The Effect of High-Intensity Ultrasound on the Physicochemical and Microbiological Properties of Mexican Panela Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment Design and High-Intensity Ultrasound (HIU)
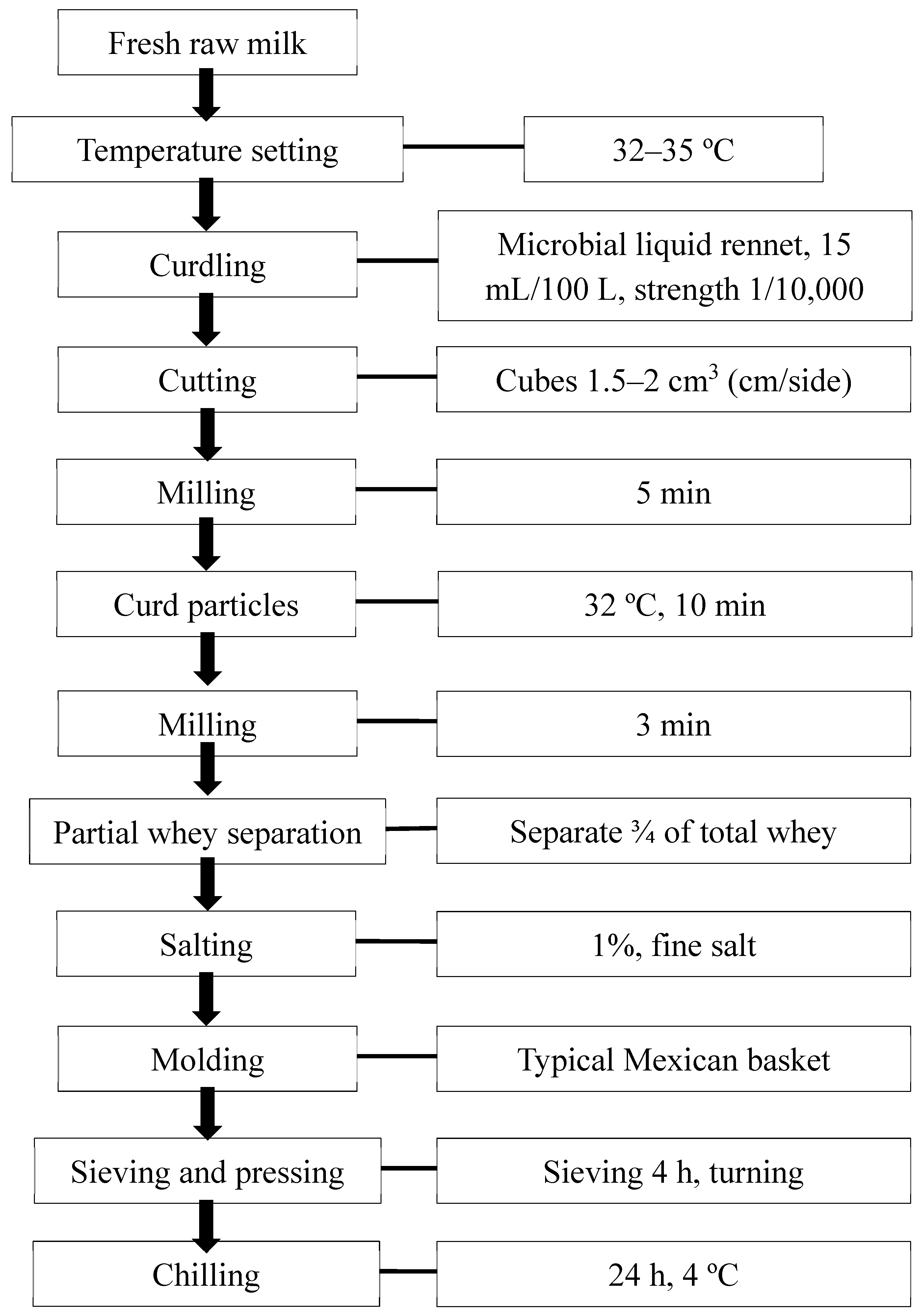
2.2. Panela Cheesemaking
2.3. Actual and Exudate Cheese Yield
2.4. Microbiological Evaluation
2.5. Physicochemical Characteristics
2.6. Data Analyses
3. Results and Discussion
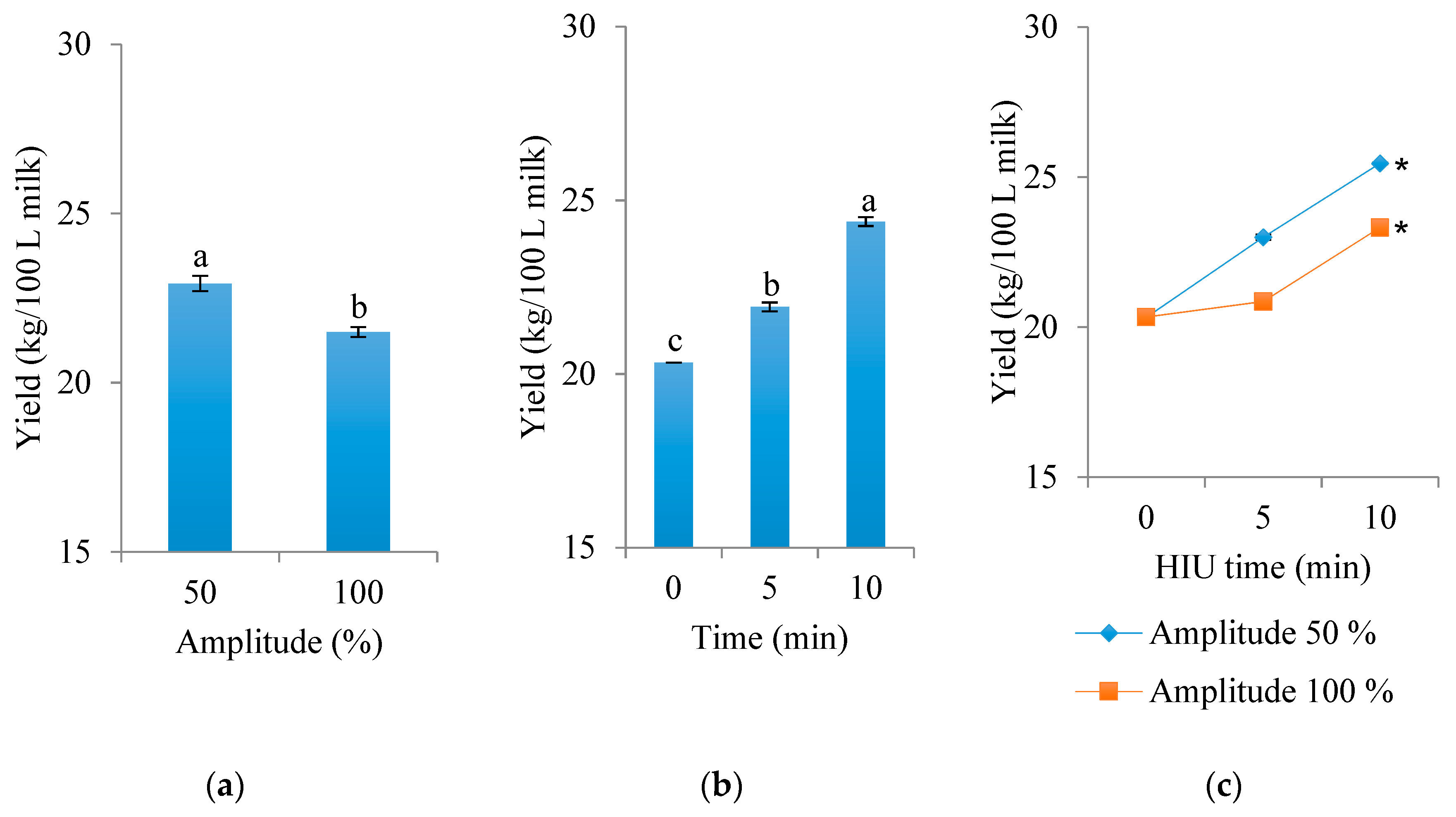
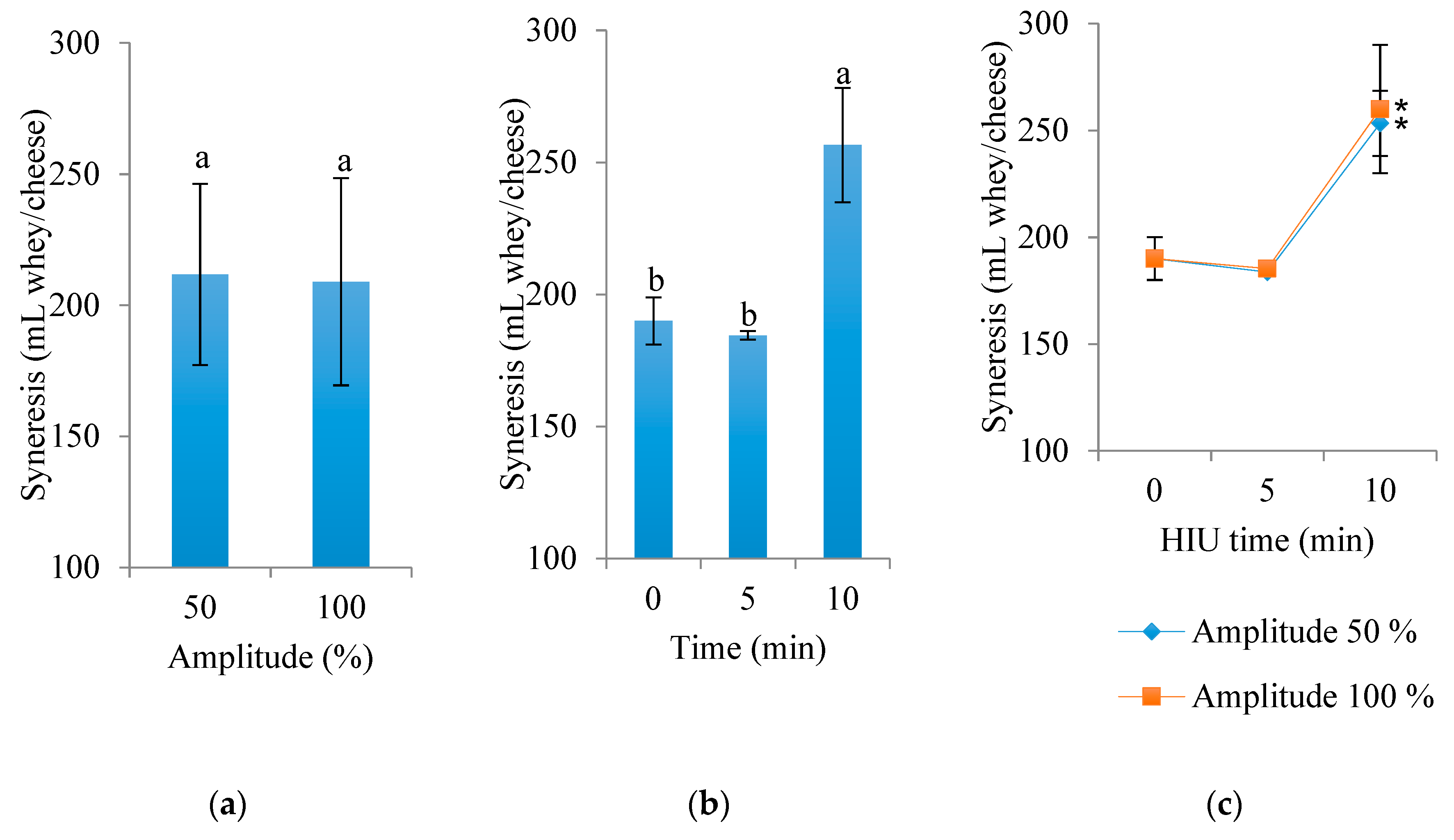
3.1. Actual Yield and Exudate of Cheese
3.2. CIE L*a*b* Color
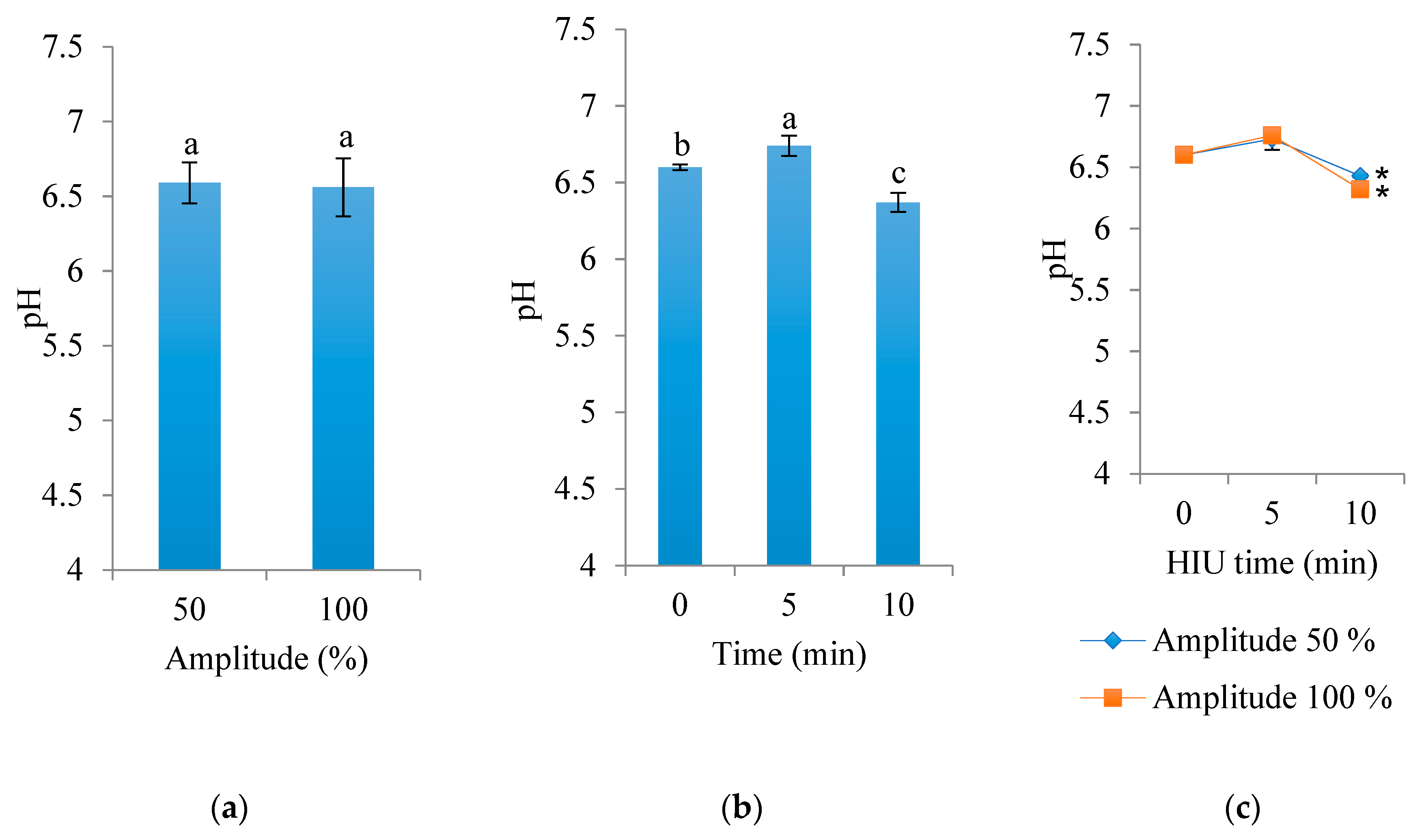
3.3. pH
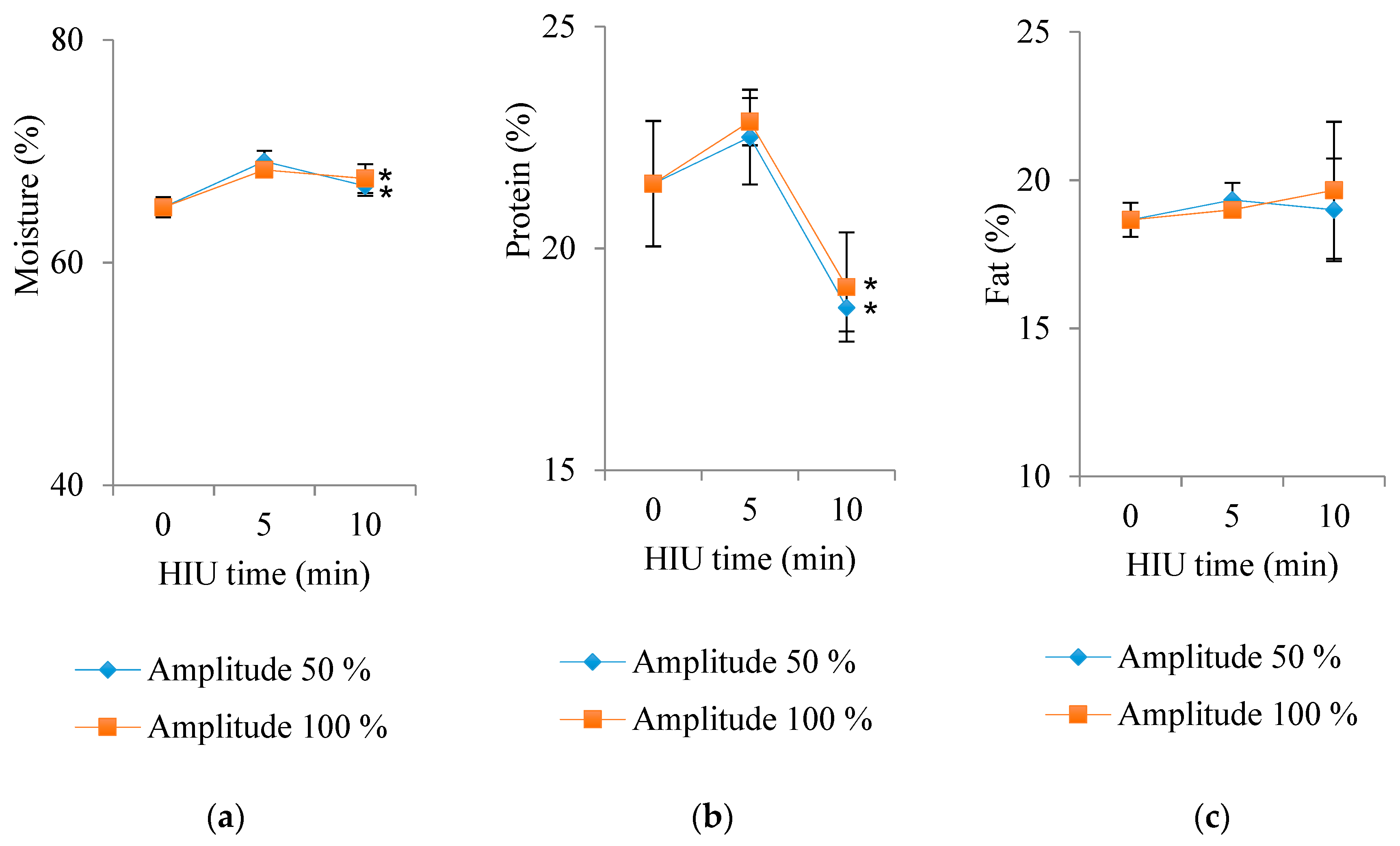
3.4. Water, Protein, and Fat Contents
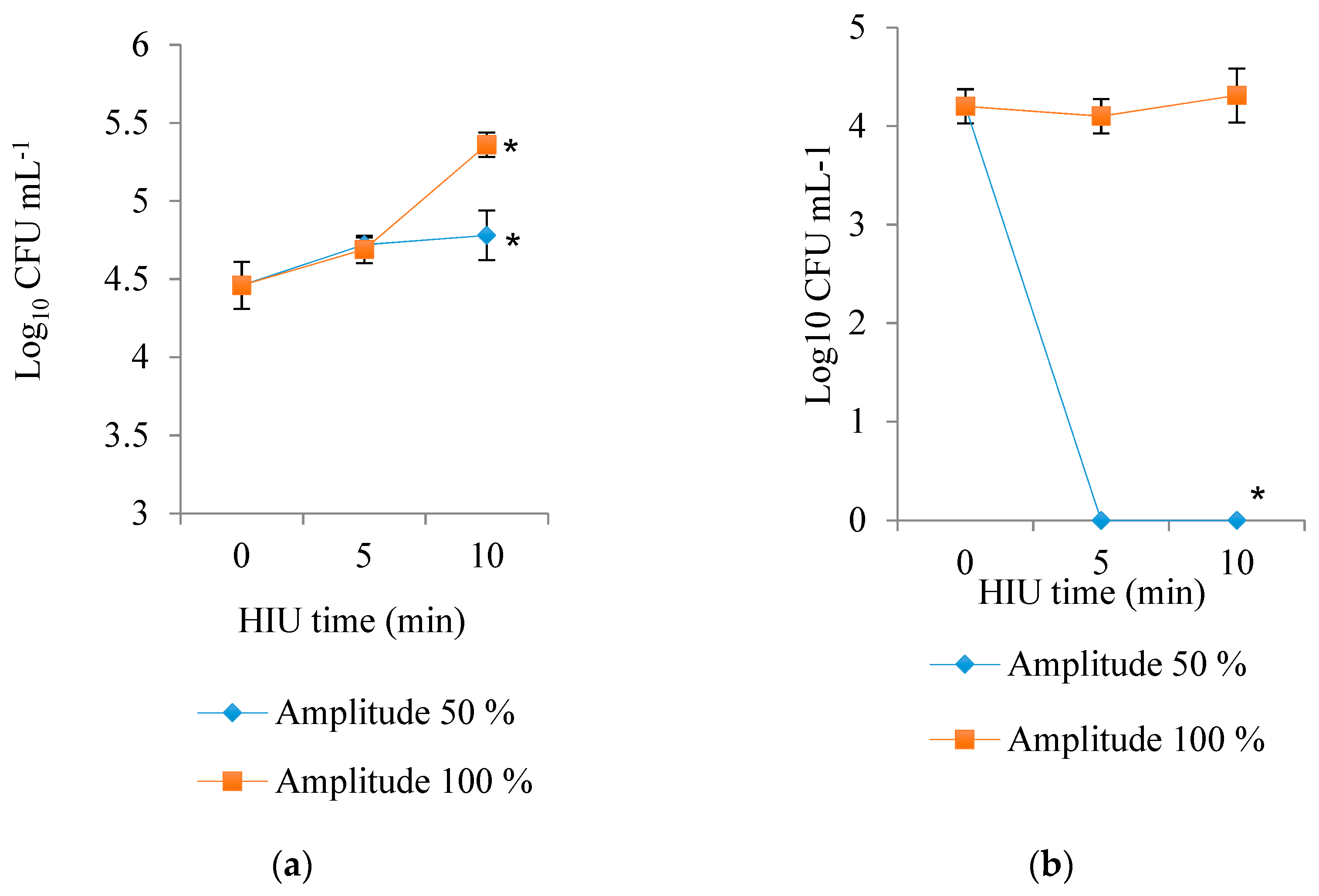
