22, 323–329. [Google Scholar] [CrossRef]
- Salazar, R.; Arambulavilla, G.; Hidalgo, F.J.; Zamora, R. Structural characteristics that determine the inhibitory role of phenolic compounds on 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) formation. Food Chem. 2014, 151, 480–486. [Google Scholar] [CrossRef]
- Lee, J.; Dong, A.; Jung, K.; Shin, H. Influence of extra virgin olive oil on the formation of heterocyclic amines in roasted beef steak. Food Sci. Biotechnol. 2011, 20, 159–165. [Google Scholar] [CrossRef]
- Zochling, S.; Murkovic, M. Formation of the heterocyclic aromatic amine PhIP: Identification of precursors and intermediates. Food Chem. 2002, 79, 125–134. [Google Scholar] [CrossRef]
- Cheng, K.; Wong, C.C.; Chao, J.; Lo, C.; Chen, F.; Chu, I.K.; Che, C.; Ho, C.; Wang, M. Inhibition of mutagenic PhIP formation by epigallocatechin gallate via scavenging of phenylacetaldehyde. Mol. Nutr. Food Res. 2009, 53, 716–725. [Google Scholar] [CrossRef]
- Shao, X.; Chen, H.; Zhu, Y.; Sedighi, R.; Ho, C.; Sang, S. Essential structural requirements and additive effects for flavonoids to scavenge methylglyoxal. J. Agric. Food Chem. 2014, 62, 3202–3210. [Google Scholar] [CrossRef]
- Wong, D.; Cheng, K.; Wang, M. Inhibition of heterocyclic amine formation by water-soluble vitamins in Maillard reaction model systems and beef patties. Food Chem. 2012, 133, 760–766. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, Y.; Wang, X.; Fan, D.; Cheng, K.; Wang, M. Unraveling the inhibitory effect of dihydromyricetin on heterocyclic aromatic amines formation. J. Sci. Food Agric. 2018, 98, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Morales, F.J. Mechanism of reactive carbonyl species trapping by hydroxytyrosol under simulated physiological conditions. Food Chem. 2015, 175, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Li, S.; Tan, D.; Pan, M.; Sang, S.; Ho, C. