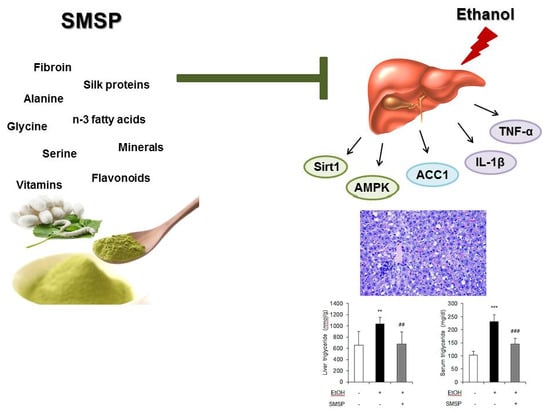
Hepatoprotective Effects of Steamed and Freeze-Dried Mature Silkworm Larval Powder against Ethanol-Induced Fatty Liver Disease in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Steamed Mature Silkworm Larval Powder
2.2. Animal and Experimental Design
2.3. Serum Analysis
2.4. Hepatic Triglyceride Analysis
2.5. Histological Analysis in the Liver
2.6. RNA Isolation and Gene Expression Analysis
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
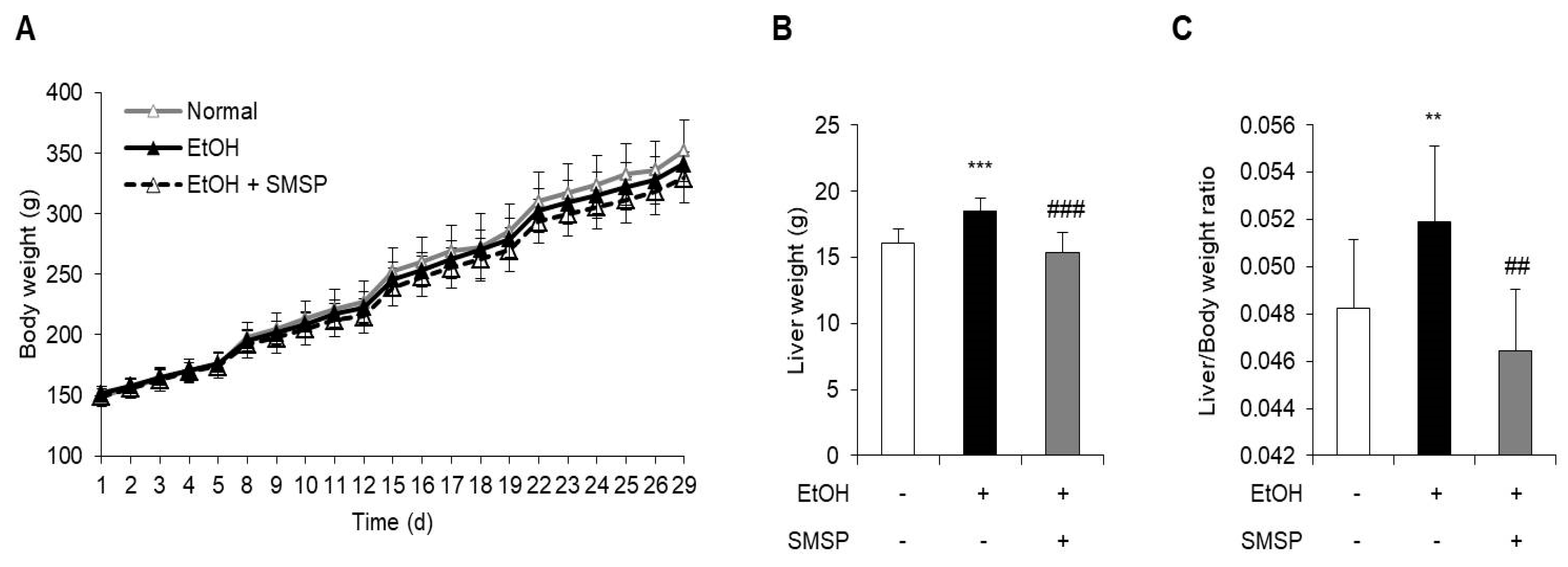
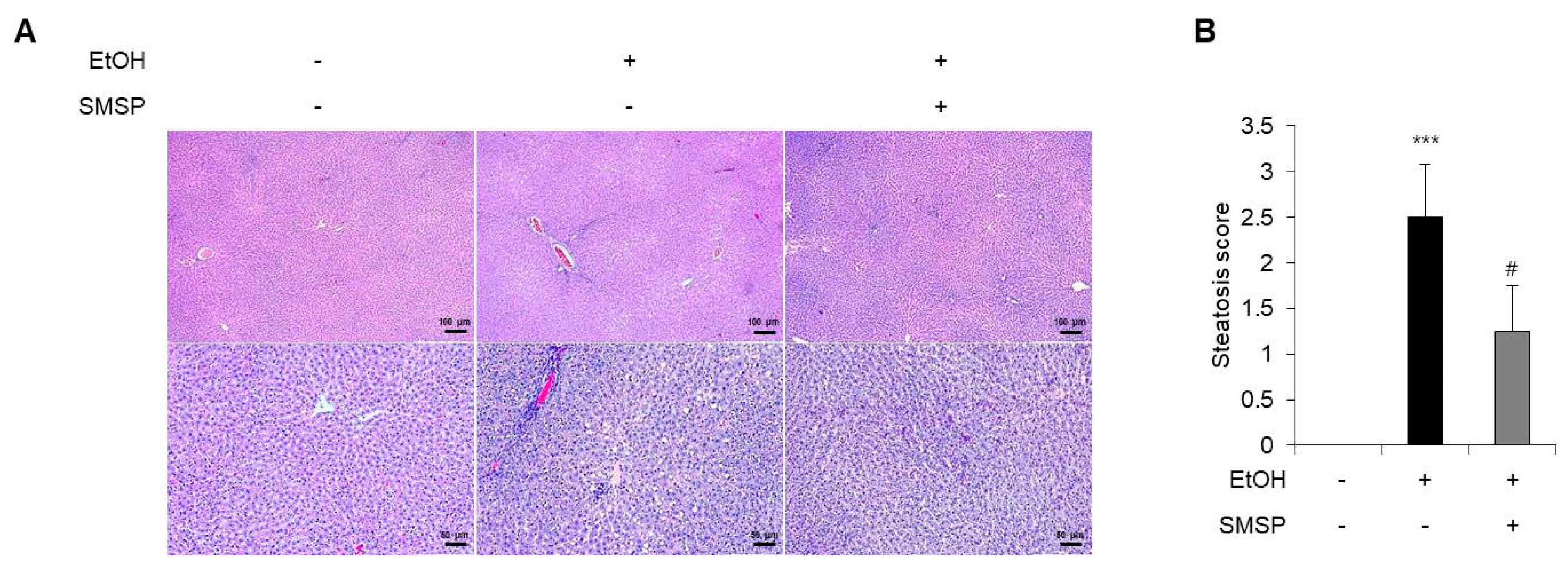
3.1. SMSP Supplementation Alleviates Hepatic Steatosis in Ethanol-Treated Rats
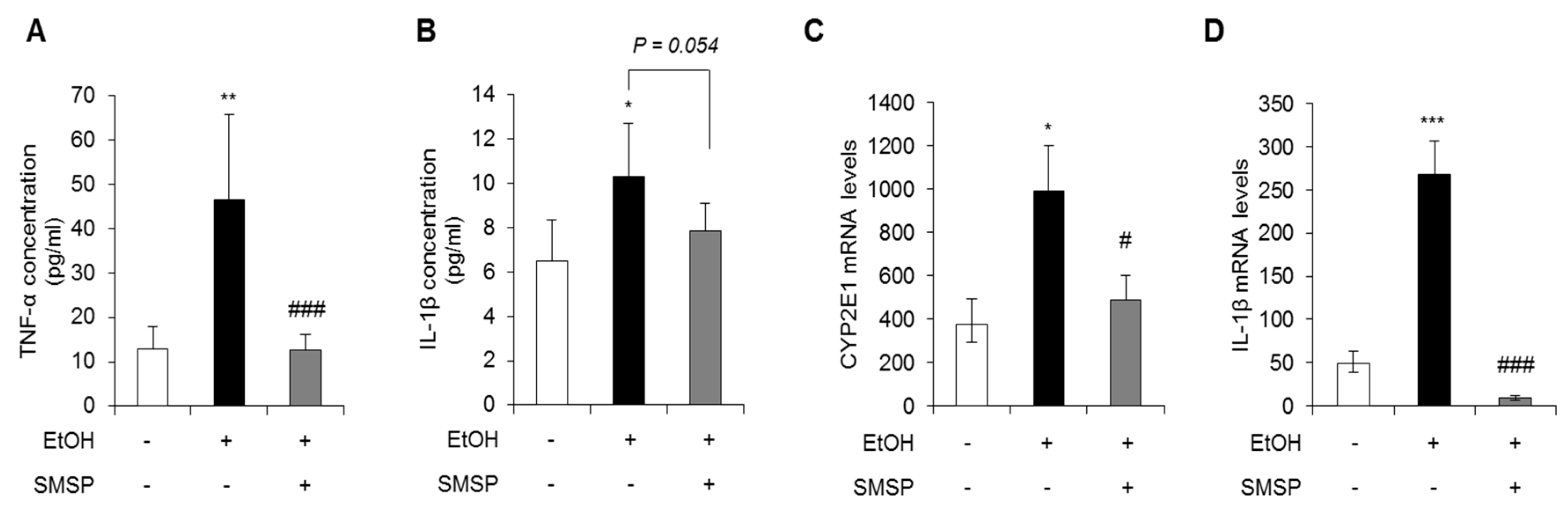
3.2. SMSP Supplementation Attenuates Inflammation in Ethanol-Treated Rats
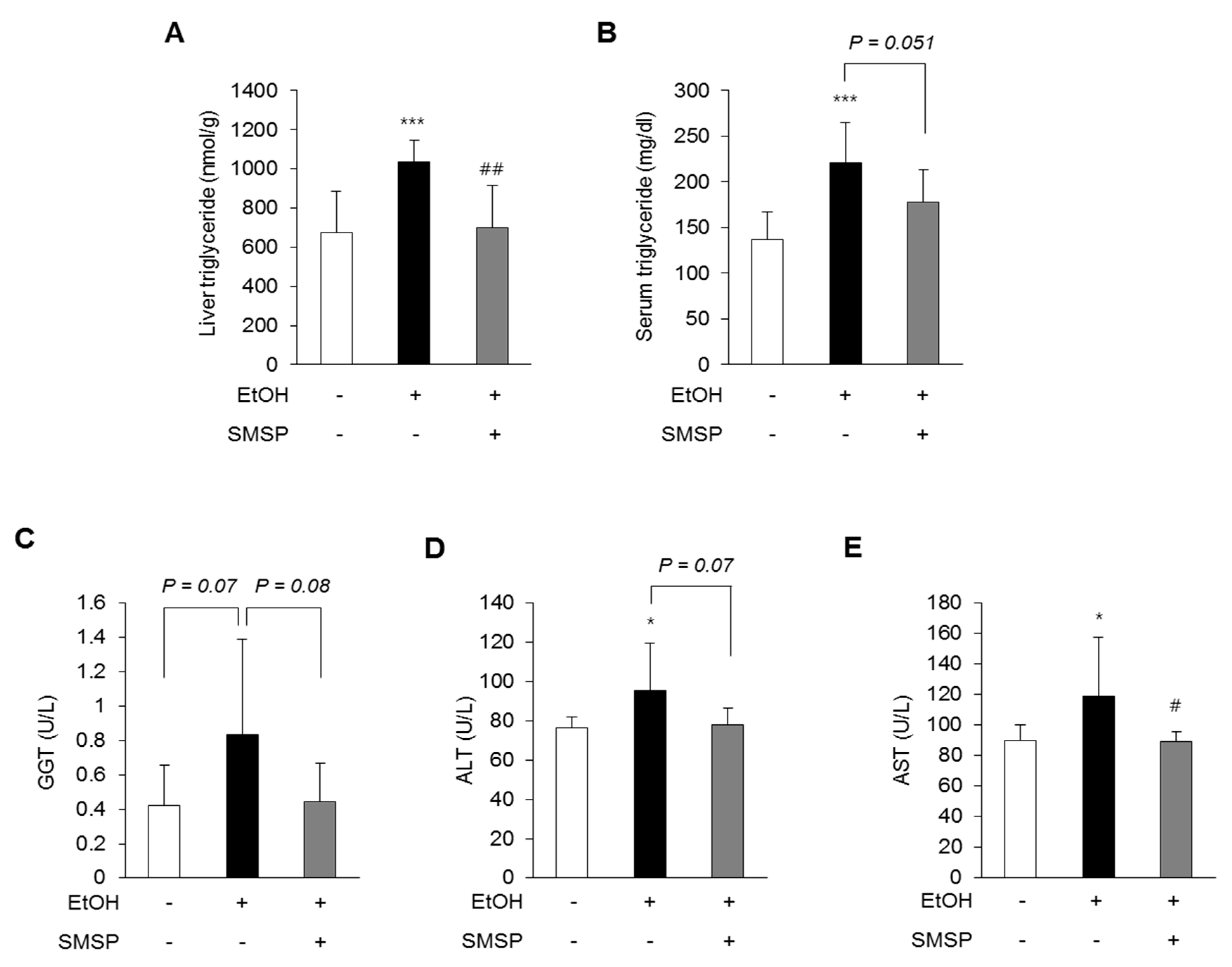
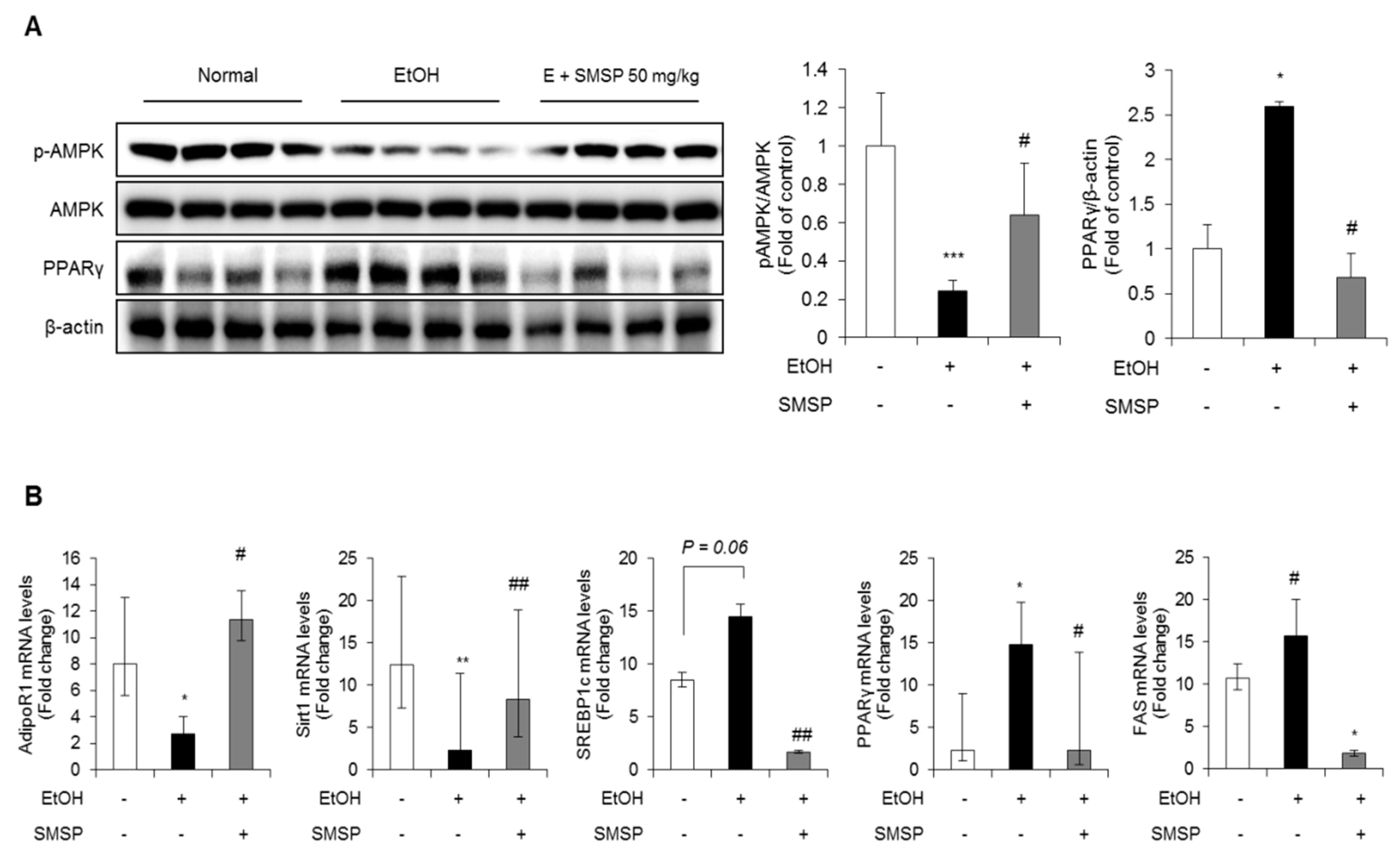
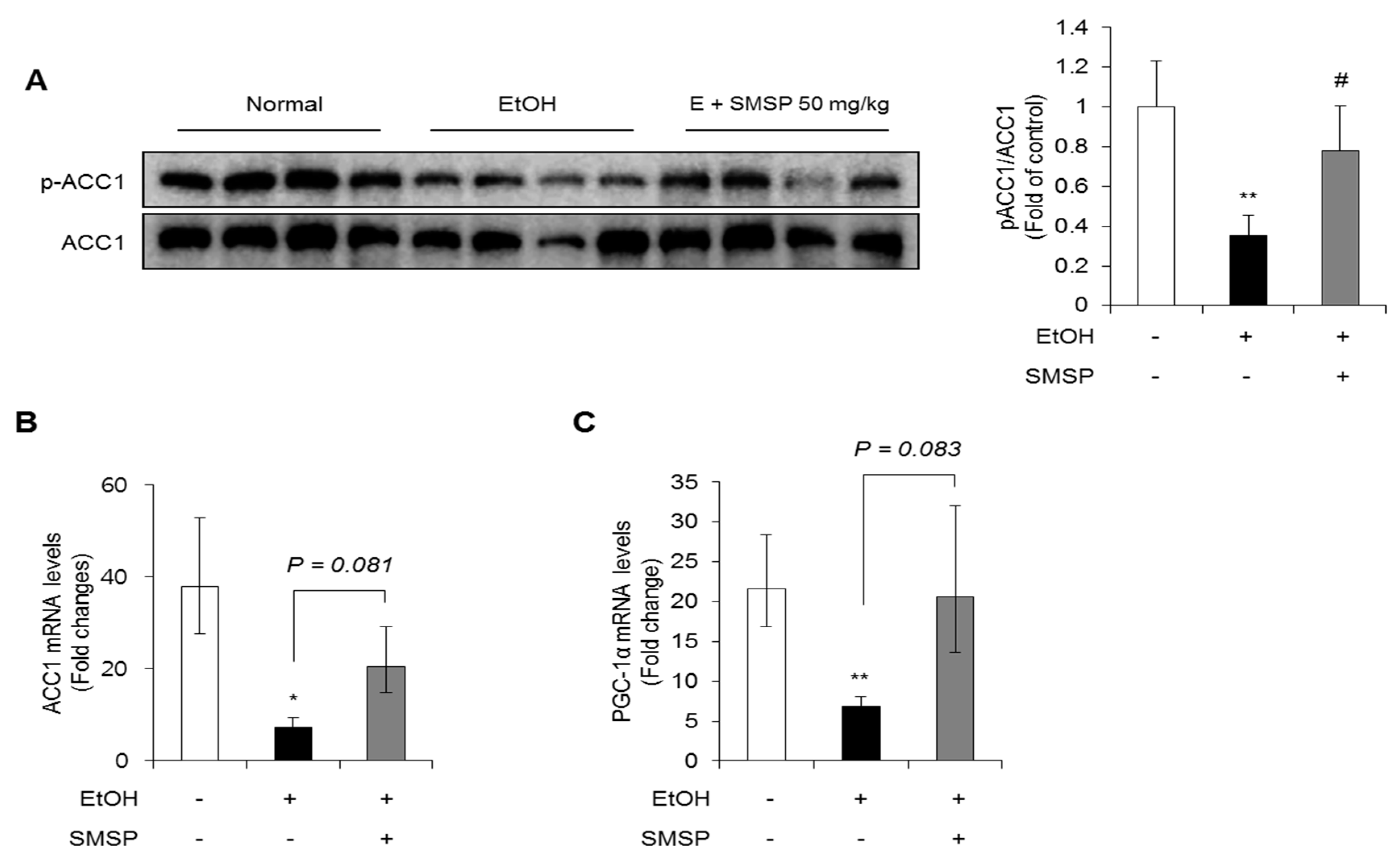
3.3. SMSP Supplementation Improves Lipid Metabolism in Ethanol-Treated Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lucey, M.R.; Schaubel, D.E.; Guidinger, M.K.; Tome, S.; Merion, R.M. Effect of alcoholic liver disease and hepatitis C infection on waiting list and posttransplant mortality and transplant survival benefit. Hepatology 2009, 50, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kawaratani, H.; Tsujimoto, T.; Douhara, A.; Takaya, H.; Moriya, K.; Namisaki, T.; Noguchi, R.; Yoshiji, H.; Fujimoto, M.; Fukui, H. