Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview
Abstract
1. Introduction
2. Industrial Production of Food Enzymes
2.1. Fermentation Processes
2.2. Purification
2.3. Immobilization
2.4. Formulation
3. GMM Used for Optimization of Food Enzyme Production
3.1. Advantages of Using Micro-Organisms to Produce FE
3.2. Characteristics of Genetically Modified Micro-Organsisms
3.2.1. Selection of Host Organism
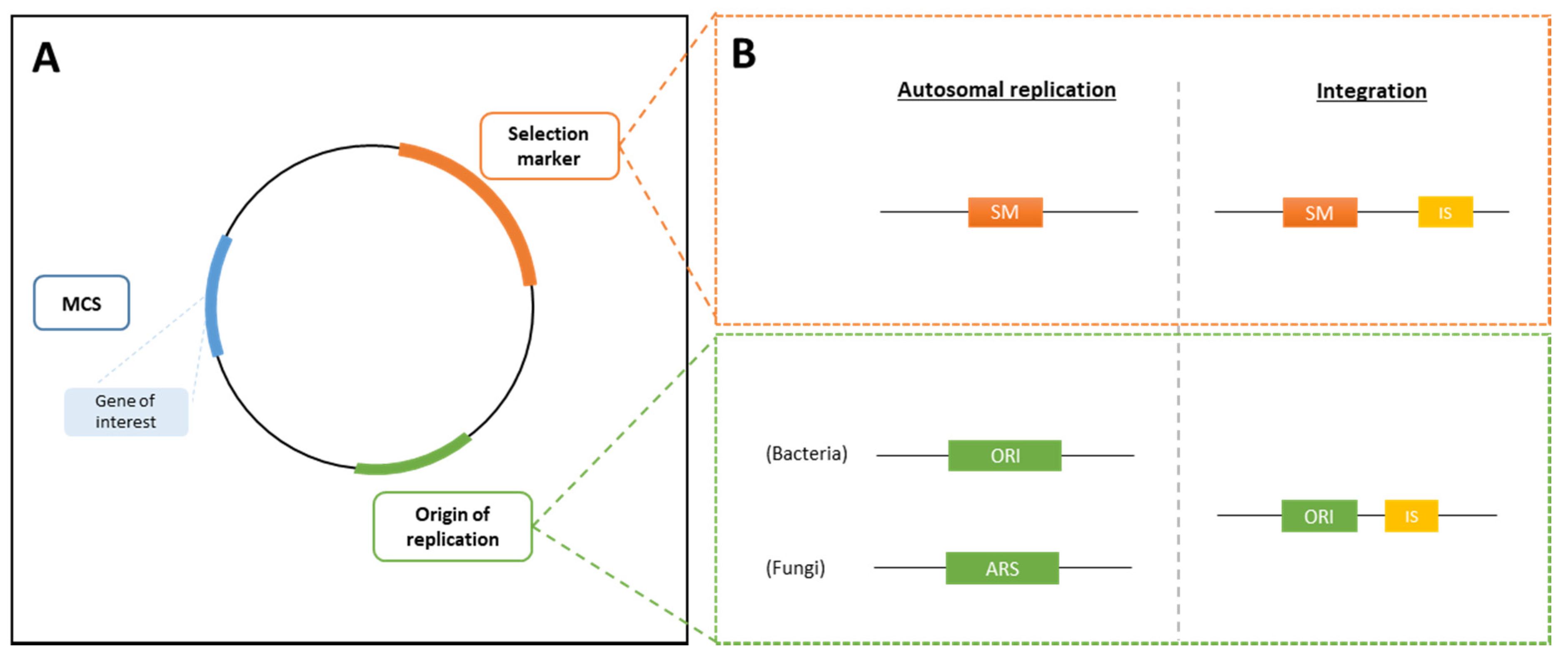
3.2.2. Expression Vectors and Transformation Processes
3.2.3. Selection Markers
3.2.4. Increased Gene Expression
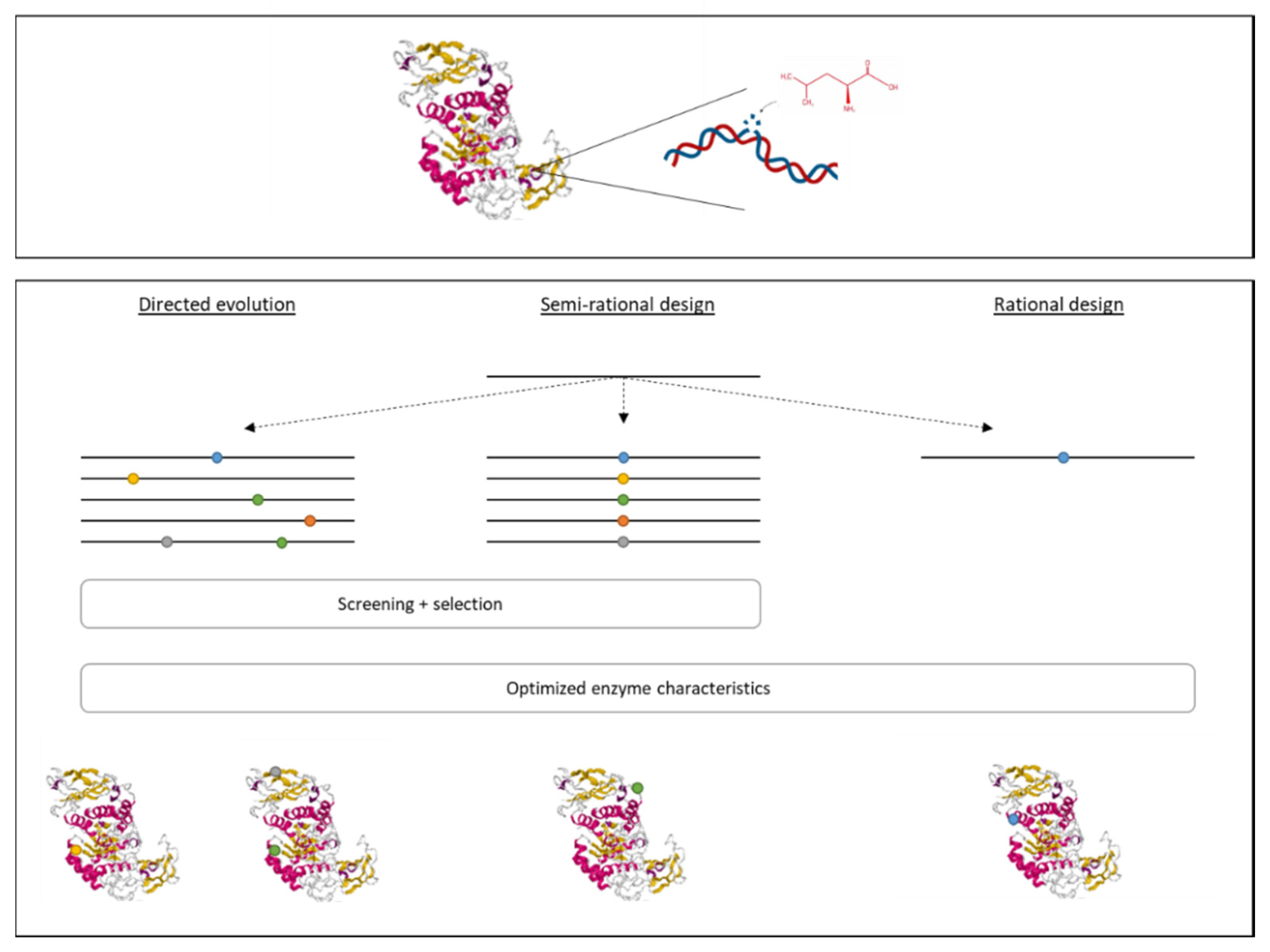
3.3. New Technologies to Introduce Genetic Modifications
3.4. Development of Recombinant Enzymes
4. European Food Enzyme Regulations and Consequent Safety Evaluations: Current Status and Challenges
4.1. Food Additive or Processing Aid
4.2. Overview of the Different European Regulations
