Comparison of Three Extraction Techniques for the Determination of Volatile Flavor Components in Broccoli
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Isolation of Volatile Components from Broccoli
2.2.1. Solvent-Assisted Flavor Evaporation (SAFE)
2.2.2. Simultaneous Distillation Extraction (SDE)
2.2.3. Solid-Phase Microextraction (SPME)
2.3. Chromatographic Analyses
2.3.1. Gas Chromatography–Olfactometry (GC-O) Analysis
2.3.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.3.3. Comprehensive Two-Dimensional Gas Chromatography Time-of-Flight Mass Spectrometry (GC×GC-ToFMS) Analysis
3. Results and Discussion
3.1. Evaluation of SAFE, SDE and SPME Extracts by GC-O
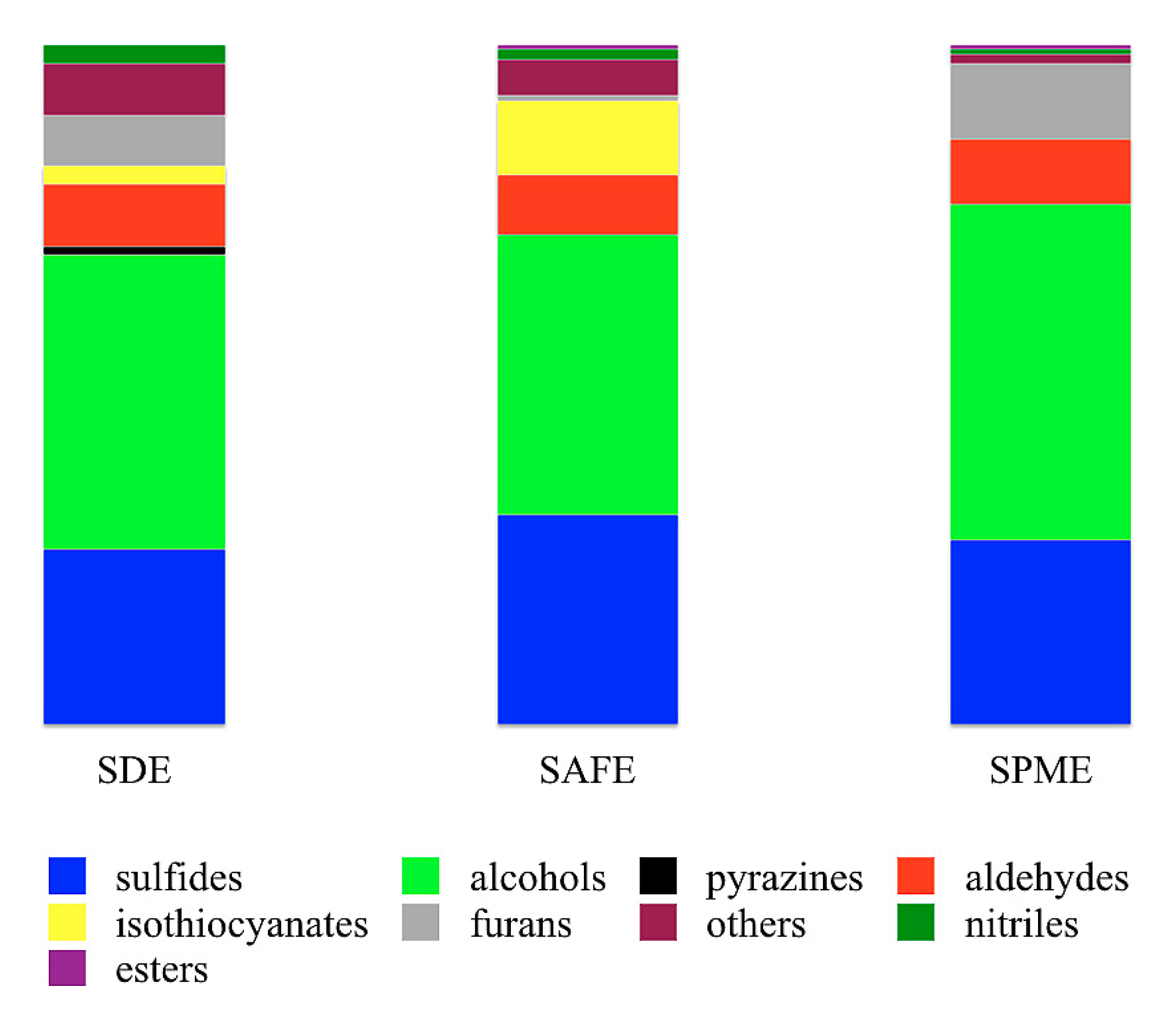
3.2. Evaluation of SAFE, SDE and SPME Extracts by GC×GC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- MacLeod, A.J.; MacLeod, G. Flavor volatiles of some cooked vegetables. J. Food Sci. 1970, 35, 734–738. [Google Scholar] [CrossRef]
- MacLeod, A.J.; MacLeod, G. The flavor volatiles of dehydrated cabbage. J. Food Sci. 1970, 35, 739–743. [Google Scholar] [CrossRef]
- Macleod, A.J.; Macleod, G. Volatiles of cooked cabbage. J. Sci. Food Agric. 1968, 19, 273–277. [Google Scholar] [CrossRef]
- Buttery, R.G.; Guadagni, D.G.; Ling, L.C.; Seifert, R.M.; Lipton, W. Additional volatile components of cabbage, broccoli, and cauliflower. J. Agric. Food Chem. 1976, 1, 24. [Google Scholar] [CrossRef]
- Valette, L.; Fernandez, X.; Poulain, S.; Loiseau, A.-M.; Lizzani-Cuvelier, L.; Levieil, R. Volatile constituents from Romanesco cauliflower. Food Chem. 2003, 80, 353–358. [Google Scholar] [CrossRef]
- Jacobsson, A.; Nielsen, T.; Sjöholm, I. Influence of temperature, modified atmosphere packaging, and heat treatment on aroma compounds in broccoli. J. Agric. Food Chem. 2004, 52, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.; Baty, C.; Le Corre, D.; Souchon, I.; Martin, N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J. Agric. Food Chem. 2002, 50, 6459–6467. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Krumbein, A.; Schonhof, I.; Hoberg, E. Comparison of two sample preparation techniques for sniffing experiments with broccoli (Brassica oleracea var. italica Plenck). Nahrung 1998, 42, 392–394. [Google Scholar] [CrossRef]