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013, 2013, 495156. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Younossi, Z.M. Treatment options for nonalcoholic fatty liver disease. Therap. Adv. Gastroenterol. 2010, 3, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. (N. Y.) 2015, 11, 167–175. [Google Scholar]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, C.; Zhao, H.; Zhao, C.; Chen, Y.; Wang, Y.; McClain, C.; Feng, W. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J. Nutr. Biochem. 2015, 26, 337–344. [Google Scholar] [CrossRef]
- Park, S.; Gammon, S.; Knippers, J.; Paulsen, S.; Rubink, D.; Winder, W. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J. Appl. Physiol. 2002, 92, 2475–2482. [Google Scholar] [CrossRef]
- Lieber, C.S. Cytochrome P-4502E1: Its physiological and pathological role. Physiol. Rev. 1997, 77, 517–544. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Matsumoto, M.; Pacold, C.M.; Cho, W.K.; Crabb, D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 2004, 127, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Deng, Q.; Kaplowitz, N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology 2004, 40, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Navasa, M.; Gordon, D.A.; Hariharan, N.; Jamil, H.; Shigenaga, J.K.; Moser, A.; Fiers, W.; Pollock, A.; Grunfeld, C.; Feingold, K.R. Regulation of microsomal triglyceride transfer protein mRNA expression by endotoxin and cytokines. J. Lipid Res. 1998, 39, 1220–1230. [Google Scholar]
- Kim, D.W.; Hwang, H.S.; Kim, D.S.; Sheen, S.H.; Heo, D.H.; Hwang, G.; Kang, S.H.; Kweon, H.; Jo, Y.Y.; Kang, S.W.; et al. Effect of silk fibroin peptide derived from silkworm Bombyx mori on the anti-inflammatory effect of Tat-SOD in a mice edema model. BMB Rep. 2011, 44, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.D.; Kim, N.S.; Kweon, H.Y.; Choi, B.H.; Yoon, S.M.; Kim, K.Y.; Koh, Y.H. Nutrient compositions of Bombyx mori mature silkworm larval powders suggest their possible health improvement effects in humans. J. Asia-Pacif. Entomol. 2016, 19, 1027–1033. [Google Scholar] [CrossRef]
- Tabunoki, H.; Ono, H.; Ode, H.; Ishikawa, K.; Kawana, N.; Banno, Y.; Shimada, T.; Nakamura, Y.; Yamamoto, K.; Satoh, J.; et al. Identification of key uric acid synthesis pathway in a unique mutant silkworm Bombyx mori model of Parkinson’s disease. PLoS ONE 2013, 8, e69130. [Google Scholar] [CrossRef]
- Igarashi, K.; Yoshioka, K.; Mizutani, K.; Miyakoshi, M.; Murakami, T.; Akizawa, T. Blood pressure-depressing activity of a peptide derived from silkworm fibroin in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 517–520. [Google Scholar] [CrossRef]