3.5. Microbial Counts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Mason, T.J. Ultrasound processing of fluid foods. In Novel Thermal and Non-Thermal Technologies for Fluid Foods, 1st ed.; Cullen, P.J., Tiwari, B.K., Valdramidis, V.P., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 135–165. [Google Scholar]
- Priego, C.F.; Luque de Castro, M.D. Analytical Applications of Ultrasound, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 1–34. [Google Scholar]
- Patish, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Abesinghe, A.M.N.L.; Islam, N.; Vidanarachchi, J.K.; Prakash, S.; Silva, K.F.S.T.; Karim, M.A. Effects of ultrasound on the fermentation profile of fermented milk products incorporated with lactic acid bacteria. Int. Dairy J. 2019, 90, 1–14. [Google Scholar] [CrossRef]
- Zisu, B.; Chandrapala, J.; Schleyer, M. Application of ultrasound to reduce viscosity and control the rate of age thickening of concentrated skim milk. Int. Dairy J. 2013, 31, 41–43. [Google Scholar] [CrossRef]
- Yanjun, S.; Jianhang, C.; Shuwen, Z.; Hongjuan, L.; Jing, L.; Lu, L.; Uluko, H.; Yanling, S.; Wenming, C.; Wupeng, G.; et al. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014, 124, 11–18. [Google Scholar] [CrossRef]
- Shanmugam, A.; Chandrapala, J.; Ashokkumar, M. The effect of ultrasound on the physical and functional properties of skim milk. Innov. Food Sci. Emerg. Technol. 2012, 16, 251–258. [Google Scholar] [CrossRef]
- Cameron, M.; McMaster, L.D.; Britz, T.J. Electron microscopic analysis of dairy microbes inactivated by ultrasound. Ultrason. Sonochem. 2008, 15, 960–964. [Google Scholar] [CrossRef]
