Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Phenolics Extracts
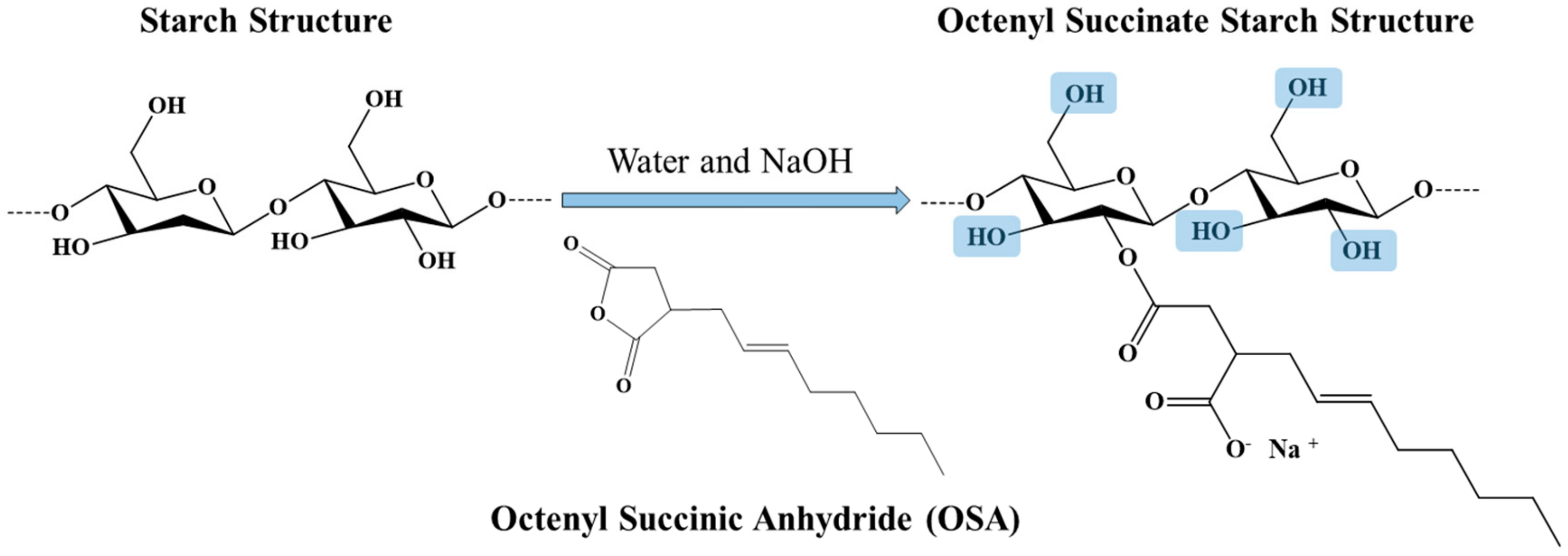
2.3. Preparation of Octenyl Succinate Starch
2.4. Preparation of Films
2.5. Characterization of Films
2.5.1. Water Resistance Properties
2.5.2. Optical Properties
2.5.3. Mechanical Properties
2.5.4. Biodegradability
2.5.5. Morphological and Structural Analysis
2.5.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Phenolic Extracts
3.2. The Thickness and Water Barrier Properties
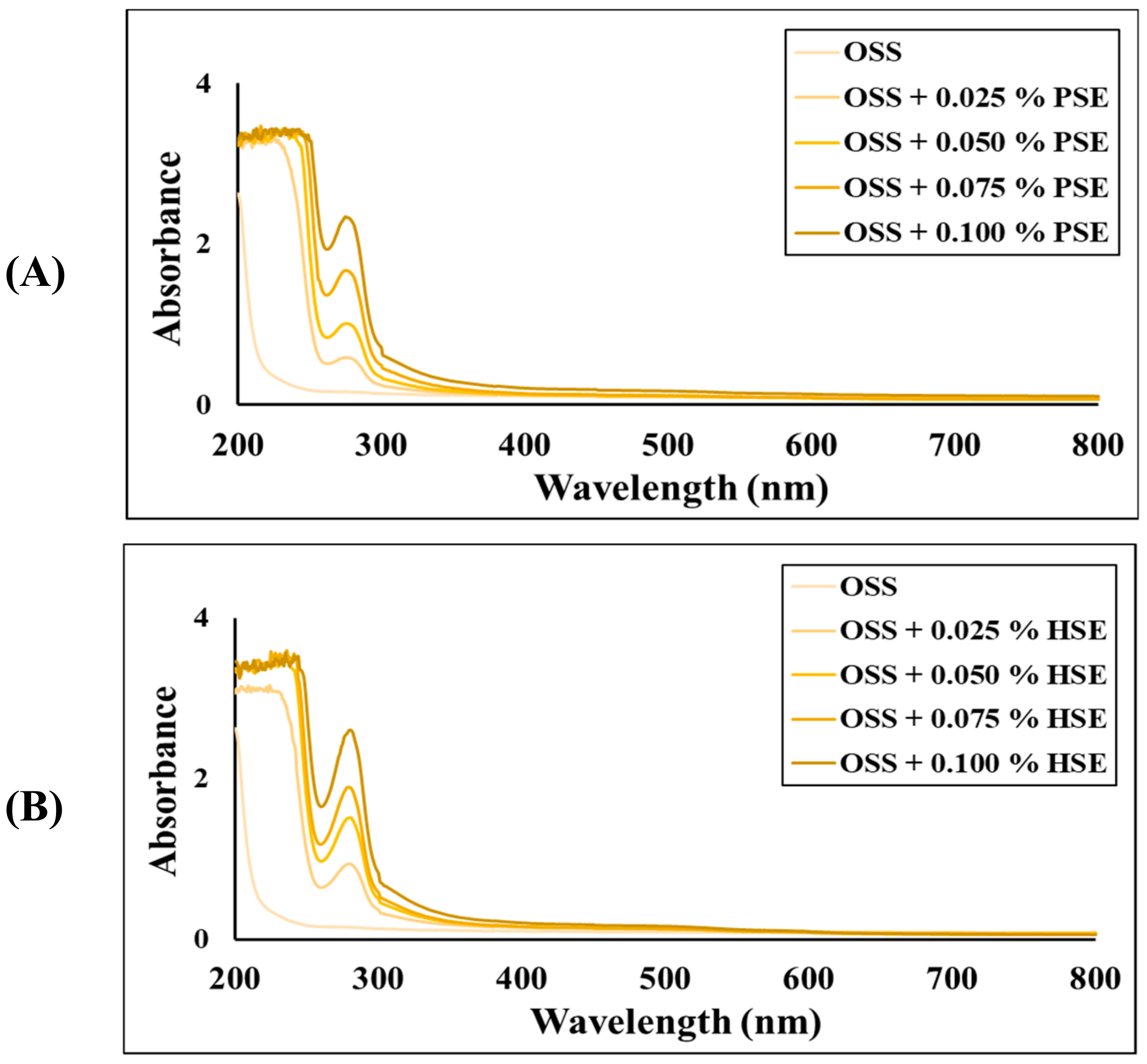
3.3. Optical Properties
3.4. Mechanical Properties
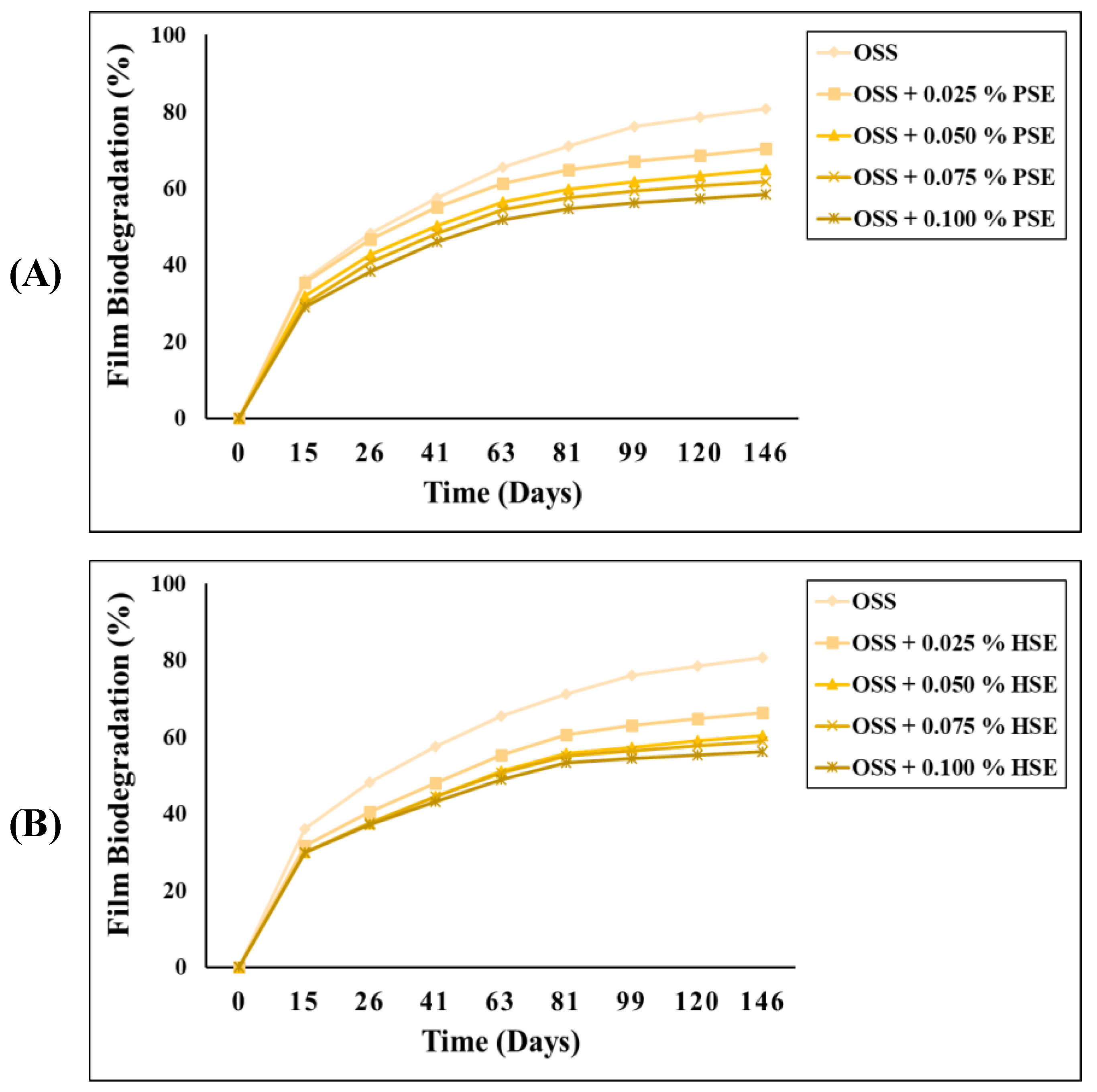
3.5. Biodegradability
3.6. Morphology Analysis
3.7. Structural Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gadhave, R.V.; Das, A.; Mahanwar, P.A.; Gadekar, P.T. Starch based bio-plastics: The future of sustainable packaging. Open J. Polym. Chem. 2018, 8, 21–33. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and science behind modified starch edible films and coatings: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. Starch Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Hemamalini, T.; Giri Dev, V.R. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.; Wang, L.; ul Abdin, Z.; Chen, Y.; Wang, J.; Zhou, W.; Yang, X.; Khan, R.U.; Zhang, H.; et al. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015, 5, 67459–67474. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Liu, J.; Zhao, G. Effects of octenylsuccination on physical, mechanical and moisture-proof properties of stretchable sweet potato starch film. Food Hydrocoll. 