- Ji, S.D.; Kim, N.S.; Lee, J.Y.; Kim, M.J.; Kweon, H.Y.; Sung, G.B.; Kang, P.D.; Kim, K.Y. Development of processing technology for edible mature silkworm. J. Sericult. Entomol. Sci. 2015, 53, 38–43. [Google Scholar]
- Hong, K.S.; Yun, S.M.; Cho, J.M.; Lee, D.Y.; Ji, S.D.; Son, J.G.; Kim, E.H. Silkworm (Bombyx mori) powder supplementation alleviates alcoholic fatty liver disease in rats. J. Funct. Foods 2018, 43, 29–36. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Bae, J.S.; Hahm, K.B.; Cha, J.Y. Endogenously synthesized n-3 polyunsaturated fatty acids in fat-1 mice ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Biochem. Pharmacol. 2012, 84, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Narayan, V.P.; Hong, E.Y.; Whang, W.K.; Park, T. Artemisia Iwayomogi Extract Attenuates High-Fat Diet-Induced Hypertriglyceridemia in Mice: Potential Involvement of the Adiponectin-AMPK Pathway and Very Low Density Lipoprotein Assembly in the Liver. Int. J. Mol. Sci. 2017, 18, 1762. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Addy, C.; Kusunoki, J.; Anderson, N.N.; Deja, S.; Fu, X.; Burgess, S.C.; Li, C.; Ruddy, M.; Chakravarthy, M.; et al. Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab. 2017, 26, 394–406 e396. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Amino acids, peptides, proteins. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 8–91. [Google Scholar]