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, F.; Chen, H.; Cheng, K.; Zykova, T.A.; Oi, N.; Lubet, R.A.; Bode, A.M.; Wang, M.; Dong, Z. 6-C-(E-phenylethenyl)-naringenin suppresses colorectal cancer growth by inhibiting cyclooxygenase-1. Cancer Res. 2014, 74, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, D.; Ru, B.; Cheng, K.; Hu, S.; Zhang, J.; Li, E.T.S.; Wang, M. 6-C-(E-phenylethenyl)naringenin induces cell growth inhibition and cytoprotective autophagy in colon cancer cells. Eur. J. Cancer 2016, 68, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, D.; Zheng, Z.; Li, E.T.S.; Chen, F.; Cheng, K.; Wang, M. 8-C-(E-phenylethenyl)quercetin from onion/beef soup induces autophagic cell death in colon cancer cells through ERK activation. Mol. Nutr. Food Res. 2017, 61, 1600437. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.; Chen, C.; Cai, S.; Hu, J. Isorhamnetin suppresses colon cancer cell growth through the PI3K-Akt-mTOR pathway. Mol. Med. Rep. 2014, 9, 935–940. [Google Scholar] [CrossRef]
- Liu, K.; Gao, H.; Wang, Q.; Wang, L.; Zhang, B.; Han, Z.; Chen, X.; Han, M.; Gao, M. Hispidulin suppresses cell growth and metastasis by targeting PIM1 through JAK2/STAT3 signaling in colorectal cancer. Cancer Sci. 2018, 109, 1369–1381. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.E.; Lee, K.W.; Son, J.E.; Seo, S.K.; Li, J.; Jung, S.K.; Heo, Y.S.; Mottamal, M.; Bode, A.M. Isorhamnetin suppresses skin cancer through direct inhibition of MEK1 and PI3-K. Cancer Prev. Res. 2011, 4, 582–591. [Google Scholar] [CrossRef]
- Gao, H.; Gao, M.; Peng, J.; Han, M.; Liu, K.; Han, Y. Hispidulin mediates apoptosis in human renal cell carcinoma by inducing ceramide accumulation. Acta Pharmacol. Sin. 2017, 38, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
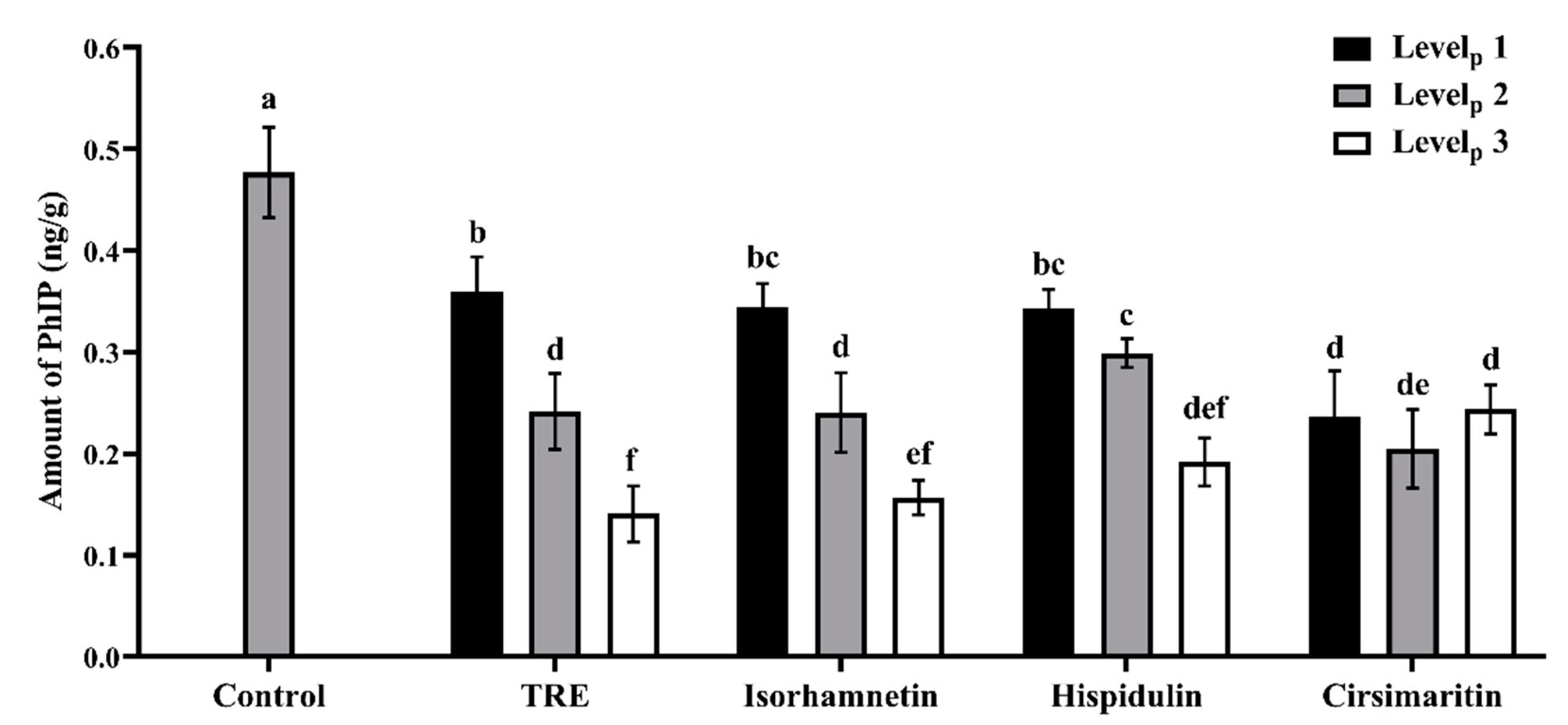
