Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Membrane Preparation
2.3. P. granatum Juice Preparation
2.4. Clarification of P. granatum Juice
2.5. Determination of Suspended Solids and Soluble Solids and pH
2.6. Total Phenols, Anthocyanins, Flavonoids, and Ascorbic Acid Content
2.7. In Vitro Antioxidant Activity
2.8. In Vitro Hypoglycaemic Properties
2.9. In Vivo Assay
2.9.1. Animals
2.9.2. Experimental Design
- Group Control (Control, n = 6): mice received 0.2 mL of water to minimize false positive results.
- Group Natural Juice (NJ, n = 6): mice received 500 mg/kg BW of natural juice dissolved into 0.2 mL of water.
- Group Clarified Juice (CJ, n = 6): mice received 500 mg/kg BW of clarified juice dissolved into 0.2 mL of water.
2.9.3. In-Life Evaluations
2.9.4. Laboratory Analyses
2.10. Statistical Analysis
3. Results
3.1. Chemical Profile
3.2. In Vitro Antioxidant and Hypoglycaemic Activities
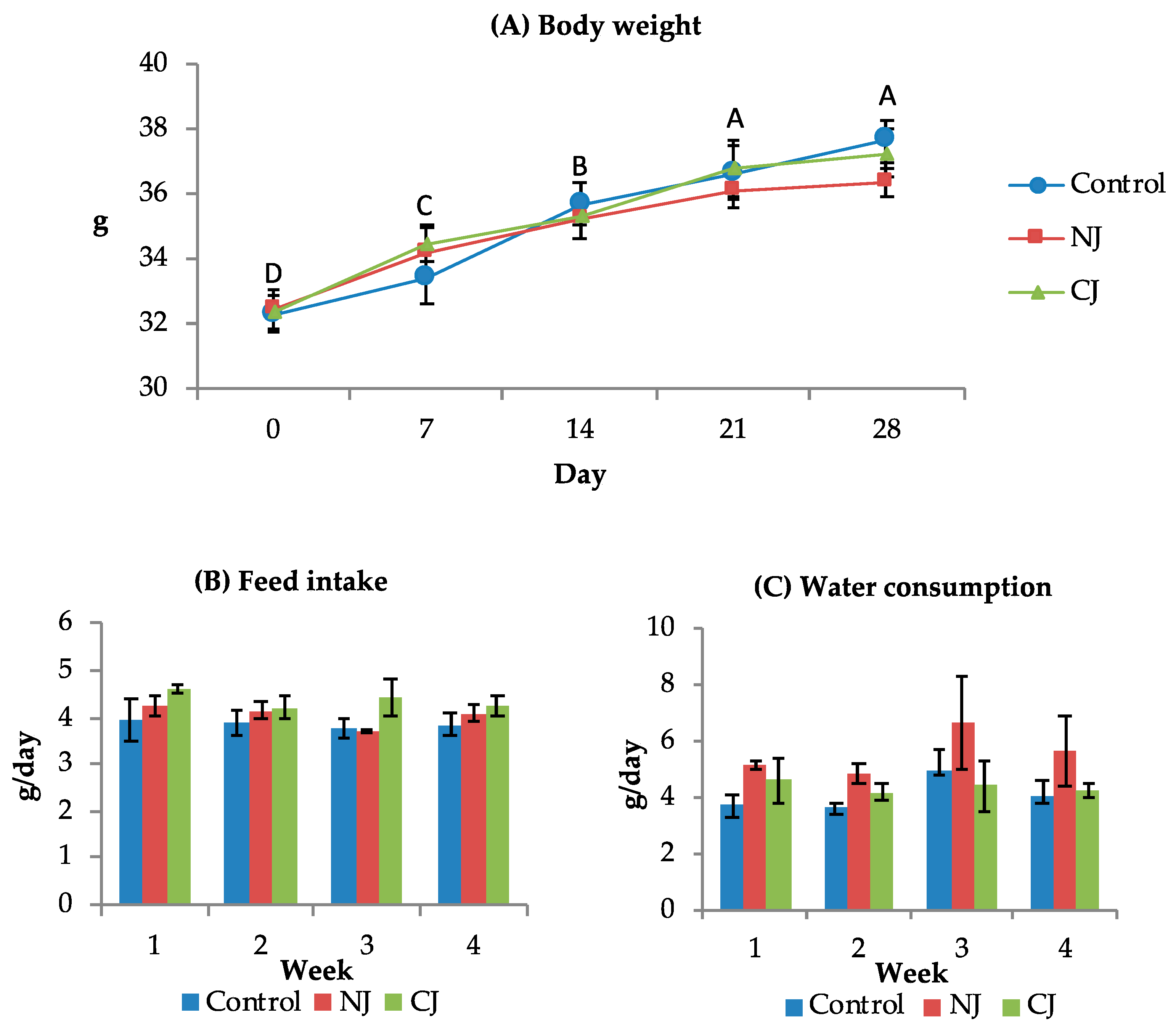
3.3. In-Life Evaluations
3.4. Blood Chemistry Parameters and Oxidative Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holland, D.; Hatib, K.; Bar-Yaakov, I. Pomegranate: Botany, horticulture, breeding. In Horticultural Review; Janick, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 127–191. [Google Scholar] [CrossRef]
- Vidal, A.; Fallarero, A.; Peña, B.R.; Medina, M.E.; Gra, B.; Rivera, F.; Gutierrez, Y.; Vuorela, P.M. Studies on the toxicity of Punica granatum L. (Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2003, 89, 295–300. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001, 158, 195–198. [Google Scholar] [CrossRef]
- Longtin, R. The pomegranate: Nature’s power fruit? J. Nat. Cancer Inst. 2003, 95, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Al-Jarallah, A.; Igdoura, F.; Zhang, Y.; Tenedero, C.B.; White, E.J.; MacDonald, M.E.; Igdoura, S.A.; Trigatti, B.L. The effect of pomegranate extract on coronary artery atherosclerosis in SR-BI/APOE double knockout mice. Atherosclerosis 2003, 228, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kılıçgün, H.; Arda, N.; Uçar, E.Ö. Identification of longevity, fertility and growth-promoting properties of pomegranate in Caenorhabditis elegans. Pharmacogn. Mag. 2015, 11, 356–359. [Google Scholar] [CrossRef]
- Zaouay, F.; Mena, P.; Garcia-Viguera, C.; Mars, M. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind. Crops Prod. 2012, 40, 81–89. [Google Scholar] [CrossRef]
- Chalfoun-Mounayar, A.; Nemr, R.; Yared, P.; Khairallah, S.; Chahine, R. Antioxidant and weight loss effects of pomegranate molasses. J. Appl. Pharm. Sci. 2012, 2, 45–50. [Google Scholar] [CrossRef]
- Rosenblat, M.; Volkova, N.; Coleman, R.; Aviram, M. Pomegranate byproduct administration to apolipoprotein E-deficient mice attenuates atherosclerosis development as a result of decreased macrophage oxidative stress and reduced cellular uptake of oxidized low-density lipoprotein. J. Agric. Food Chem. 2006, 54, 1928–1935. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef]