- Chandrapala, J.; Martin, G.J.; Zisu, B.; Kentish, S.; Ashokkumar, M. The effects of ultrasound on casein micelle integrity. J. Dairy Sci. 2012, 95, 6882–6890. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Arellano, M.; Pichot, R.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of dairy proteins. Food Hydrocoll. 2014, 42, 386–396. [Google Scholar] [CrossRef]
- Liu, Z.; Juliano, P.; Williams, R.P.; Niere, J.; Augustin, M.A. Ultrasound improves the renneting properties of milk. Ultrason. Sonochem. 2014, 21, 2131–2137. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, S.; Uluko, H.; Liu, L.; Lu, J.; Xue, H.; Kong, F.; Lv, J. Effect of ultrasound pretreatment on rennet-induced coagulation properties of goat’s milk. Food Chem. 2014, 165, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, O.; Yilmaz, Y.; Gokce, O.; Ertan, K. Effect of ultrasound power on physicochemical and rheological properties of yoghurt drink produced with thermosonicated milk. Emir. J. Food Agric. 2016, 28, 235–241. [Google Scholar] [CrossRef]
- Sfakianakis, P.; Tzia, C. Flavor and sensory characteristics of yogurt derived from milk treated by high intensity ultrasound. In Nutrition, Functional and Sensory Properties of Foods, 1st ed.; Ho, C.-T., Mussinan, C., Shahidi, F., Contis, E., Eds.; Royal Society of Chemistry: Great Britain, UK, 2013; pp. 92–97. [Google Scholar]
- Overton, T.W.; Lu, T.; Bains, N.; Leeke, G.A. Reduction of aerobic and lactic acid bacteria in dairy desludge using an integrated compressed CO2 and ultrasonic process. Dairy Sci. Technol. 2015, 95, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Grewell, D.; Annandarajah, C.; Benner, L.; Clark, S. Quality characteristics and plasmin activity of thermosonicated skim milk and cream. J. Dairy Sci. 2015, 98, 6678–6691. [Google Scholar] [CrossRef]
- Villegas, G.A. Los Quesos Mexicanos, 1st ed.; CIESTAAM: Ciudad de Mexico, Mexico, 1993; pp. 9–68. [Google Scholar]
- Villegas, G.A. Tecnología Quesera, 2nd ed.; Trillas: Mexico City, Mexico, 2012; pp. 135–218. [Google Scholar]
- Bermúdez-Aguirre, D.; Mobbs, T.; Barbosa-Cánovas, G.V. Ultrasound Applications in Food Processing. In Ultrasound Technologies for Food and Bioprocessing, 1st ed.; Feng, H., Barbosa-Cánovas, G., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 65–105. [Google Scholar]
- AOAC Official Method 926.08, Determination of Moisture in Cheese. Available online: http://www.cankaowuzhi.com/document/guowai/AOAC926_08.pdf (accessed on 14 August 2019).
