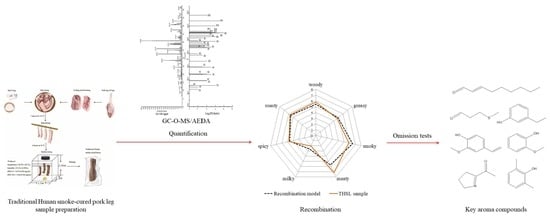
Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments
Abstract
1. Introduction
2. Materials and Methods
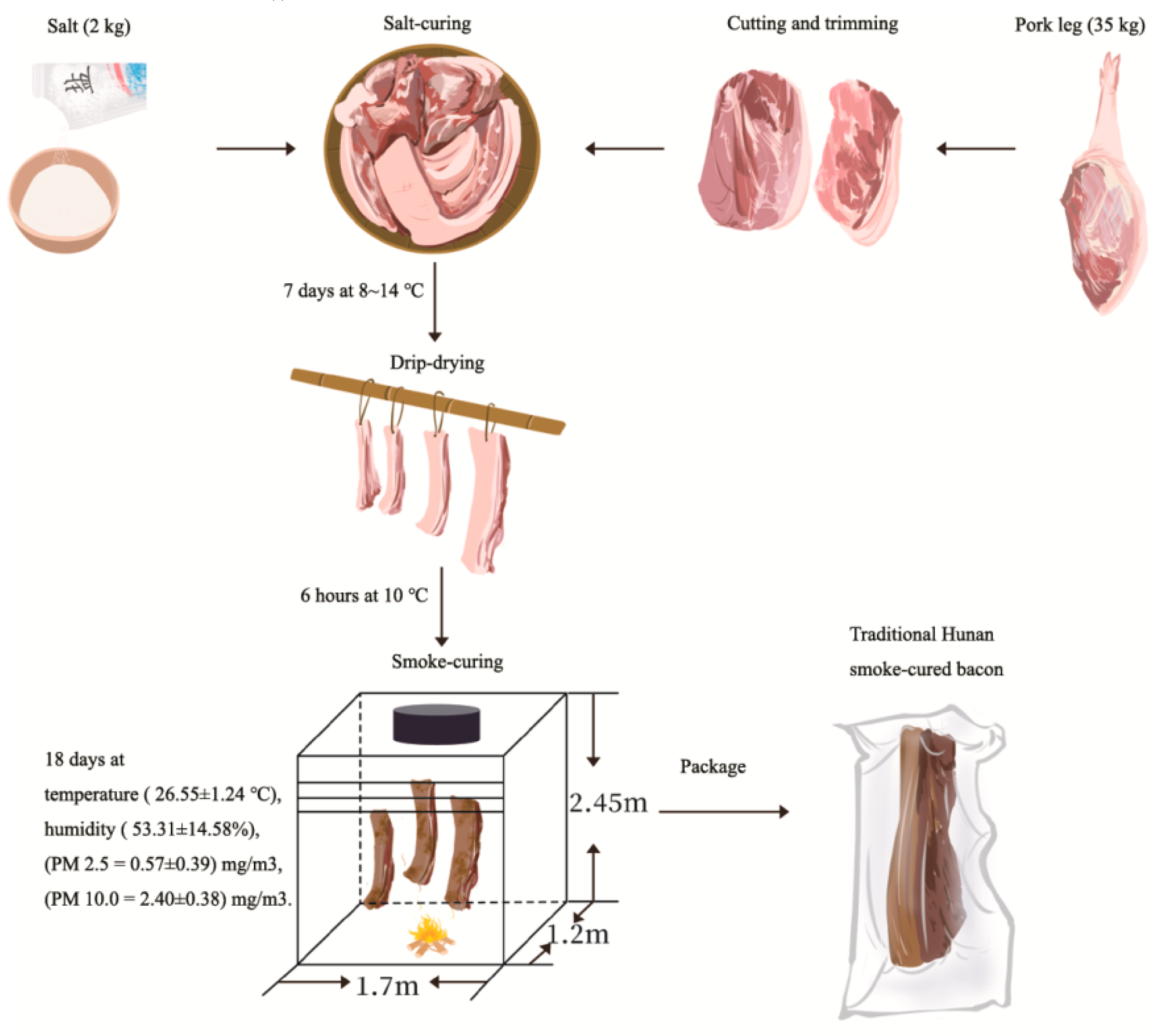
2.1. Sample Preparation
2.2. Chemicals
2.3. Sensory Evaluation Analysis
2.4. Separation of Volatile Compounds
2.5. GC–MS and GC–O–MS Analysis
2.6. Aroma Extraction Dilution Analysis (AEDA)
2.7. Identification and Quantification
2.8. Calculation of the Odor Activity Value
2.9. Aroma Recombination
2.10. Omission Tests
2.11. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation
3.2. GC–O/AEDA Analysis
3.3. Quantitation and Calculation of the OAV
3.4. Recombination Result
3.5. Omission Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zeng, W.; Wen, W.; Deng, Y.; Tian, Y.; Sun, H.; Sun, Q. Chinese ethnic meat products: Continuity and development. Meat Sci. 2016, 120, 37–46. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass spectrometry-based metabolomics of volatiles as a new tool for understanding aroma and flavour chemistry in processed food products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1988, 62, 415–424. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Huang, T.; Li, F.; Sun, J. Lipolysis and lipid oxidation during processing of Chinese traditional smoke-cured bacon. Food Chem. 2014, 149, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.C.; Sun, B.G.; Wang, S.B. Aromatic Constituents from Chinese Traditional Smoke-curing Bacon of Mini-pig. J. Food Sci. Technol. 2008, 14, 329–340. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wang, Y.; Pan, D.; Sun, Y.; Cao, J. Study on the volatile compounds generated from lipid oxidation of Chinese bacon (unsmoked) during processing. Eur. J. Lipid Sci. Technol. 2017, 119, 1600512. [Google Scholar] [CrossRef]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Edit. 2014, 53, 7124–7143. [Google Scholar] [CrossRef] [PubMed]
- Kosowska, M.; Majcher, M.A.; Jelen, H.H.; Fortuna, T. Key aroma compounds in smoked cooked Lion. J. Agric. Food Chem. 2018, 66, 3683–3695. [Google Scholar] [CrossRef]