- de Pinho, P.G.; Valentão, P.; Gonçalves, R.F.; Sousa, C.; Andrade, P.B. Volatile composition of Brassica oleracea L. var. costata DC leaves using solid-phase microextraction and gas chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Majcher, M.; Jeleń, H.H. Comparison of suitability of SPME, SAFE and SDE methods for isolation of flavor compounds from extruded potato snacks. J. Food Compos. Anal. 2009, 22, 606–612. [Google Scholar] [CrossRef]
- Wardencki, W.; Chmiel, T.; Dymerski, T. Gas Chromatography-Olfactometry (GC-O), Electronic Noses (e-Noses) and Electronic Tongues (e-Tongues) for in Vivo Food Flavour Measurement.” In Instrumental Assessment of Food Sensory Quality; Elsevier: Amsterdam, The Netherlands, 2013; pp. 195–229. [Google Scholar]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent Assisted Flavour Evaporation (SAFE)—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Wieczorek, M.; Jeleń, H. Volatile Compounds of Selected Raw and Cooked Brassica Vegetables. Molecules 2019, 24, 391. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Dec Kratz, P. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Lindsay, R.C.; Rippe, J.K. Enzymic generation of methanethiol to assist in the flavor development of Cheddar cheese and other foods. In Biogeneration of Aromas; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1986; pp. 286–308. [Google Scholar]
- Hansen, M.; Buttery, R.G.; Stern, D.; Cantwell, M.; Ling, L. Broccoli storage under low-oxygen atmosphere: Identification of higher boiling volatiles. J. Agric. Food Chem. 1992, 40, 850–852. [Google Scholar] [CrossRef]
- Vermeulen, C.; Gijs, L.; Collin, S. Sensorial Contribution and formation pathways of thiols: A Review. Food Rev. Int. 2005, 21, 69–137. [Google Scholar] [CrossRef]
- Chin, H.W.; Lindsay, R.C. Volatile sulfur compounds formed in disrupted tissues of different cabbage cultivars. J. Food Sci. 1993, 8, 835–839. [Google Scholar] [CrossRef]
- McGorrin, R.J. The significance of volatile sulfur compounds in food flavors. An overview. In Volatile Sulfur Compounds in Food; ACS Symposium Series; ACS: Washington, DC, USA, 2011; pp. 3–31. [Google Scholar]
- Mazelis, M. Demonstration and characterization of custeine sulfoxide lyase in the cruciferae. Phytochemistry 1963, 2, 15–22. [Google Scholar] [CrossRef]
- Kjær, A.; Madsen, J.Ǿ.; Maeda, Y.; Ozawa, Y.; Uda, Y. Volatiles in distillates of fresh radish of Japanese and Kenyan origin. Agric. Biol. Chem. 1978, 42, 1715–1721. [Google Scholar]
| Odour Type | Name | SAFE | SDE | SPME | RI DB5 | Major Fragment Ions (m/z/) |
|---|---|---|---|---|---|---|