- Baklouti, S.; Ellouze-Ghorbel, R.; Mokni, A.; Chaabouni, S. Clarification of pomegranate juice by ultrafiltration: Study of juice quality and of the fouling mechanism. Fruits 2012, 67, 215–225. [Google Scholar] [CrossRef][Green Version]
- Bagci, P.O. Effective clarification of pomegranate juice: A comparative study of pretreatment methods and their influence on ultrafiltration flux. J. Food Eng. 2014, 141, 58–64. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Tasselli, F. Clarification of pomegranate juice (Punica granatum L.) by hollow fibre membranes: Analyses of membrane fouling and performance. J. Chem. Technol. Biotechnol. 2015, 90, 859–866. [Google Scholar] [CrossRef]
- Echavarria, A.P.; Torras, C.; Pagan, J.; Ibarz, A. Fruit juice processing and membrane technology application. Food Eng. Rev. 2011, 3, 136–158. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Clarification and concentration of pomegranate juice (Punica granatum L.) using membrane processes. J. Food Eng. 2011, 107, 366–373. [Google Scholar] [CrossRef]
- Onsekizoglu, P. Production of high quality clarified pomegranate juice concentrate by membrane processes. J. Membr. Sci. 2013, 442, 264–271. [Google Scholar] [CrossRef]
- Aliasghari Aghdam, M.; Mirsaeedghazi, H.; Aboonajmi, M.; Kianmehr, M.H. The effect of ultrasound waves on the efficiency of membrane clarification of pomegranate juice. Int. J. Food Sci. Technol. 2015, 50, 892–898. [Google Scholar] [CrossRef]
- Galiano, F.; Figoli, A.; Conidi, C.; Menichini, F.; Bonesi, M.; Loizzo, M.R.; Cassano, A.; Tundis, R. Functional properties of Punica granatum L. juice clarified by hollow fiber membranes. Processes 2016, 4, 21. [Google Scholar] [CrossRef]
- Colantuono, A.; Vitaglione, P.; Manzo, N.; Blaiotta, G.; Montefusco, I.; Marrazzo, A.; Pizzolongo, F.; Romano, R. Evaluation of microfiltration and heat treatment on the microbiological characteristics, phenolic composition and volatile compound profile of pomegranate (Punica granatum L.) juice. J. Sci. Food Agric. 2018, 98, 3324–3332. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible flowers: A rich source of phytochemicals with antioxidant and hypoglycemic properties. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef]
- Klein, B.P.; Perry, A.K. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J. Food Sci. 1982, 47, 941–945. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; De Luca, D.; de Cindio, B.; Menichini, F. Comparative study on the chemical composition, antioxidant properties and hypoglycaemic activities of two Capsicum annuum L. cultivars (Acuminatum small and Cerasiferum). Plant Foods Hum. Nutr. 2011, 66, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; Menichini, F.; Loizzo, M.R. Antioxidant and hypoglycaemic activities and their relationship to phytochemicals in Capsicum annuum cultivars during fruit development. LWT-Food Sci. Technol. 2013, 53, 370–377. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Mousavi, S.M.; Aroujalian, A.; Navidbakhsh, M. Clarification of pomegranate juice by microfiltration with PVDF membranes. Desalination 2010, 3, 243–248. [Google Scholar] [CrossRef]