- Pu, D.D.; Sun, J.; Chen, H.T.; Sun, B.G.; Zhang, Y.Y. Comparative Analysis of Volatile Flavor Compounds of Cooked Hunan and Guangdong Bacon by Simultaneous Distillation and Extraction Combine with Gas Chromatography-Mass Spectrometry (SDE-GC-MS) and Gas Chromatography-Olfactometry (GC-O). Food Sci. 2015, 36, 131–136. [Google Scholar]
- Yu, N.; Sun, B.G. Flavour substances of Chinese traditional smoke-curing bacon. Food Chem. 2005, 89, 227–233. [Google Scholar]
- Yu, N.; Sun, B.G.; Tian, D.; Qu, W. Analysis of volatile compounds in traditional smoke-curing bacon (CSCB) with different fiber coatings using SPME. Food Chem. 2008, 110, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.B.; Wu, J.F.; Xia, Y.Y.; Tu, D.W. Changes of Volatile Flavor Compounds in Traditional Chinese Bacon during Cold Smoking. Food Sci. 2009, 30, 79–83. [Google Scholar]
- Zhang, S.L.; Wang, S.W.; Chen, X.Y.; Song, Z.X.; Hao, B.R.; Qiao, X.L.; Chen, W.H.; Qu, C. Changes in volatile flavor components during hunan cured meat processing. Food Sci. 2015, 36, 215–219. [Google Scholar]
- Zhong, Y.R.; Zhou, H.; Lou, A.H.; Lou, Q.W.; Liu, C.G. Comparison between Volatile Compounds in Fast, Factory Dry-smoked and Traditional Air Dry-smoked Xiang-xi Chinese Bacon. Mod. Food Sci. Technol. 2015, 31, 361–371. [Google Scholar]
- Saldaña, E.; Saldarriaga, L.; Cabrera, J.; Siche, R.; Behrens, J.H.; Selani, M.M.; Contreras-Castillo, C.J. Relationship between volatile compounds and consumer-based sensory characteristics of bacon smoked with different Brazilian woods. Food Res. Int. 2018, 119, 839–849. [Google Scholar] [CrossRef]
- Poligné, A.; Collignan, G. Trystram, Effects of Salting, Drying, Cooking, and Smoking Operations on Volatile Compound Formation and Color Patterns in Pork. J. Food Sci. 2010, 67, 2976–2986. [Google Scholar] [CrossRef]
- Li, Y.Y.; Song, Y.Q.; Li, H.N.; Guo, W.P.; Wang, S.W. Analysis of Main Rancid Compounds from Cured Meat Products by solid-phase microextraction-Gas Chromatography-Mass Spectrometry. Meat Res. 2014, 28, 11–14. [Google Scholar]
- Li, X.; Zhu, J.; Li, C.; Ye, H.; Wang, Z.; Wu, X.; Xu, B. Evolution of Volatile Compounds and Spoilage Bacteria in Smoked Bacon during Refrigeration Using an E-Nose and GC-MS Combined with Partial Least Squares Regression. Molecules 2018, 23, 3286. [Google Scholar] [CrossRef]