| sulfur, burnt | methanethiol * | + | + | + | >500 | 47 (100) 48(89); 45(47); 46(12); 15(10) |
| putrid, cabbage | dimethyl sulfide * | - | + | + | >500 | 62(100); 47(95); 45(41); 46(36); 61(33) |
| buttery | 2,3-butanedione * | + | + | + | >600 | 43(100); 86(18); 46(88); 15(44); 44(28) |
| rancid, sulfur | 2/3- methyl butanal | + | + | - | 655 | 41(100); 29(93); 57(88); 58(58); 27(42); |
| garlic | methyl thiocyanate * | + | + | + | 665 | 73(100); 72(58); 45(36); 44(32); 46(32) |
| buttery | 2,3-pentanedione * | + | - | - | 707 | 43(100); 29(61); 57(33); 27(26); 100(11) |
| garlic | dimethyl disulfide * | - | + | + | 720 | 94(100); 79(57); 45(48); 46(25); 47(19) |
| cabbage | unknown | + | - | - | 790 | - |
| grass | hexanal * | + | + | + | 795 | 44(100); 56(81); 41(69); 43(55); 57(38) |
| garlic | unknown | + | + | + | 804 | - |
| cabbage, sweaty | 2/3-methyl butanoic acid | + | - | + | 847 | 74(100); 57(64); 29(62); 41(53); 27(32) |
| grassy, green | 3-hexen-1-ol * | + | + | + | 848 | 41(100); 67(59); 39(39); 55(34); 69(27) |
| rancid | Z-(4)-heptenal | + | + | + | 898 | 44(100); 70(94); 43(84); 41(67); 55(59) |
| boiled potatoes | methional * | + | + | + | 905 | 44(100); 104(51); 47(43); 76(33); 45(28) |
| popcorn | 2-acetyl-1-pyrroline | - | + | - | 920 | 43(100); 41(54); 42(24); 83(13); 39(11) |
| cabbage | unknown | + | - | - | 951 | - |
| putrid, cabbage | dimethyl trisulfide * | + | + | + | 975 | 126(100); 45(59); 79(51); 47(36); 64(22) |
| geranium | 1,5-octadien-3-one | + | + | + | 986 | - |
| cabbage | S-methylmethanethiosulphinate | + | - | - | 995 | 64(100); 47(74); 110(61); 45(46) 32(39) |
| broccoli | unknown | + | + | - | 1032 | - |
| honey | phenylacetaldehyde * | + | + | - | 1046 | 91(100); 92(29); 120(28); 68(18); 39(74) |
| earthy, roasted | 2-ethyl-3,5-dimethylpyrazine | + | + | + | 1054 | 135(100); 136(78); 42(36); 39(28); 54(20) |
| sauerkraut | S-methyl methanethisulphonate * | + | - | - | 1071 | 47(100); 81(87); 63(73); 79(68); 126(66) |
| green peas | 2-isopropyl-3-methoxy pyrazine * | + | + | + | 1094 | 137(100); 152(42); 124(26); 138(12); 109(11) |
| broth like | unknown | + | - | - | 1119 | - |
| hop | unknown | + | + | + | 1133 | - |
| roasted | unknown | + | + | - | 1148 | - |
| cucumber | (E)-2-nonenal * | + | + | + | 1159 | 43(100); 41(99); 29(76); 55(76); 70(72) |
| earthy, roatsted | 2-s-butyl-3-methoxypyrazine | + | + | + | 1178 | 138(100); 124(65); 151(45) 137(31); 123(17) |