- Qin, G.; Lü, X.; Wei, W.; Li, J.; Cui, R.; Hu, S. Microfiltration of kiwifruit juice and fouling mechanism sing fly-ash-based ceramic membranes. Food Bioprod. Process. 2015, 96, 278–284. [Google Scholar] [CrossRef]
- Valero, M.; Vegara, S.; Martí, N.; Saura, D. Clarification of pomegranate juice at industrial scale. J. Food Process. Technol. 2014, 5, 324–330. [Google Scholar] [CrossRef]
- Al-Muammar, M.N.; Khan, F. Obesity: The preventive role of the pomegranate (Punica granatum). Nutrition 2012, 28, 595–604. [Google Scholar] [CrossRef]
- Patel, C.; Dadhaniya, P.; Hingorani, L.; Soni, M.G. Safety assessment of pomegranate fruit extract: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2008, 46, 2728–2735. [Google Scholar] [CrossRef]
- Cerdá, B.; Llorach, R.; Cerón, J.J.; Espin, J.C.; Tomas-Barberan, F.A. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur. J. Nutr. 2003, 42, 18–28. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Morittu, V.M.; Musco, N.; Mastellone, V.; Bonesi, M.; Britti, D.; Infascelli, F.; Loizzo, R.L.; Tundis, R.; Sicari, V.; Tudisco, R.; et al. In vitro and in vivo studies of Cucurbita pepo L. flowers: Chemical profile and bioactivity. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Morittu, V.M.; Pero, M.E.; Musco, N.; Mastellone, V.; Tudisco, R.; Provenzano, E.; Britti, D.; Menichini, F.; Infascelli, F.; Lombardi, P. Potential beneficial and/or adverse effects of Capsicum annuum L. (cv. Fiesta) at two stage of ripening in CD-1 mice. Nat. Prod. Res. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Swendseid, M.E. Plasma beta-carotene response in humans after meals supplemented with dietary pectin. Am. J. Clin. Nutr. 1992, 55, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Shishavan, N.G.; Abbasi, M.M.; Afshar, R.A.; Milani, P.Z.; Yahyavi, F. The effects of pomegranate (Punica granatum L.) peel methanolic extract on methotrexate induced changes in hepatic antioxidant enzymes of rats. Jundishapur. J. Nat. Pharm. Prod. 2017, 12, e57499. [Google Scholar] [CrossRef]
- Razani, Z.; Dastani, M.; Kazerani, H.R. Cardioprotective effects of pomegranate (Punica granatum) juice in patients with ischemic heart disease. Phytother. Res. 2017, 31, 1731–1738. [Google Scholar] [CrossRef]
- Moneim, A.E.; Dkhil, M.A.; Al-Quraishy, S. Studies on the effect of pomegranate (Punica granatum) juice and peel on liver and kidney in adult male rats. J. Med. Plants Res. 2011, 5, 5083–5088. [Google Scholar]
- Esmaillzadeh, A.; Tahbaz, F.; Gaieni, I.; Alavi-Majd, H.; Azadbakht, L. Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. Int. J. Vitamin Nutr. Res. 2006, 76, 147–151. [Google Scholar] [CrossRef]
| Parameter | Natural Juice | Clarified Juice |
|---|---|---|
| pH | 4.1 ± 0.1 | 4.0 ± 0.1 |
| Suspended solids (% w/w) | 4.2 ± 0.1 | - |
| Total soluble solids (°Brix) | 22.1 ± 0.4 | 21.9 ± 0.4 |
| Total phenols a | 1989.7 ± 28.3 | 1919.1 ± 32.3 |
| Total flavonoids b | 288.7 ± 7.1 | 276.8 ± 6.2 |
| Total anthocyanins c | 121.3 ± 3.1 | 116.1 ± 3.5 |