- Liu, H.L.; Sun, B.G. Effect of fermentation processing on the flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Cai, B.; Liu, B.Z.; Su, Q.D. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components. J. Chromatog. A 2011, 930, 1–7. [Google Scholar] [CrossRef]
- Hübschmann, H.J. Handbook of GC-MS: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Pu, D.D.; Zhang, H.Y.; Zhang, Y.Y.; Sun, B.G.; Ren, F.Z.; Chen, H.T. Characterization of the key aroma compounds in white bread by aroma extract dilution analysis, quantitation, and sensory evaluation experiments. J. Food Process. Preserv. 2009, 43, e13933. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Pu, D.D.; Sun, B.G.; Ren, F.Z.; Zhang, Y.Y.; Chen, H.T. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hernandez, M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Hitzel, A.; Pöhlmann, M.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic aromatic hydrocarbons (PAH) and phenolic substances in meat products smoked with different types of wood and smoking spices. Food Chem. 2013, 139, 955–962. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Suo, H.; Zhao, X.; Kan, J. Aroma and flavor characteristics of commercial Chinese traditional bacon from different geographical regions. J. Sens. Stud. 2018, 34, e12475. [Google Scholar] [CrossRef]
- Bi, Y.; Zhou, G.H.; Pan, D.D.; Wang, Y.; Dang, Y.L.; Liu, J.H.; Jiang, M.F.; Cao, J.X. The effect of coating incorporated with black pepper essential oil on the lipid deterioration and aroma quality of Jinhua ham. J. Food Meas. Charact. 2019, 13, 2740–2750. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S. The structural and thermal characteristics of wheat straw hemicellulose. J. Anal. Appl. Pyrol. 2010, 88, 134–139. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Wongpornchai, S.; Sriseadka, T.; Choonvisase, S. Identification and Quantitation of the Rice Aroma Compound, 2-Acetyl-1-pyrroline, in Bread Flowers (Vallaris glabra Ktze). J. Agric. Food Chem. 2003, 51, 457–462. [Google Scholar] [CrossRef]