- AOAC Official Method 2001.14, Determination of Nitrogen (Total) in Cheese. Available online: http://www.wdfxw.net›goDownFiles (accessed on 14 August 2019).
- ISO 3433:2008 [IDF 222:2008] Cheese—Determination of fat Content—Van Gulik Method. Available online: https://www.iso.org/standard/46336.html (accessed on 14 August 2019).
- Villamiel, M.; van Hamersveld, E.H.; de Jong, P. Review: Effect of ultrasound processing on the quality of dairy products. Milchwissenschaft 1999, 54, 69–73. [Google Scholar]
- Dufossé, L.; Galaup, L. Color of dairy foods. In Handbook of Dairy Foods Analysis, 1st ed.; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA; pp. 581–602.
- Bermúdez-Aguirre, D.; Mawson, R.; Versteeg, K.; Barbosa-Cánovas, G.V. Composition parameters, physical-chemical characteristics and shelf-life of whole milk after thermal and thermo-sonication treatments. J. Food Qual. 2009, 32, 283–302. [Google Scholar] [CrossRef]
- Ertugay, M.F.; Sengül, M.; Sengül, M. Effect of ultrasound treatment on milk homogenisation and particle size distribution of fat. Turk. J. Vet. Anim. Sci. 2004, 28, 303–308. [Google Scholar]
- Jalilzadeh, A.; Hesari, J.; Peighambardoust, S.H.; Javidipour, I. The effect of ultrasound treatment on microbial and physicochemical properties of Iranian ultrafiltered feta-type cheese. J. Dairy Sci. 2018, 101, 5809–5820. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Guven, M.; Fox, P.F.; McSweeney, P.L. Influence of starters on chemical, biochemical, and sensory changes in Turkish White-brined cheese during ripening. J. Dairy Sci 2005, 88, 3460–3474. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, T.M.; Geurts, T.J. Dairy Science and Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 641–676. [Google Scholar]
- Uluko, H.; Zhang, S.; Liu, L.; Tsakama, M.; Lu, J.; Lv, J. Effect of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J. Funct. Foods 2015, 18, 1138–1146. [Google Scholar] [CrossRef]
- Supeno, P.K. Sonochemical formation of nitrate and nitrite in water. Ultrason. Sonochem. 2000, 7, 109–113. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Lelas, V.; Mason, T.J.; Krešić, G.; Badanjak, M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009, 93, 386–393. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Bhaskaracharya, R.; Kentish, S.; Lee, J.; Palmer, M.; Zisu, B. The ultrasonic processing of dairy products—An overview. Dairy Sci. Technol. 2009, 90, 147–168. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Kentish, S.; Ashokkumar, M. Influence of ultrasound on chemically induced gelation of micellar casein systems. J. Dairy Res. 2013, 80, 138–143. [Google Scholar] [CrossRef]
- Shanmugam, A.; Ashokkumar, M. Functional properties of ultrasonically generated flaxseed oil-dairy emulsions. Ultrason. Sonochem. 2014, 21, 1649–1657. [Google Scholar] [CrossRef]
- Villamiel, M.; de Jong, P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J. Agric. Food Chem. 2000, 48, 472–478. [Google Scholar] [CrossRef]
- Barukčić, I.; Jakopović, K.L.; Herceg, Z.; Karlović, S.; Bozanić, R. Influence of high intensity ultrasound on microbial reduction, physic-chemical characteristics and fermentation of sweet whey. Innov. Food Sci. Emerg. Technol. 2015, 27, 94–101. [Google Scholar] [CrossRef]
- Lucey, J.A.; Munro, P.A.; Singh, H. Rheological properties and microstructure of acid milk gels as affected by fat content and heat treatment. J. Food Sci. 2006, 63, 660–664. [Google Scholar] [CrossRef]
- Sams, A.R.; Feria, R. Microbial Effects of Ultrasonication of Broiler Drumstick Skin. J. Food Sci. 1991, 56, 247–248. [Google Scholar] [CrossRef]