2015, 46, 226–232. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Lei, L.; Zhao, G. Combined effects of octenylsuccination and oregano essential oil on sweet potato starch films with an emphasis on water resistance. Int. J. Biol. Macromol. 2018, 115, 547–553. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- De Araújo, G.K.P.; de Souza, S.J.; da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Medina Jaramillo, C.; González Seligra, P.; Goyanes, S.; Bernal, C.; Famá, L. Biofilms based on cassava starch containing extract of yerba mate as antioxidant and plasticizer. Starch Stärke 2015, 67, 780–789. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.A.; dos Santos, D.F.; Pilatti-Riccio, D.; Deon, V.G.; dos Santos, G.H.F.; Pinto, V.Z. Yerba mate extract in active starch films: Mechanical and antioxidant properties. J. Food Process. Preserv. 2019, 43, e13897. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica Júnior, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Bolling, B.W.; Shahidi, F. Nuts and their co-products: The impact of processing (roasting) on phenolics, bioavailability, and health benefits—A comprehensive review. J. Funct. Foods 2016, 26, 88–122. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; Urrea-López, R.; de la Rosa, L.A. Bioactive components and health effects of pecan nuts and their byproducts: A review. J. Food Bioact. 2018, 1, 56–92. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Yılmaz, C.; Durmaz, G.; Gökmen, V. Hazelnut skin powder: A new brown colored functional ingredient. Food Res. Int. 2014, 65, 291–297. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Liao, M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind. Crop. Prod. 2013, 49, 837–843. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Ovando-Martinez, M.; Whitney, K.; Ozsisli, B.; Simsek, S. Physicochemical Properties of Octenyl Succinic Esters of Cereal, Tuber and Root Starches. J. Food Process. Preserv. 2017, 41, e12872. [Google Scholar] [CrossRef]
- Whitney, K.; Reuhs, B.L.; Ovando Martinez, M.; Simsek, S. Analysis of octenylsuccinate rice and tapioca starches: Distribution of octenylsuccinic anhydride groups in starch granules. Food Chem. 2016, 211, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Flores, P.B.; Ochoa-Reyes, E.; Ornelas-Paz, J.D.J.; Tirado-Gallegos, J.M.; Bello-Pérez, L.A.; Rubio-Ríos, A.; Cárdenas-Felix, R.G. Caracterización fisicoquímica, mecánica y estructural de películas de almidones oxidados de avena y plátano adicionadas con betalaínas. Agrociencia 2015, 49, 483–498. [Google Scholar]
- Nouri, L.; Mohammadi Nafchi, A. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int. J. Biol. Macromol. 2014, 66, 254–259. [Google Scholar] [CrossRef]
- Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement; ASTM-D7334-08; ASTM International: West Conshohocken, PA, USA, 2013.