- Wei, X.; Handoko, D.D.; Pather, L.; Methven, L.; Elmore, J.S. Evaluation of 2-acetyl-1-pyrroline in foods, with an emphasis on rice flavor. Food Chem. 2017, 232, 531–544. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Quantitative analysis of aroma compounds in wheat and rye bread crusts using a stable isotope dilution assay. J. Agric. Food Chem. 1987, 35, 252–257. [Google Scholar] [CrossRef]
- Takakura, Y.; Sakamoto, T.; Hirai, S.; Masuzawa, T.; Wakabayashi, H.; Nishimura, T. Characterization of the key aroma compounds in beef extract using aroma extract dilution analysis. Meat Sci. 2014, 97, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Rubin, L.J.; D’Souza, L.A.; Teranishi, R.; Buttery, R.G. Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. Crit. Rev. Food Sci. 1986, 24, 141–243. [Google Scholar] [CrossRef] [PubMed]
- Song, H.L.; Cadwallader, K.R. Aroma Components of American Country Ham. J. Food Sci. 2007, 73, 29–35. [Google Scholar] [CrossRef] [PubMed]

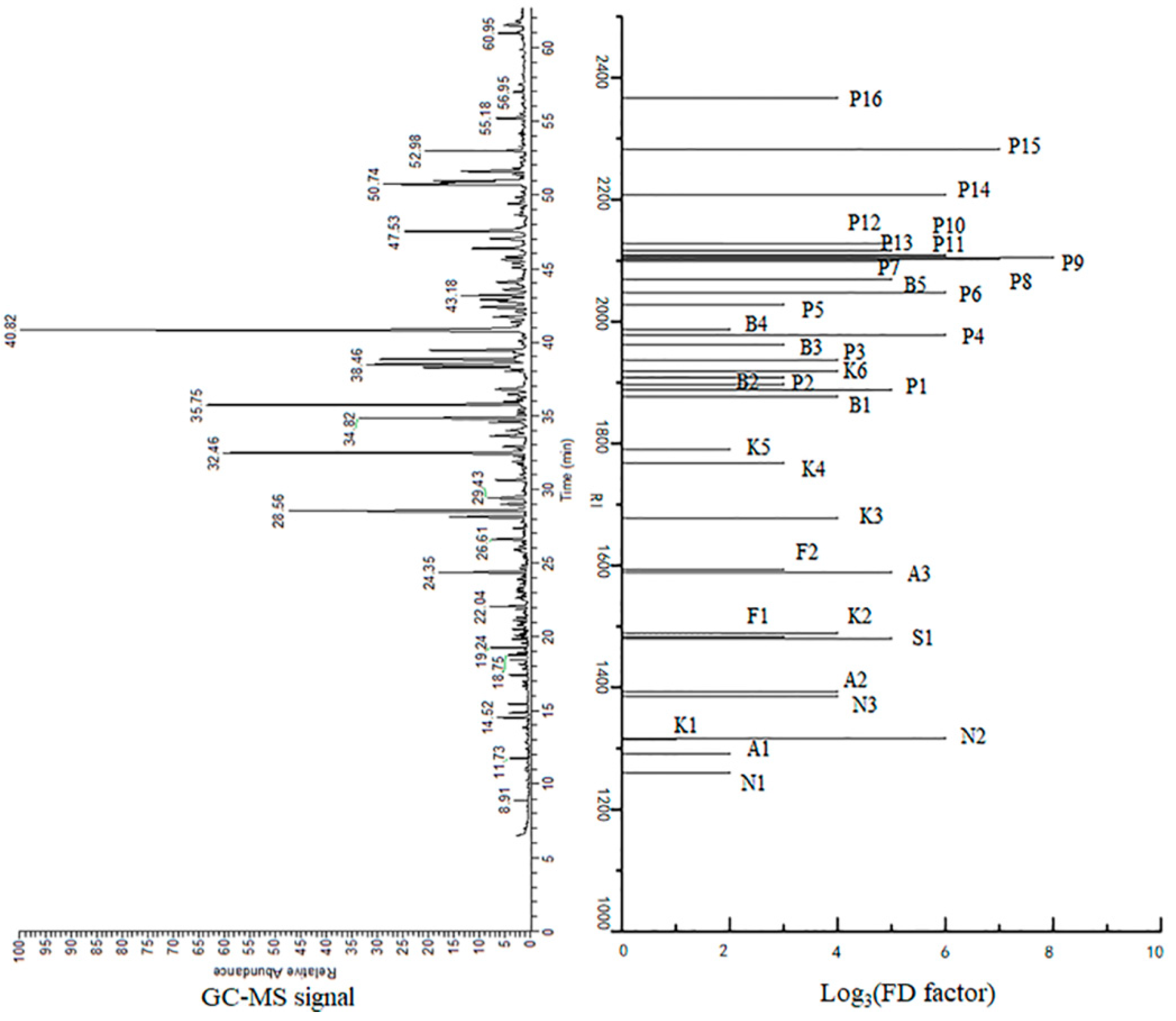
| Samples | THSL-5 | THSL-6 | THSL-11 | THSL-12 | THSL-17 | THSL-18 | THSL-19 | THSL-20 | THSL-25 | THSL-26 |
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | 0 | 0 | 0 | 3 | 8 | 14 | 10 | 7 | 2 | 1 |
| No. | Compounds | RI | Identification | FD Factors | Odorant Descriptor | |
|---|---|---|---|---|---|---|
| TG-WAX | TG-5MS | |||||
| Nitrogen compounds (3) | ||||||
| N1 | 2-Methylpyrazine | 1260 | 863 | MS, RI, S, O | 9 | roasty, nutty |
| N2 | 2-Acetyl-1-pyrroline | 1316 | 921 | MS, RI, S, O | 729 | popcorn, grain, roasty |
| N3 | 2,3,5-Trimethylpyrazine | 1385 | 1024 | MS, RI, S, O | 81 | roasty, earthy |
| Aldehydes (3) | ||||||
| A1 | Octanal | 1291 | 1023 | MS, RI, S, O | 9 | green, citrus |
| A2 | (E)-2-Octenal | 1393 | 1078 | MS, RI, S, O | 81 | green leaf |
| A3 | (E)-2-Nonenal | 1589 | 1177 | MS, RI, S, O | 243 | peanut, almond, fatty |
| Ketones (6) | ||||||
| K1 | 1-Octen-3-one | 1315 | - | MS, RI, S, O | 3 | mushroom |
| K2 | 3-Methyl-2-cyclopenten-1-one | 1489 | 990 | MS, RI, S, O | 81 | sweet, fruity |
| K3 | 3-Ethyl-2-cyclopenten-1-one | 1677 | - | MS, RI, S, O | 81 | caramel-like |