- Drenjančević, I.; Kralik, G.; Kralik, Z.; Mihalj, M.; Stupin, A.; Novak, S.; Grčević, M. The effect of dietary intake of Omega-3 polyunsaturated fatty acids on cardiovascular health: Revealing potentials of functional food. Superfood Funct. Food-The Dev. Superfoods Their Roles Med. 2017. [Google Scholar] [CrossRef]
- Cho, J.M.; Kim, K.Y.; Ji, S.D.; Kim, E.H. Protective effect of boiled and freeze-dried mature silkworm larval powder against diethylnitrosamine-induced hepatotoxicity in mice. J. Cancer Prev. 2016, 21, 173. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yun, S.M.; Song, M.Y.; Ji, S.D.; Son, J.G.; Kim, E.H. Administration of steamed and freeze-dried mature silkworm larval powder prevents hepatic fibrosis and hepatocellular carcinogenesis by blocking TGF-β/STAT3 signaling cascades in rats. Cells 2020, in press. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Cequera, A.; Garcia de Leon Mendez, M.C. [Biomarkers for liver fibrosis: Advances, advantages and disadvantages]. Rev. Gastroenterol. Mex. 2014, 79, 187–199. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.; Kim, S.H. Green tea extract (Camellia sinensis) fermented by Lactobacillus fermentum attenuates alcohol-induced liver damage. Biosci. Biotechnol. Biochem. 