| pepper like | 2-isobutyl-3-methoxy pyrazine | + | + | + | 1185 | 108(100); 135(14); 107(10); 67(9); 41(9) |
| broccoli | hexyl isothiocyanate * | + | + | + | 1209 | 43(100); 12(61); 41(46); 72(27); 29(24) |
| broth like | unknown | - | + | + | 1217 | - |
| soup | (E)-2-decenal * | + | + | - | 1236 | 43(100); 41(88); 55(70); 70(70); 29(67) |
| fatty | (E, E) -2,4-decadienal * | + | + | - | 1323 | 81(100); 41(51); 39(22); 27(22); 29(21) |
| Compound | Molecular Weight | SDE % | SAFE % | SPME % |
|---|---|---|---|---|
| methanethiol | 48 | S | s | 0.26 |
| ethanenitrile | 55 | S | s | 0.26 |
| dimethyl sulfide | 62 | S | s | 2.42 |
| 1,3-pentadiene, (E)- | 68 | 0.76 | 0.03 | nd |
| 1,3-pentadiene, (Z)- | 68 | 0.80 | nd | nd |
| 1,4-pentadiene | 68 | 0.13 | nd | 0.03 |
| butanenitrile | 69 | 0.61 | nd | nd |
| thiocyanic acid, methyl ester | 73 | 2.30 | 1.12 | 4.38 |
| 1-butanol | 74 | 0.32 | 0.04 | nd |
| methyl ethyl sulfide | 76 | nd | nd | 0.02 |
| dimethyl sulfoxide | 78 | 2.93 | 5.38 | 0.04 |
| pyrazine | 80 | 0.28 | nd | nd |
| 2-pentenenitrile | 81 | 0.18 | nd | nd |
| methallyl cyanide | 81 | nd | 0.05 | nd |
| furan, 2-methyl | 82 | s | s | 0.06 |
| butanenitrile, 3-methyl- | 83 | 0.47 | nd | nd |
| 1-penten-3-one | 84 | 0.55 | 0.85 | 0.73 |
| 2-pentenal, (E)- | 84 | 0.67 | 1.20 | 0.26 |
| 1-penten-3-ol | 86 | 4.84 | 9.31 | 10.01 |
| 2-penten-1-ol. (E)- | 86 | 11.86 | 14.34 | 10.03 |
| butanal, 2-methyl- | 86 | nd | 0.51 | nd |
| pentanal | 86 | 0.38 | 1.52 | nd |
| sulfide, allyl methyl | 88 | 0.02 | nd | nd |
| dimethyl sulfone | 94 | 0.05 | 1.62 | 0.51 |
| disulfide, dimethyl | 94 | 5.50 | 3.19 | 13.00 |
| pyrazine. methyl- | 94 | 0.40 | 0.01 | nd |
| 2,4-hexadienal, (E,E)- | 96 | 0.60 | 0.28 | 0.19 |
| furan, 2-ethyl- | 96 | 1.84 | 0.65 | 10.29 |
| furfural | 96 | 3.76 | nd | nd |
| hexanenitrile | 97 | 0.09 | 0.06 | 0.19 |
| pentanenitrile, 4-methyl- | 97 | s | s | 0.01 |
| 2-hexenal, (E)- | 98 | 1.39 | 1.06 | 3.63 |
| 3-hexanal | 98 | nd | 0.55 | 0.57 |
| thiophene, 2-methyl- | 98 | 0.05 | 0.04 | 0.03 |
| thiophene, 3-methyl- | 98 | 0.02 | nd | nd |
| 2-hexen-1-ol, (E)- | 100 | 8.51 | 6.02 | 0.02 |
| 3-hexen-1-ol, (Z)- | 100 | 5.62 | 3.51 | 23.58 |
| hexanal | 100 | 0.52 | 0.31 | 3.10 |
| isopropyl isothiocyanate | 101 | 0.03 | nd | nd |
| 1-hexanol | 102 | 9.70 | 6.48 | 4.91 |
| S-methyl propanethioate | 104 | 0.03 | nd | nd |
| benzaldehyde | 106 | 0.51 | 1.09 | 0.46 |
| pyrazine, ethenyl- | 106 | 0.02 | nd | nd |