4.3. Qualified Presumption of Safety Status
4.4. Criteria and Challenges Regarding Microbial Presence in Food Enzyme Preparations
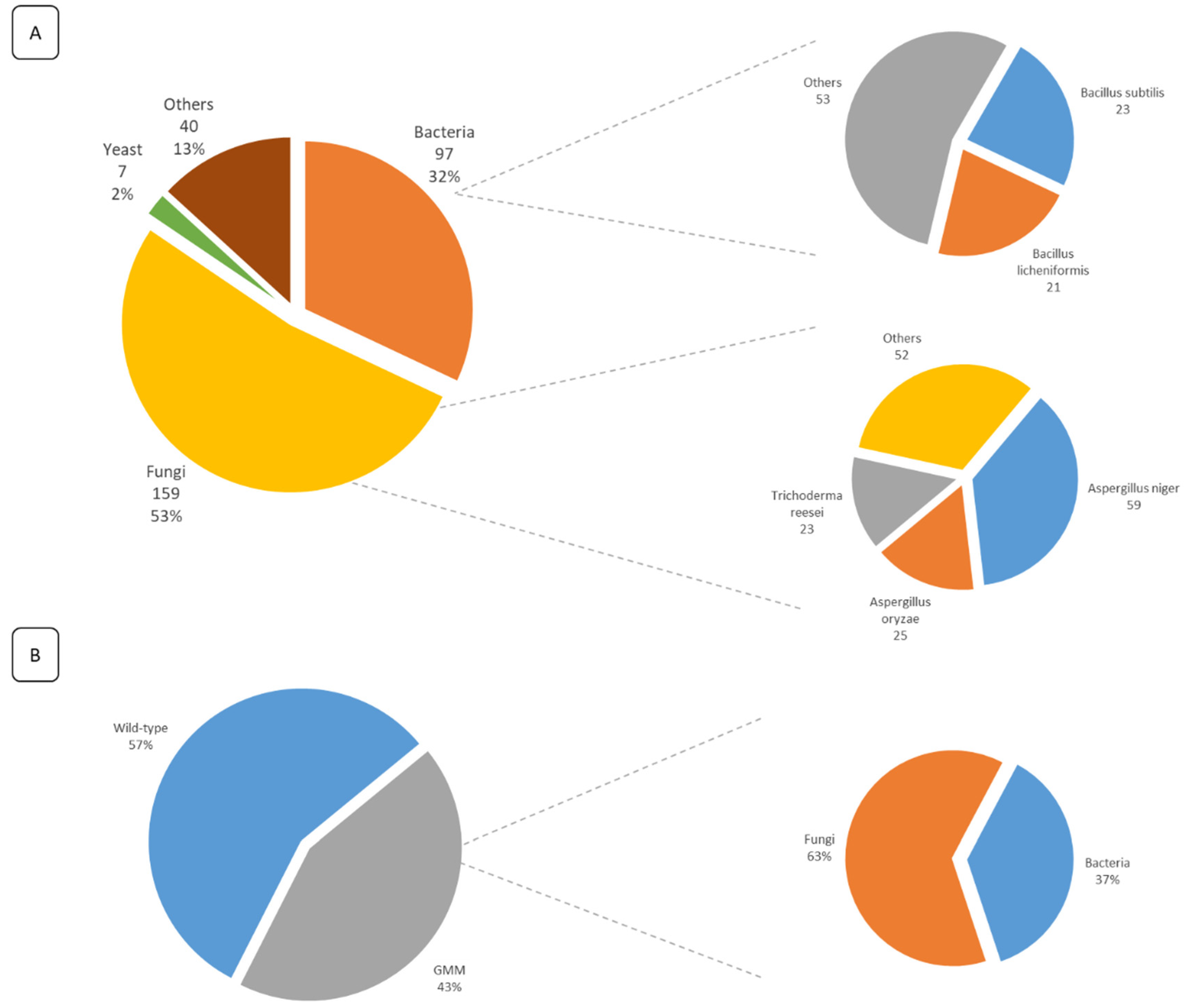
5. Overview of the Submitted FE Dossiers and Identification of Key Information
6. Food Enzymes and Related GMO Regulations
6.1. Issues Related to the Presence of GMM in the Food Chain
6.2. Case Study: Unauthorized GMM in A Commercialized Protease Product
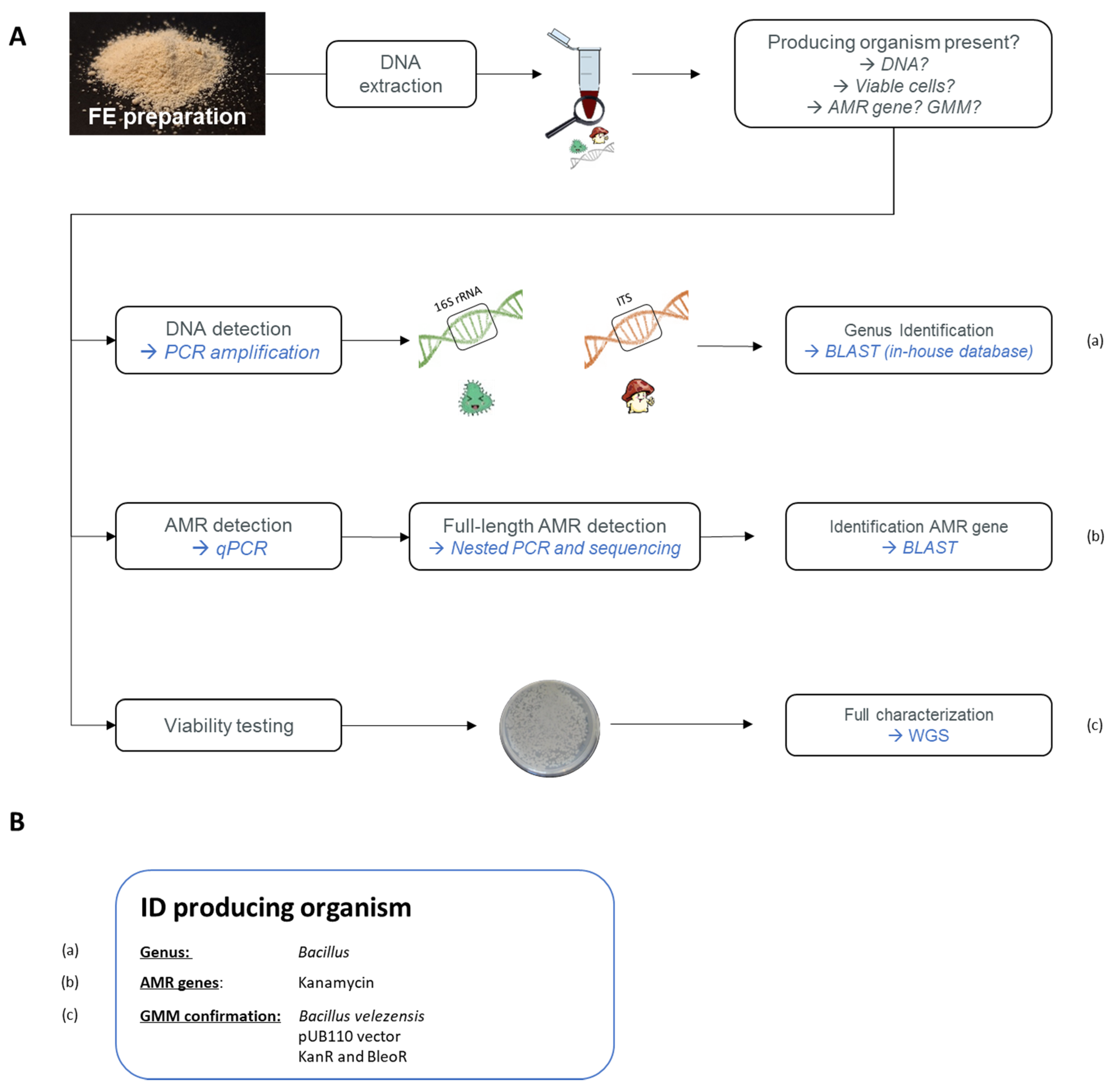
6.2.1. Developed Strategy to Detect GMM in Food
6.2.2. Application of the Proposed Strategy
6.3. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Industrial Enzymes Market by Type (Amylases, Cellulases, Proteases, Lipases, and Phytases), Application (Food & Beverages, Cleaning Agents, and Animal Feed), Source (Microorganism, Plant, and Animal), and Region—Global Forecast to 2022. Market Research Report. 