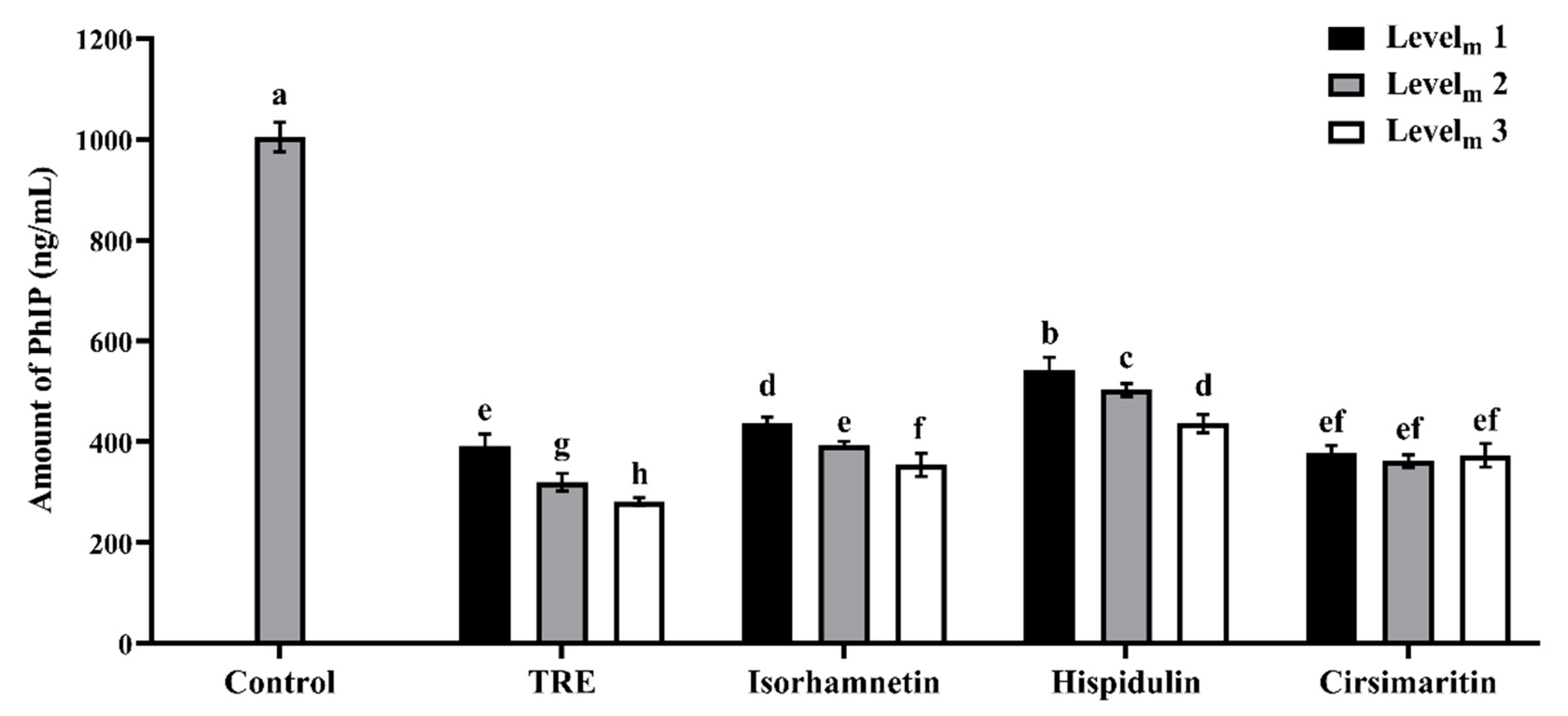

| Groups | Lamb Patty | Model System | ||||
|---|---|---|---|---|---|---|
| Levelp 1 | Levelp 2 | Levelp 3 | Levelm 1 | Levelm 2 | Levelm 3 | |
| TRE | 0.15 mg/g | 0.30 mg/g | 0.45 mg/g | 12.5 mg/mL | 25.0 mg/mL | 37.5 mg/mL |
| Isorhamnetin | 6.0 μg/g | 12.0 μg/g | 18.0 μg/g | 0.03 mmol | 0.16 mmol | 0.32 mmol |
| Hispidulin | 3.0 μg/g | 6.0 μg/g | 9.0 μg/g | 0.03 mmol | 0.08 mmol | 0.16 mmol |
| Cirsimaritin | 1.5 μg/g | 3.0 μg/g | 4.5 μg/g | 0.02 mmol | 0.03 mmol | 0.06 mmol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Wang, W.; Bao, Y.; Zhu, Y.; Zhang, Y.; Lu, Y.; Peng, Z.; Zhou, G. Isorhamnetin and Hispidulin from Tamarix ramosissima Inhibit 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) Formation by Trapping Phenylacetaldehyde as a Key Mechanism. Foods 2020, 9, 420. https://doi.org/10.3390/foods9040420
Ren X, Wang W, Bao Y, Zhu Y, Zhang Y, Lu Y, Peng Z, Zhou G. Isorhamnetin and Hispidulin from Tamarix ramosissima Inhibit 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) Formation by Trapping Phenylacetaldehyde as a Key Mechanism. Foods. 2020; 9(4):420. https://doi.org/10.3390/foods9040420
Chicago/Turabian StyleRen, Xiaopu, Wei Wang, Yingjie Bao, Yuxia Zhu, Yawei Zhang, Yaping Lu, Zengqi Peng, and Guanghong Zhou. 2020. "Isorhamnetin and Hispidulin from Tamarix ramosissima Inhibit 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) Formation by Trapping Phenylacetaldehyde as a Key Mechanism" Foods 9, no. 4: 420. https://doi.org/10.3390/foods9040420
APA StyleRen, X., Wang, W., Bao, Y., Zhu, Y., Zhang, Y., Lu, Y., Peng, Z., & Zhou, G. (2020). Isorhamnetin and Hispidulin from Tamarix ramosissima Inhibit 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) Formation by Trapping Phenylacetaldehyde as a Key Mechanism. Foods, 9(4), 420. https://doi.org/10.3390/foods9040420