| K4 | 4,4-Dimethyl-2-cyclopenten-1-one | 1768 | 964 | MS, RI, O | 27 | caramel-like, bitter |
| K5 | 3-Methylacetophenone | 1790 | - | MS, RI, S, O | 9 | floral, sweet |
| K6 | 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 1919 | 1136 | MS, RI, S, O | 81 | sweet, caramel-like |
| Sulfur compound (1) | ||||||
| S1 | Methional | 1480 | 905 | MS, RI, S, O | 243 | cooked potato |
| Furan compounds (2) | ||||||
| F1 | 2-Acetylfuran | 1483 | 938 | MS, RI, S, O | 27 | sweet, roast |
| F2 | 5-Methyl furfural | 1593 | 986 | MS, RI, S, O | 27 | green, sweet, grass |
| Unknow compounds (3) | ||||||
| U1 | Unknown1 | - | - | O | 81 | bitter, medicine |
| U2 | Unknown2 | - | - | O | 27 | cucumber, green, |
| U3 | Unknown3 | - | - | O | 9 | green |
| Aromatic compounds (5) | ||||||
| B1 | 1-Methylnaphthalene | 1877 | 1300 | MS, RI, S, O | 81 | medicinal, sweet, vanilla-like |
| B2 | 2-Methylnaphthalene | 1908 | 1299 | MS, RI, S, O | 27 | bitter |
| B3 | 2-Ethylnaphthalene | 1962 | 1402 | MS, RI, S, O | 27 | burning |
| B4 | 3,4-Dimethoxytoluene | 1987 | 1281 | MS, RI, O | 9 | green, dried grass |
| B5 | 3,4,5-Trimethoxytoluene | 2069 | - | MS, RI, S, O | 243 | bitter, earth, pungent |
| Phenolic compounds (16) | ||||||
| P1 | Guaiacol | 1888 | 1104 | MS, RI, S, O | 243 | woody, sweet, smoky |
| P2 | 2-Methoxy-6-methylphenol | 1897 | 1257 | MS, RI, O | 27 | woody, sweet |
| P3 | 2,6-Dimethylphenol | 1937 | 1122 | MS, RI, S, O | 81 | smoky, burning |
| P4 | 4-Methyl guaiacol | 1978 | 1208 | MS, RI, S, O | 729 | sweet, wood, caramel-like, smoky |
| P5 | 2-Methylphenol | 2028 | 1076 | MS, RI, S, O | 27 | vanilla-like, woody |
| P6 | 4-Ethyl guaiacol | 2048 | 1292 | MS, RI, S, O | 729 | wood, smoky, caramel-like |
| P7 | 2,5-Dimethylphenol | 2099 | 1168 | MS, RI, S, O | 243 | butyric acid, stink, leather |
| P8 | 3,4-Dimethylphenol | 2102 | 1194 | MS, RI, S, O | 2187 | butyric acid, stinky, leathery |
| P9 | 3-Ethylphenol | 2105 | 1158 | MS, RI, S, O | 6561 | leathery, smoky |
| P10 | 2-Methoxy-4-propyl-phenol | 2108 | 1382 | MS, RI, S, O | 729 | green, cool, fresh |
| P11 | 3-Methylphenol | 2109 | 1095 | MS, RI, S, O | 729 | burning, leathery, stinky |