2016. Available online: https://www.marketsandmarkets.com/Market-Reports/industrial-enzymes-market-237327836.html (accessed on 6 January 2019).
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, M.; Mittal, A.; Kumar, P. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Kokare, C. Microbial Enzymes of Use in Industry. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings. Off. J. Eur. Union 2008, 354, 16–33. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1332/2008 of the European Parliament and of the Council of 16 December 2008 on food enzymes and amending Council Directive 83/417/EEC, Council Regulation (EC) No 1493/1999, Directive 2000/13/EC, Council Directive 2001/112/EC and Regulation (EC) No 258/97. Off. J. Eur. Union 2008, 354, 7–15. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1334/2008 of the european parliament and of the council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Off. J. Eur. Union 2008, 354, 34–50. [Google Scholar]
- Zhang, Y.; Geary, T.; Simpson, B.K. Genetically modified food enzymes: A review. Curr. Opin. Food Sci. 2019, 25, 14–18. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Pandey, A. Production, Purification, and Application of Microbial Enzymes; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme Immobilization: An Overview on Methods, Support Material, and Applications of Immobilized Enzymes, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Fernandes, P. Enzymes in Food Processing: A Condensed Overview on Strategies for Better Biocatalysts. Enzym. Res. 2010, 2010, 1–19. [Google Scholar] [CrossRef] [PubMed]
- JECFA. General Specifications and Considerations for Enzyme Preparations. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/enzymes/en/ (accessed on 16 January 2020).