| hexanedinitrile | 108 | 0.02 | 0.01 | nd |
| pyrazine, 2.3-dimethyl- | 108 | 0.10 | nd | nd |
| pyrazine, 2.5-dimethyl- | 108 | 0.20 | nd | nd |
| 2,4-heptadienal, (E,E)- | 110 | 2.05 | 1.21 | 0.26 |
| furan, 2-ethyl-5-methyl- | 110 | 0.10 | nd | 0.01 |
| furan, 2-propyl- | 110 | 0.32 | nd | 0.02 |
| hexanenitrile, 5-methyl- | 111 | 0.10 | 0.03 | 0.28 |
| 2(5H)-furanone, 5-ethyl- | 112 | 0.71 | 1.65 | 0.14 |
| 3-hepten-2-one | 112 | 0.52 | 0.04 | nd |
| thiophene, 2-ethyl- | 112 | 0.46 | 0.38 | 2.87 |
| 1-butene, 4-isothiocyanato- | 113 | 1.75 | 9.83 | 0.01 |
| heptanal | 114 | 0.45 | 0.08 | 0.15 |
| octane | 114 | 0.26 | nd | nd |
| butane, 1-isothiocyanato- | 115 | nd | nd | 0.01 |
| butane, 2-isothiocyanato- | 115 | 0.10 | nd | nd |
| butanenitrile, 4-(methylthio)- | 115 | 0.06 | nd | nd |
| isobutyl isothiocyanate | 115 | nd | nd | 0.01 |
| benzyl nitrile | 117 | 0.13 | 0.21 | 0.05 |
| indole | 117 | 1.67 | 0.03 | nd |
| butanethioic acid, S-methyl ester | 118 | 0.04 | nd | nd |
| benzeneacetaldehyde | 120 | 0.69 | 0.64 | 0.72 |
| pyrazine, (1-methylethenyl)- | 120 | 0.01 | nd | nd |
| phenylethyl alcohol | 122 | nd | 0.66 | 0.17 |
| pyrazine, trimethyl- | 122 | 0.05 | nd | nd |
| 1,2,4-trithiolane | 124 | 0.11 | 1.33 | 0.06 |
| 2,4-octadienal, (E,E)- | 124 | 0.66 | nd | nd |
| cyclohexane, isocyanato- | 125 | 0 | 0.01 | nd |
| butane, 1-isothiocyanato-3-methyl- | 126 | 0 | 0.01 | 0.01 |
| dimethyl trisulfide | 126 | 2.33 | 2.57 | 3.37 |
| S-methyl methanethiosulphonate | 126 | 2.80 | 9.53 | 0.08 |
| cyclopentyl isothiocyanate | 127 | 0.01 | 0.06 | nd |
| hexane, 1-isocyanato- | 127 | nd | 0.03 | 0.01 |
| 3-hexen-1-ol, formate. (Z)- | 128 | 2.13 | 0.77 | 0.82 |
| butanoic acid, 2-propenyl ester | 128 | nd | 0.58 | nd |
| heptane, 2.4-dimethyl- | 128 | nd | 0.28 | nd |
| octanal | 128 | 0.10 | nd | nd |
| 2-methylbutyl isothiocyanate | 129 | 0.14 | nd | nd |
| pentanenitrile, 5-(methylthio)- | 129 | nd | 0.51 | nd |
| n-pentyl isothiocyanate | 129 | 0.05 | 0.02 | 0.01 |
| hexanoic acid, methyl ester | 130 | nd | nd | 0.49 |
| 1H-indole, 3-methyl- | 131 | 0.43 | 0.01 | 0.01 |
| benzenepropanenitrile | 131 | 0.89 | 0.34 | 0.08 |
| S-methyl pentanethioate | 132 | 0.01 | 0.03 | nd |
| benzyl isocyanate | 133 | nd | 0.01 | nd |
| benzothiazole | 135 | 0.05 | 0.75 | nd |
| 3-ethyl-1,5-octadiene | 138 | 0.63 | 0.17 | 0.18 |
| benzene, 1,2-dimethoxy- | 138 | nd | 0.51 | 0.21 |
| furan, 2-pentyl- | 138 | 1.32 | 0.09 | 0.73 |