2012, 76, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Hong, K.S.; Yun, S.M.; Song, M.Y.; Ji, S.D.; Son, J.G.; Kim, E.H. Mature silkworm powder reduces blood alcohol concentration and liver injury in ethanol-treated rats. Int. J. Ind. Entomol. 2017, 35, 123–128. [Google Scholar]
- Kubes, P.; Mehal, W.Z. Sterile inflammation in the liver. Gastroenterology 2012, 143, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Wilmer, A.; Vogel, W.; Herold, M.; Nolchen, B.; Judmaier, G.; Huber, C. Serum levels of cytokines in chronic liver diseases. Gastroenterology 1992, 103, 264–274. [Google Scholar] [CrossRef]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.-Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef]
- Lu, Y.; Zhuge, J.; Wang, X.; Bai, J.; Cederbaum, A.I. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology 2008, 47, 1483–1494. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Investig. 2003, 112, 91–100. [Google Scholar] [CrossRef]
- You, M.; Considine, R.V.; Leone, T.C.; Kelly, D.P.; Crabb, D.W. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 2005, 42, 568–577. [Google Scholar] [CrossRef]
- Chen, X.; Sebastian, B.M.; Nagy, L.E. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E621–E628. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, Z.; Deaciuc, I.; Chen, T.; McClain, C.J. Inhibition of adiponectin production by homocysteine: A potential mechanism for alcoholic liver disease. Hepatology 2008, 47, 867–879. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Guo, H.; Yuan, Z.; Wang, T.; Zhang, L.; Jiang, Z. Emodin alleviates hepatic steatosis by inhibiting sterol regulatory element binding protein 1 activity by way of the calcium/calmodulin-dependent kinase kinase-AMP-activated protein kinase-mechanistic target of rapamycin-p70 ribosomal S6 kinase signaling pathway. Hepatol. Res. 2017, 47, 683–701. [Google Scholar] [PubMed]
- Rogers, C.Q.; Ajmo, J.M.; You, M. Adiponectin and alcoholic fatty liver disease. IUBMB Life 2008, 60, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef]
| Gene | Forward | Reverse | Size (bp) |
|---|---|---|---|
| CYP2E1 | AAA CAG GGT AAT GAG GCC CG | AGG CTG GCC TTT GGT CTT TT | 75 |
| IL-1β | CAC CTT CTT TTC CTT CAT CTT TG | GTC GTT GCT TGT CTC TCC TTG TA | 240 |
| AdipoR1 | AGG TCA AAC GTG ACG GCT C | TTA GGC CTG TCG ACT CTC CA | 71 |
| SIRT1 | CCC AGA TCC TCA AGC CAT GTT | TTG TGT GTG TGT TTT TCC CCC | 120 |
| SREBP1c | CCA TGG ACG AGC TAC CCT TC | GGC ACT GGC TCC TCT TTG AT | 382 |
| PPARγ | AGA AGG CTG CAG CGC TAA AT | GGC CTG TTG TAG AGT TGG GT | 405 |
| FAS | TCG ACT TCA AAG GAC CCA GC | GGC AAT ACC CGT TCC CTG AA | 228 |
| ACC1 | CTT GGG GTG ATG CTC CCA TT | GCT GGG CTT AAA CCC CTC AT | 116 |