- Fernandes, P.; Carvalho, F. Microbial Enzymes for the Food Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Saranraj, P.; Naidu, M.A. Microbial Pectinases: A Review. Glob. J. Tradit. Med. Syst. 2014, 3, 1–9. [Google Scholar]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Hjort, C. Industrial Enzyle Production for Food Applications; Woodhead Publishing Limitedp: Cambridge, UK, 2007. [Google Scholar]
- Chang, M.; Chu, X.; Lv, J.; Li, Q.; Tian, J.; Wu, N. Improving the thermostability of acidic pullulanase from bacillus naganoensis by rational design. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic Enzymes. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef]
- Olempska-Beer, Z.S.; Merker, R.I.; Ditto, M.D.; DiNovi, M.J. Food-processing enzymes from recombinant microorganisms—A review. Regul. Toxicol. Pharmacol. 2006, 45, 144–158. [Google Scholar] [CrossRef]
- Trono, D. Recombinant Enzymes in the Food and Pharmaceutical Industries; Elsevier B.V.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Hui, Y.H.; Nip, W.K.; Nollet, L.M.L.; Paliyath, G.; Simpson, B.K. Food Biochemistry and Food Processing; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rieder, L.; Teuschler, N.; Ebner, K.; Glieder, A. Eukaryotic Expressin Systems for Industrial Enzymes. In Industrial Enzyme Applications; Wiley: Hoboken, NJ, USA, 2015; Volume 95, pp. 345–351. [Google Scholar]
- Yan, S.; Wu, G. Bottleneck in secretion of α-amylase in Bacillus subtilis. Microb. Cell Fact. 2017, 16, 124. [Google Scholar] [CrossRef]
- Meyer, V. Genetic engineering of filamentous fungi—Progress, obstacles and future trends. Biotechnol. Adv. 2008, 26, 177–185. [Google Scholar] [CrossRef]
- European Commission. Food Enzyme Applications Submitted to the Commission within the Legal Deadline; From 11 September 2011 to 11 March 2015; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- EFSA. Panel on Food Contact Material, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on lipase from a genetically modified strain of Aspergillus oryzae (strain NZYM-AL). EFSA J. 2014, 12, 3778. [Google Scholar] [CrossRef]
- Van Dijck, P.W.M.; Selten, G.C.M.; Hempenius, R.A. On the safety of a new generation of DSM Aspergillus niger enzyme production strains. Regul. Toxicol. Pharmacol. 2003, 38, 27–35. [Google Scholar] [CrossRef]
- Clyne, R.K.; Kelly, T.J. Identification of autonomously replicating sequence (ARS) elements in eukaryotic cells. Methods Companion Methods Enzymol. 1997, 13, 221–233. [Google Scholar] [CrossRef]
- Vile, R. Selectable markers for eukaryotic cells. Methods Mol. Biol. 1992, 8, 49–60. [Google Scholar] [PubMed]
- Pronk, J.T. Treatments for Osteoarthritis in Pets Continue to Evolve. Appl. Environ. Microbiol. 2002, 68, 2095–2100. [Google Scholar] [CrossRef]
- Lopes, T.S.; Klootwijk, J.; Veenstra, A.E.; van der Aar, P.C.; van Heerikhuizen, H.; Raúe, H.A.; Planta, R.J. High-copy-number integration into the ribosomal DNA of Saccharomyces cerevisiae: A new vector for high-level expression. Gene 1989, 79, 199–206. [Google Scholar] [CrossRef]
- Sunga, A.J.; Tolstorukov, I.; Cregg, J.M. Posttransformational vector amplification in the yeast Pichia pastoris. FEMS Yeast Res. 2008, 8, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Fitz, E.; Wanka, F.; Seiboth, B. The promoter toolbox for recombinant gene expression in trichoderma reesei. Front. Bioeng. Biotechnol. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Christensen, T.; Woeldike, H.; Boel, E.; Mortensen, S.B.; Hjortshoej, K.; Thim, L.; Hansen, M.T. High level expression of recombinant genes in Aspergillus oryzae. Nat. Publ. Gr. 1988, 6, 1419–1422. [Google Scholar] [CrossRef]
- Liu, T.; Wang, T.; Li, X.; Liu, X. Improved heterologous gene expression in Trichoderma reesei by cellobiohydrolase i gene (cbh1) promoter optimization. Acta Biochim. Biophys. Sin. 2008, 40, 158–165. [Google Scholar] [CrossRef]
- Zou, G.; Shi, S.; Jiang, Y.; van den Brink, J.; de Vries, R.P.; Chen, L.; Zhang, J.; Ma, L.; Wang, C.; Zhou, Z. Construction of a cellulase hyper-expression system in Trichoderma reesei by promoter and enzyme engineering. Microb. Cell Fact. 2012, 11, 1–12. [Google Scholar] [CrossRef]