| Ascorbic acid d | 132.0 ± 2.5 | 90.0 ± 1.8 |
| Sample | Antioxidant Activity | Hypoglycemic Activity | ||||
|---|---|---|---|---|---|---|
| DPPH Test (IC50 µg/mL) | FRAP Test # (µM Fe(II)/g) | β-Carotene Bleaching Test (IC50 µg/mL) | α-Amylase Inhibitory Assay (IC50 µg/mL) | α-Glucosidase Inhibitory Assay (IC50 µg/mL) | ||
| 30 min | 60 min | |||||
| NJ | 782.6 ± 3.7 A | 5.1 ± 1.0 C | 51.5 ± 1.9 A | 55.7 ± 1.7 A | 67.1 ± 2.3 B | 80.1 ± 2.8 A |
| CJ | 734.2 ± 2.8 B | 14.1 ± 0.6 B | 19.7 ± 1.1 B | 44.1 ± 1.0 B | 76.6 ± 2.2 A | 68.6 ± 2.0 B |
| Positive control * | 5.0 ± 0.8 C | 63.2 ± 4.5 A | 1.0 ± 0.04 C | 1.0 ± 0.03 C | 50.0 ± 0.9 C | 35.5 ± 1.2 C |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| R squared | 1.000 | 0.9927 | 0.9975 | 0.9984 | 0.9803 | 0.9918 |
| Item | Control | NJ | CJ | P | R squared | ||||
|---|---|---|---|---|---|---|---|---|---|
| ALT | U/L | 61 ± 3.4 | A | 49 ± 5.9 | B | 53 ± 8.0 | AB | 0.0081 | 0.473 |
| AST | U/L | 73 ± 6.6 | A | 59 ± 5.1 | B | 67 ± 6.9 | AB | 0.0058 | 0.497 |
| ALP * | U/L | 90 ± 43.8 | 158 ± 48.0 | 134 ± 58.4 | 0.0802 | 0.276 | |||
| TP | g/dL | 5.7 ± 0.5 | 5.7 ± 0.1 | 6.1 ± 0.3 | 0.1627 | 0.231 | |||
| ALB | mg/dL | 3.3 ± 0.3 | 3.4 ± 0.2 | 3.3 ± 0.1 | 0.6976 | 0.047 | |||
| UREA | mg/dL | 111 ± 6.3 | A | 91 ± 14.6 | A | 69 ± 17.8 | B | 0.0003 | 0.659 |
| CREA * | mg/dL | 0.24 ± 0.1 | 0.25 ± 0.0 | 0.26 ± 0.1 | 0.6925 | 0.011 | |||
| LDH | U/L | 3458 ± 1250 | 2094 ± 852 | 3429 ± 1607 | 0.1405 | 0.230 | |||
| CPK | U/L | 341 ± 192 | A | 80 ± 42 | B | 91 ± 19 | B | 0.0017 | 0.573 |
| CHOL * | mg/dL | 210 ± 12 | 200 ± 14 | 210 ± 39 | 0.8026 | 0.039 | |||
| TRIG | mg/dL | 335 ± 46 | a | 295 ± 59 | ab | 248 ± 44 | b | 0.0279 | 0.379 |
| d-ROMs | U CARR | 151 ± 30 | a | 149 ± 35 | a | 102 ± 20 | b | 0.0187 | 0.412 |
| BAP | μmol/L | 3896 ± 277 | b | 4607 ± 772 | ab | 4813 ± 229 | a | 0.0322 | 0.434 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morittu, V.M.; Mastellone, V.; Tundis, R.; Loizzo, M.R.; Tudisco, R.; Figoli, A.; Cassano, A.; Musco, N.; Britti, D.; Infascelli, F.; et al. Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane. Foods 2020, 9, 242. https://doi.org/10.3390/foods9020242
Morittu VM, Mastellone V, Tundis R, Loizzo MR, Tudisco R, Figoli A, Cassano A, Musco N, Britti D, Infascelli F, et al. Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane. Foods. 2020; 9(2):242. https://doi.org/10.3390/foods9020242
Chicago/Turabian StyleMorittu, Valeria Maria, Vincenzo Mastellone, Rosa Tundis, Monica Rosa Loizzo, Raffaella Tudisco, Alberto Figoli, Alfredo Cassano, Nadia Musco, Domenico Britti, Federico Infascelli, and et al. 2020. "Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane" Foods 9, no. 2: 242. https://doi.org/10.3390/foods9020242
APA StyleMorittu, V. M., Mastellone, V., Tundis, R., Loizzo, M. R., Tudisco, R., Figoli, A., Cassano, A., Musco, N., Britti, D., Infascelli, F., & Lombardi, P. (2020). Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane. Foods, 9(2), 242. https://doi.org/10.3390/foods9020242