| PGC-1α | TAA ACT GAG CTA CCC TTG GG | CTC GAC ACG GAG AGT TAA AGG AA | 89 |
| 18s rRNA | GCA ATT ATT CCC CAT GAA CG | GGC CTC ACT AAA CCA TCC AA | 111 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-Y.; Hong, K.-S.; Song, M.-Y.; Yun, S.-M.; Ji, S.-D.; Son, J.-G.; Kim, E.-H. Hepatoprotective Effects of Steamed and Freeze-Dried Mature Silkworm Larval Powder against Ethanol-Induced Fatty Liver Disease in Rats. Foods 2020, 9, 285. https://doi.org/10.3390/foods9030285
Lee D-Y, Hong K-S, Song M-Y, Yun S-M, Ji S-D, Son J-G, Kim E-H. Hepatoprotective Effects of Steamed and Freeze-Dried Mature Silkworm Larval Powder against Ethanol-Induced Fatty Liver Disease in Rats. Foods. 2020; 9(3):285. https://doi.org/10.3390/foods9030285
Chicago/Turabian StyleLee, Da-Young, Kyung-Sook Hong, Moon-Young Song, Sun-Mi Yun, Sang-Deok Ji, Jong-Gon Son, and Eun-Hee Kim. 2020. "Hepatoprotective Effects of Steamed and Freeze-Dried Mature Silkworm Larval Powder against Ethanol-Induced Fatty Liver Disease in Rats" Foods 9, no. 3: 285. https://doi.org/10.3390/foods9030285
APA StyleLee, D.-Y., Hong, K.-S., Song, M.-Y., Yun, S.-M., Ji, S.-D., Son, J.-G., & Kim, E.-H. (2020). Hepatoprotective Effects of Steamed and Freeze-Dried Mature Silkworm Larval Powder against Ethanol-Induced Fatty Liver Disease in Rats. Foods, 9(3), 285. https://doi.org/10.3390/foods9030285