- Rajamanickam, V.; Metzger, K.; Schmid, C.; Spadiut, O. A novel bi-directional promoter system allows tunable recombinant protein production in Pichia pastoris. Microb. Cell Fact. 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Yang, S.; Sleight, S.C.; Sauro, H.M. Rationally designed bidirectional promoter improves the evolutionary stability of synthetic genetic circuits. Nucleic Acids Res. 2013, 41, 1–7. [Google Scholar] [CrossRef]
- Vogl, T.; Kickenweiz, T.; Pitzer, J.; Sturmberger, L.; Weninger, A.; Biggs, B.W.; Köhler, E.M.; Baumschlager, A.; Fischer, J.E.; Hyden, P.; et al. Engineered bidirectional promoters enable rapid multi-gene co-expression optimization. Nat. Commun. 2018, 9, 3589. [Google Scholar] [CrossRef]
- Song, R.; Zhai, Q.; Sun, L.; Huang, E.; Zhang, Y.; Zhu, Y.; Guo, Q.; Tian, Y.; Zhao, B.; Lu, H. CRISPR/Cas9 genome editing technology in filamentous fungi: Progress and perspective. Appl. Microbiol. Biotechnol. 2019, 103, 6919–6932. [Google Scholar] [CrossRef]
- Donohoue, P.D.; Barrangou, R.; May, A.P. Advances in Industrial Biotechnology Using CRISPR-Cas Systems. Trends Biotechnol. 2018, 36, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Börner, R.A.; Kandasamy, V.; Axelsen, A.M.; Nielsen, A.T.; Bosma, E.F. Genome editing of lactic acid bacteria: Opportunities for food, feed, pharma and biotech. FEMS Microbiol. Lett. 2019, 366, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, L.; Wu, J. Enhanced extracellular pullulanase production in Bacillus subtilis using protease-deficient strains and optimal feeding. Appl. Microbiol. Biotechnol. 2018, 102, 5089–5103. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, X.; Wu, J. Multigene disruption in undomesticated Bacillus subtilis ATCC 6051a using the CRISPR/Cas9 system. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Cerezo, S.; Kun, R.S.; de Vries, R.P.; Garrigues, S. CRISPR/Cas9 technology enables the development of the filamentous ascomycete fungus Penicillium subrubescens as a new industrial enzyme producer. Enzym. Microb. Technol. 2020, 133, 109463. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Useful Microbial Enzymes—An Introduction; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- The European Commission. Commission Regulation (EU) N° 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) N° 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union 2011, 295, 1–177. [Google Scholar]
- Cerutti, G.; Boudot, J.; Bournigal, J.M.; Rousseau, L. Arrêté du 19 Octobre 2006 relatif à L’emploi d’auxIliaires Technologiques Dans la Fabrication de Certaines Denrées Alimentaires. 2006. Available online: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=LEGITEXT000020667468#LEGISCTA000020667473 (accessed on 9 August 2019).
- Fenger, A. Bekendtgørelse om tilsætninger mv. til fødevarer. Available online: https://www.retsinformation.dk/eli/lta/2018/1247 (accessed on 22 January 2020).
- Publications Office of the EU. Commission Regulation (EU) No 231/2012 of 9 March 2012 Laying Down Specifications for Food Additives Listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council; Publications Office of the EU: Brussels, Belgium, 2012. [Google Scholar]
- The Council of the European Union. Council regulation (EC) No 1493/1999 of 17 may 1999 on the common organisation of the market in wine. Off. J. Eur. Communities 1999, 179, 1–84. [Google Scholar]
- The European Commission. Regulation (Eu) No 234/2011 of 10 March 2011 implementing Regulation (EC) No 1331/2008 of the European Parliament and of the Council establishing a common authorisation procedure for food additives, food enzymes and food flavourings. Off. J. Eur. Union 2011, 2011, 15–24. [Google Scholar]
- The European Commission. Commission Implementing. Regulation (EU) No 562/2012 of 27 June 2012 amending Commission Regulation (EU) No 234/2011 with regard to specific data required for risk assessment of food enzymes. Off. J. Eur. Union 2012, 562, 21–23. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2000/13/EC on the approximation of the laws of the Member States relating to the labelling, presentation and advertising of foodstuffs. Off. J. Eur. Union 2000, 109, 29–42. [Google Scholar]