| thiophene, 3.4-diethyl- | 140 | 0.03 | nd | nd |
| cyclohexane, isothiocyanato- | 141 | nd | nd | nd |
| 2-hexen-1-ol, acetate, (Z)- | 142 | 0.47 | nd | nd |
| nonanal | 142 | 0.23 | 0.29 | 0.10 |
| 4-methylpentyl isothiocyanate | 143 | nd | 0.06 | nd |
| hexane, 1-isothiocyanato- | 143 | 0.09 | 0.04 | 0.01 |
| benzenebutanenitrile | 145 | 0.01 | nd | nd |
| sulforaphane nitrile | 145 | nd | 0.22 | nd |
| 1H-indole, 1-methoxy- | 147 | 0.50 | nd | nd |
| benzene, (isothiocyanatomethyl)- | 149 | 0.11 | 0.41 | 0.01 |
| methyl pentyl disulfide | 150 | 0.23 | 0.01 | nd |
| 2,4-decadienal, (E,E)- | 152 | 0.67 | 0.07 | 0.01 |
| furan, 2-hexyl- | 152 | 0.03 | nd | nd |
| pyrazine, 2-methoxy-3-(1-methylethyl)- | 152 | 0.01 | nd | nd |
| 1H-indole-3-acetonitrile | 156 | 0.28 | 1.15 | nd |
| decanal | 156 | 0.18 | 0.07 | 0.01 |
| tetrasulfide, dimethyl | 158 | 4.98 | 3.71 | 0.06 |
| methyl n-octyl sulfide | 160 | 0.08 | 0.01 | nd |
| benzene, (2-isothiocyanatoethyl)- | 163 | 0.52 | 0.43 | nd |
| methyl n-hexyl disulfide | 164 | 0.03 | nd | nd |
| sulforaphane | 177 | nd | 0.03 | nd |
| sulfide, methyl 1-methyl-2-butenyl | 178 | 0.01 | nd | nd |
| butanoic acid, 3-hexenyl ester. (E)- | 184 | nd | nd | 0.07 |
| pentasulfide, dimethyl | 190 | 3.61 | 0.41 | nd |
| tetradecane | 198 | 0.13 | 0.28 | nd |
| pentadecane | 212 | 0.06 | 0.04 | nd |
| heptadecane | 240 | 0.13 | 0.22 | nd |
| hexadecanal | 240 | 0.06 | 0.03 | nd |
| heptacosane | 380 | 0.16 | nd | nd |
| sulfurous acid, octadecyl pentyl ester | 404 | 0.16 | 0.81 | nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, M.N.; Majcher, M.; Jeleń, H. Comparison of Three Extraction Techniques for the Determination of Volatile Flavor Components in Broccoli. Foods 2020, 9, 398. https://doi.org/10.3390/foods9040398
Wieczorek MN, Majcher M, Jeleń H. Comparison of Three Extraction Techniques for the Determination of Volatile Flavor Components in Broccoli. Foods. 2020; 9(4):398. https://doi.org/10.3390/foods9040398
Chicago/Turabian StyleWieczorek, Martyna Natalia, Małgorzata Majcher, and Henryk Jeleń. 2020. "Comparison of Three Extraction Techniques for the Determination of Volatile Flavor Components in Broccoli" Foods 9, no. 4: 398. https://doi.org/10.3390/foods9040398
APA StyleWieczorek, M. N., Majcher, M., & Jeleń, H. (2020). Comparison of Three Extraction Techniques for the Determination of Volatile Flavor Components in Broccoli. Foods, 9(4), 398. https://doi.org/10.3390/foods9040398