| P12 | 3,5-Dimethoxyphenol | 2117 | 1189 | MS, RI, S, O | 243 | rubbery, butyric acid |
| P13 | 2,3-Dimethoxyphenol | 2128 | - | MS, RI, S, O | 243 | rubbery, butyric acid |
| P14 | 2-Methoxy-4-vinylphenol | 2208 | 1329 | MS, RI, S, O | 729 | vanilla-like, smoky, woody |
| P15 | 2,6-Dimethoxyphenol | 2283 | 1370 | MS, RI, S, O | 2187 | leathery, green |
| P16 | trans-2-Methoxy-4-propenyl-phenol | 2367 | 1382 | MS, RI, O | 81 | leathery, smoky |
| No. | Odorants | Concentration (μg/kg) | Threshold (μg/kg) | OAV |
|---|---|---|---|---|
| A1 | (E)-2-Nonenal | 279.75 ± 11.13 | 0.19 a | 1472 |
| A2 | (E)-2-Octenal | 30.42 ± 1.54 | 4.00 b | 8 |
| A3 | Octanal | 76.77 ± 22.61 | 3.40 a | 23 |
| Total | Aldehydes | 386.94 | ||
| P1 | Guaiacol | 1225.46 ± 72.44 | 2.50 a | 490 |
| P3 | 2,6-Dimethylphenol | 2455.15 ± 191.36 | 14.20 c | 173 |
| P4 | 4-Methyl guaiacol | 1395.64 ± 62.82 | 25.00 a | 56 |
| P5 | 2-Methylphenol | 2316.82 ± 248.34 | 45.00 a | 52 |
| P6 | 4-Ethyl guaiacol | 534.46 ± 41.54 | 16.00 a | 33 |
| P7 | 2,5-Dimethylphenol | 811.64 ± 55.08 | 400.00 c | 2 |
| P8 | 3,4-Dimethylphenol | 653.04 ± 59.91 | 17.00 c | 38 |
| P9 | 3-Ethylphenol | 344.81 ± 5.97 | 1.70 a | 203 |
| P10 | 2-Methoxy-4-propyl-phenol | 510.51 ± 80.98 | 157.00 c | 3 |
| P11 | 3-Methylphenol | 96.84 ± 14.66 | 15.00 a | 6 |
| P12 | 3,5-Dimethoxyphenol | 4368.41 ± 435.16 | 140.00 c | 31 |
| P13 | 2,3-Dimethoxyphenol | 1100.40 ± 30.37 | 170.00 c | 6 |
| P14 | 2-Methoxy-4-vinylphenol | 2761.99 ± 39.96 | 5.10 a | 542 |
| P15 | 2,6-Dimethoxyphenol | 9784.39 ± 852.69 | 263.00 a | 37 |
| Total | Phenolic compounds | 28,359.56 | ||
| N1 | 2-Methylpyrazine | 142.89 ± 55.24 | 250.00 a | <1 |
| N2 | 2-Acetyl-1-pyrroline | 38.88 ± 5.32 | 0.12 b | 324 |
| N3 | 2,3,5-Trimethylpyrazine | 204.69 ± 52.35 | 71.00 a | 3 |
| Total | Nitrogen compounds | 386.46 | ||
| B1 | 1-Methylnaphthalene | 693.08 ± 48.08 | 10.75 c | 65 |
| B2 | 2-Methylnaphthalene | 206.49 ± 9.39 | 4.00 c | 52 |
| B3 | 2-Ethylnaphthalene | 222.57 ± 7.78 | 4.96 c | 55 |
| B5 | 3,4,5-Trimethoxytoluene | 2275.00 ± 29.69 | 120.00 c | 19 |
| Total | Aromatic compounds | 3397.14 | ||