- Herman, L.; Chemaly, M.; Cocconcelli, P.S.; Fernandez, P.; Klein, G.; Peixe, L.; Prieto, M.; Querol, A.; Suarez, J.E.; Sundh, I.; et al. The qualified presumption of safety assessment and its role in EFSA risk evaluations: 15 years past. FEMS Microbiol. Lett. 2019, 366, 1–7. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA J. 2017, 15, 177. [Google Scholar]
- European Food Safety Authority. Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA J. 2020, 18, 1–56. [Google Scholar]
- Sewalt, V.; Shanahan, D.; Gregg, L.; La Marta, J.; Carillo, R. The Generally Recognized as Safe (GRAS) process for industrial microbial enzymes. Ind. Biotechnol. 2016, 12, 295–302. [Google Scholar] [CrossRef]
- Pariza, M.W.; Johnson, E.A. Evaluating the safety of microbial enzyme preparations used in food processing: Update for a new century. Regul. Toxicol. Pharmacol. 2001, 33, 173–186. [Google Scholar] [CrossRef]
- European Food Safety Authority. Administrative Guidance to applicants on the suitability check of applications for authorisation of food enzymes submitted under Regulation (EC) No 1332/2008. EFSA Support. Publ. 2014, 638, 17. [Google Scholar]
- European Food Safety Authority. Explanatory Note for the Guidance of the Scientific Panel of Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on the Submission of a Dossier on Food Enzymes. EFSA Support. Publ. 2014, 689, 1–22. [Google Scholar]
- Anadón, A.; Bell, D.; Binderup, M.L.; Bursch, W.; Castle, L.; Crebelli, R.; Engel, K.H.; Franz, R.; Gontard, N.; Haertlé, T.; et al. Guidance of the Scientific Panel of Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on the Submission of a Dossier on Food Enzymes for Safety Evaluation by the Scientific Panel of Food Contact Material, Enzymes, Flavourings and Proc. EFSA J. 2009, 1305, 1–26. [Google Scholar]
- EFSA; Panel on Genetically Modified Organisms (GMO). Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use. EFSA J. 2011, 9, 1–53. [Google Scholar]
- Silano, V.; Baviera, J.M.B.; Bolognesi, C.; Brüschweiler, B.J.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mortensen, A.; et al. Characterisation of microorganisms used for the production of food enzymes. EFSA J. 2019, 17, 1–13. [Google Scholar]
- The European Parliament and the Council of the European Union. The new directive 2001/18/EC on the deliberate release of genetically modified organisms into the environment: Changes and perspectives. Off. J. Eur. Communities 2001, 10, 309–320. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1829/2003 of the European Parliament and of the council on genetically modified food and feed. Off. J. Eur. Union 2003, 268, 1–23. [Google Scholar]
- FOD Volksgezondheid. Contractueel Onderzoek; Informatiebrochure Oproep 2017; FOD Volksgezondheid: Brussels, Belgium, 2017. [Google Scholar]
- Deckers, M.; Vanneste, K.; Winand, F.; DeKeersmaecker, S.C.J.; Denayer, S.; Heyndrickx, M.; Deforce, D.; Fraiture, M.A.; Roosens, N.H.C. Strategy for the identification of micro-organisms producing food and feed products: Bacteria producing food enzymes as study case. Food Chem. 2020, 305, 125431. [Google Scholar] [CrossRef]
- Fraiture, M.A.; Deckers, M.; Papazova, N.; Roosens, N.H.C. Detection strategy targeting a chloramphenicol resistance gene from genetically modified bacteria in food and feed products. Food Control 2020, 108, 106873. [Google Scholar] [CrossRef]
- Fraiture, M.; Deckers, M.; Papazova, N.; Roosens, N.H.C. Strategy to control the presence of antimicrobial resistance genes in food and feed bacterial fermentation products. Int. J. Food Microbiol. 2020. Submitted. [Google Scholar]
- Fraiture, M. Next-generation sequencing: A key tool to identify unauthorized genetically modified microorganisms in food enzyme preparations. Sci. Rep. 2020. Submitted. [Google Scholar]
- Likotrafiti, E.; Oniciuc, E.; Prieto, M.; Santos, J.; Lopez, S.; Alvarez-Ordonez, A. Risk assessment of antimicrobial resistance along the food chain through culture-independent methodologies. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Genetically Modified Organisms on the use of antibiotic resistance genes as marker genes in genetically modified plants. EFSA J. 2004, 48, 1–18. [Google Scholar]
| Dossier Status | |
|---|---|
| Submitted dossiers (Within legal deadline) | 303 |