- Pitt, W.G.; Ross, S.A. Ultrasound increases the rate of bacterial cell growth. Biotechnol. Prog. 2003, 19, 1038–1044. [Google Scholar] [CrossRef]
- Herceg, Z.; Juraga, E.; Sobota-Šalamon, B.; Rezek-Jambrak, A. Inactivation of mesophilic bacteria in milk by means of high intensity ultrasound using response surface methodology. Czech. J. Food Sci. 2012, 30, 108–117. [Google Scholar] [CrossRef]
- Ganesan, B.; Martini, S.; Solorio, J.; Walsh, M.K. Determining the effects of high intensity ultrasound on the reduction of microbes in milk and orange juice using response surface methodology. Int. J. Food Sci. 2015, 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing—Food quality assurance and food safety. Trends Food Sci. Technol. 2012, 26, 88–98. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Herceg, Z.; Jambrak, R.; Lelas, V.; Thagard, S.M. The effect of hogh intensity ultrasound treatment on the amount of Staphylococcus aureus and Escherichia coli in milk. Food Technol. Biotechnol. 2012, 50, 46–52. [Google Scholar]
- Cameron, M.; Mcmaster, L.D.; Britz, T.J. Impact of ultrasound on dairy spoilage microbes and milk components. Dairy Sci. Technol. 2009, 89, 83–98. [Google Scholar] [CrossRef]
- Pagán, R.; Mañas, P.; Raso, J.; Condón, S. Bacterial resistance to ultrasonic waves under pressure at Nonlethal (manosonication) and lethal (Manothermosonication) Temperatures. Appl. Environ. Microbiol. 1999, 65, 297–300. [Google Scholar] [CrossRef]
- Noci, F.; Walkling-Ribeiro, D.A.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. Effect of thermosonication, pulsed electric field and their combination on inactivation of Listeria innocua in milk. Intern. Dairy J. 2009, 19, 30–35. [Google Scholar] [CrossRef]
| Titratable Acidity (°D) | Fat (%) | Protein (%) | Lactose (%) | Non-Fatty Solids (%) |
|---|---|---|---|---|
| 16 | 3.87 | 3.11 | 4.79 | 8.6 |
| Factor | CIE Space L*, a*, and b* | ||||
|---|---|---|---|---|---|
| Amplitude (%) | Luminosity (L*) | a* | b* | Hue | Chrome |
| 50 | 92.74 ± 2.77 a | −2.27 ± 1.92 a | 11.37 ± 1.21 a | 101.4 ± 1.46 a | 11.6 ± 2.21 a |
| 100 | 92.4 ± 3.89 a | −2.32 ± 1.61 a | 11.76 ± 1.13 a | 101.1 ± 2.27 a | 12.0 ± 1.82 a |
| Sonication time (min) | |||||
| 0 | 92.49 ± 3.55 a | −2.51 ± 1.76 b | 10.77 ± 1.31 b | 103.1 ± 2.10 a | 11.06 ± 2.08 a |
| 5 | 92.48 ± 3.13 a | −2.63 ± 1.94 b | 11.68 ± 1.31 b | 102.6 ± 2.43 a | 11.97 ± 2.20 a |
| 10 | 92.75 ± 3.13 a | −1.75 ± 1.94 a | 12.25 ± 1.31 a | 98.07 ± 2.43 b | 12.38 ± 2.20 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Lopez, L.M.; Juarez-Morales, M.G.; Garcia-Galicia, I.A.; Alarcon-Rojo, A.D.; Huerta-Jimenez, M. The Effect of High-Intensity Ultrasound on the Physicochemical and Microbiological Properties of Mexican Panela Cheese. Foods 2020, 9, 313. https://doi.org/10.3390/foods9030313
Carrillo-Lopez LM, Juarez-Morales MG, Garcia-Galicia IA, Alarcon-Rojo AD, Huerta-Jimenez M. The Effect of High-Intensity Ultrasound on the Physicochemical and Microbiological Properties of Mexican Panela Cheese. Foods. 2020; 9(3):313. https://doi.org/10.3390/foods9030313
Chicago/Turabian StyleCarrillo-Lopez, Luis M., Monica G. Juarez-Morales, Ivan A. Garcia-Galicia, Alma D. Alarcon-Rojo, and Mariana Huerta-Jimenez. 2020. "The Effect of High-Intensity Ultrasound on the Physicochemical and Microbiological Properties of Mexican Panela Cheese" Foods 9, no. 3: 313. https://doi.org/10.3390/foods9030313
APA StyleCarrillo-Lopez, L. M., Juarez-Morales, M. G., Garcia-Galicia, I. A., Alarcon-Rojo, A. D., & Huerta-Jimenez, M. (2020). The Effect of High-Intensity Ultrasound on the Physicochemical and Microbiological Properties of Mexican Panela Cheese. Foods, 9(3), 313. https://doi.org/10.3390/foods9030313