| S1 | Methional | 98.58 ± 8.18 | 1.80 a | 55 |
| Total | Sulfur compound | 98.58 | ||
| K2 | 3-Methyl-2-cyclopenten-1-one | 96.63 ± 52.23 | 300.00 b | <1 |
| K5 | 3-Methylacetophenone | 236.84 ± 20.57 | 123.25 c | 2 |
| K6 | 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 205.09 ± 86.55 | 53.35 c | 4 |
| Total | Ketone compounds | 538.56 | ||
| F1 | 2-Acetylfuran | 595.46 ± 35.50 | 1000.00 a | <1 |
| F2 | 5-Methyl furfural | 133.84 ± 4.43 | 50.00 b | 3 |
| Total | Furan compounds | 729.30 |
| No. | Aroma Profile Descriptions | Omitted Compounds | Correct Number in All | Significance |
|---|---|---|---|---|
| M1 | Smoky, leathery | All of the phenolic compounds | 15/15 | *** |
| M2 | Caramel-like, sweet | All of the ketone compounds | 7/15 | - |
| M3 | Green, grass, fatty | All of the aldehyde compounds | 14/15 | *** |
| M4 | Bitter, leathery | All of the aromatic compounds | 8/15 | - |
| M5 | Popcorn, grain, roasty | 2-acetyl-1-pyrroline | 14/15 | *** |
| M6 | Peanut, almond, fatty | (E)-2-nonenal | 14/15 | *** |
| M7 | green, citrus | octanal | 10/15 | - |
| M8 | Cooked potato | methional | 12/15 | ** |
| M9 | Vanilla-like, smoky, woody | 2-methoxy-4-vinylphenol | 14/15 | *** |
| M10 | Woody, sweet, smoky | guaiacol | 13/15 | *** |
| M11 | Leathery, smoky | 3-ethylphenol | 13/15 | ** |
| M12 | Leathery, green | 2,6-dimethylphenol | 12/15 | ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, D.; Zhang, Y.; Zhang, H.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments. Foods 2020, 9, 413. https://doi.org/10.3390/foods9040413
Pu D, Zhang Y, Zhang H, Sun B, Ren F, Chen H, Tang Y. Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments. Foods. 2020; 9(4):413. https://doi.org/10.3390/foods9040413
Chicago/Turabian StylePu, Dandan, Yuyu Zhang, Huiying Zhang, Baoguo Sun, Fazheng Ren, Haitao Chen, and Yizhuang Tang. 2020. "Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments" Foods 9, no. 4: 413. https://doi.org/10.3390/foods9040413
APA StylePu, D., Zhang, Y., Zhang, H., Sun, B., Ren, F., Chen, H., & Tang, Y. (2020). Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments. Foods, 9(4), 413. https://doi.org/10.3390/foods9040413