| Withdrawn by applicant | 8 |
| Not accepted | 5 |
| Submitted dossiers (After legal deadline) | 11 |
| Evaluated dossiers | 74 |
| Ongoing evaluations | 216 |
| Enzyme | Enzyme Class | Dossier Count | Application of the Enzyme in the Food Industry | Dossier Count |
|---|---|---|---|---|
| Alpha-amylase | 3 | 31 | Bakery products and other cereal-based products (e.g., pasta, noodles, snacks) | 70 |
| Triacylglycerol lipase | 3 | 21 | Dairy processing (whey processing) | 63 |
| Xylanase | 3 | 21 | Flavoring production | 51 |
| Beta-galactosidase (lactase) | 3 | 12 | Beer and other cereal-based beverages | 49 |
| Glucoamylase | 3 | 12 | Starch processing | 42 |
| Protease | 3 | 10 | Cereal-based distilled alcoholic beverages | 37 |
| Endo-1,3(4)-β-glucanase | 3 | 9 | Fruit and vegetable processing | 36 |
| Cellulase | 3 | 8 | Protein processing | 34 |
| Cyclomaltodextrin glucanotransferase | 2 | 7 | Yeast processing | 32 |
| Polygalacturonase | 3 | 7 | Processing of oils and fats | 18 |
| Genus | Species | QPS | Genus | Species | QPS | Genus | Species | QPS |
|---|---|---|---|---|---|---|---|---|
| Arthrobacter | ramosus | No | Pullulanibacillus | naganoensis | No | Leptographium | procerum | No |
| Bacillus | licheniformis | Yes | Streptomyces | violaceoruber | No | Mucor | javanicus | No |
| Bacillus | subtillis | Yes | Streptomyces | murinus | No | Penicillium | roqueforti | No |
| Bacillus | circulans | No | Streptomyces | netropsis | No | Penicillium | camemberti | No |
| Bacillus | pumilus | Yes | Streptomyces | mobaraensis | No | Penicillium | multicolor | No |
| Bacillus | amyloliquefaciens | Yes | Streptomyces | rubiginosus | No | Penicillium | citrinium | No |
| Bacillus | flexus | Yes | Aspergillus | oryzae | No | Penicillium | decumbens | No |
| Cellulosimicrobium | cellulans | No | Aspergillus | niger | No | Penicillium | chrysogenum | No |
| Chryseobacterium | proteolyticum | No | Aspergillus | nigeragg. | No | Penicillium | funiculosum | No |
| Corynebacterium | glutamicum | No | Aspergillus | niger macrosporus | No | Rhizomucor | miehei | No |
| Escherichia | coli | No | Aspergillus | niger awamori | No | Rhizopus | oryzae | No |
| Geobacillus | stearothermophilus | Yes | Aspergillus | fijiensis | No | Rhizopus | niveus | No |
| Geobacillus | pallidus | No | Aspergillus | acidus | No | Talaromyces | pinophilus | No |
| Geobacillus | caldoproteolyticus | No | Aspergillus | aculeatus | No | Talaromyces | emersonii | No |
| Klebsiella | pneumoniae | No | Aspergillus | melleus | No | Trametes | hirsuta | No |
| Lactobacillus | fermentum | Yes | Chaetomium | gracile | No | Trichoderma | reesei | No |
| Lactococcus | lactis | Yes | Chaetomium | erraticum | No | Trichoderma | citrinoviride | No |
| Leuconostoc | citreum | Yes | Cryphonectria | parasitica | No | Trichoderma | viride | No |
| Microbacterium | imperiale | Yes | Sporobolomyces | singularis | No | |||
| Paenibacillus | macerans | No | Disporotrichum | dimorphosporum | No | Candida | cylindracea | Yes |
| Paenibacillus | alginolyticus | No | Boletus | edulis | No | Candida | rugosa | No |
| Protaminobacter | rubrum | No | Fusarium | venenatum | No | Kluyveromyces | lactis | Yes |
| Pseudomonas | fluorescens | No | Hansenula | polymorpha | No | Pichia | pastori | Yes |
| Pseudomonas | amyloderamosa | No | Humicola | insolens | No | Saccharomyces | cerevisiae | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deckers, M.; Deforce, D.; Fraiture, M.-A.; Roosens, N.H.C. Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview. Foods 2020, 9, 326. https://doi.org/10.3390/foods9030326
Deckers M, Deforce D, Fraiture M-A, Roosens NHC. Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview. Foods. 2020; 9(3):326. https://doi.org/10.3390/foods9030326
Chicago/Turabian StyleDeckers, Marie, Dieter Deforce, Marie-Alice Fraiture, and Nancy H.C. Roosens. 2020. "Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview" Foods 9, no. 3: 326. https://doi.org/10.3390/foods9030326
APA StyleDeckers, M., Deforce, D., Fraiture, M.-A., & Roosens, N. H. C. (2020). Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview. Foods, 9(3), 326. https://doi.org/10.3390/foods9030326