- Standard Test Methods for Water Vapor Transmission of Materials; ASTM-E96/E96M-15; ASTM International: West Conshohocken, PA, USA, 2015.
- Kanmani, P.; Rhim, J.-W. Antimicrobial and physical-mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef]
- Standard Test Method for High Speed Puncture Properties of Plastics Using Load and Displacement Sensors; ASTM-D7192-18; ASTM International: West Conshohocken, PA, USA, 2018.
- Standard test method for tear resistance (graves tear) of plastic film and sheeting. In American Society for Testing and Materials; ASTM-D1004-13; ASTM International: West Conshohocken, PA, USA, 2013.
- Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM-D882-18; ASTM International: West Conshohocken, PA, USA, 2018.
- Anderson, C.; Simsek, S. How do arabinoxylan films interact with water and soil? Foods 2019, 8, 213. [Google Scholar] [CrossRef]
- Standard Guide to Scanner and Tip Related Artifacts in Scanning Tunneling Microscopy and Atomic Force Microscopy; ASTM-E2382-04; ASTM International: West Conshohocken, PA, USA, 2012.
- Kureck, I.; Policarpi, P.D.B.; Toaldo, I.M.; Maciel, M.V.D.O.B.; Bordignon-Luiz, M.T.; Barreto, P.L.M.; Block, J.M. Chemical Characterization and Release of Polyphenols from Pecan Nut Shell [Carya illinoinensis (Wangenh) C. Koch] in Zein Microparticles for Bioactive Applications. Plant Foods Hum. Nutr. 2018, 73, 137–145. [Google Scholar] [CrossRef]
- Contini, M.; Baccelloni, S.; Frangipane, M.T.; Merendino, N.; Massantini, R. Increasing espresso coffee brew antioxidant capacity using phenolic extract recovered from hazelnut skin waste. J. Funct. Foods 2012, 4, 137–146. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic Compounds and Antioxidant Activity of Kernels and Shells of Mexican Pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Taş, N.G.; Gökmen, V. Bioactive compounds in different hazelnut varieties and their skins. J. Food Compos. Anal. 2015, 43, 203–208. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.-T.; Guerra Dias, A.R.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef]
- U Nisa, I.; Ashwar, B.A.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.A. Development of potato starch based active packaging films loaded with antioxidants and its effect on shelf life of beef. J. Food Sci. Technol. 2015, 52, 7245–7253. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Rafienia, M.; Navid, S.; Agheb, M. Physicochemical, antimicrobial and cytotoxic characteristics of corn starch film containing propolis for wound dressing. J. Polym.Environ. 2018, 26, 3345–3351. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Physico-Mechanical Properties of Starch-Based Films Containing Naturally Derived Antimicrobial Agents. Packag. Technol. Sci. 2014, 27, 149–159. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.-K.; Go, E.; Song, K.B. Application of adzuki bean starch in antioxidant films containing cocoa nibs extract. Polymers 2018, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Medina Jaramillo, C.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef]
- Hamdi, M.; Nasri, R.; Li, S.; Nasri, M. Bioactive composite films with chitosan and carotenoproteins extract from blue crab shells: Biological potential and structural, thermal, and mechanical characterization. Food Hydrocoll. 2019, 89, 802–812. [Google Scholar] [CrossRef]
- Prado, A.C.P.D.; Manion, B.A.; Seetharaman, K.; Deschamps, F.C.; Barrera Arellano, D.; Block, J.M. Relationship between antioxidant properties and chemical composition of the oil and the shell of pecan nuts [Caryaillinoinensis (Wangenh) C. Koch]. Ind. Crop. Prod. 2013, 45, 64–73. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A. Plasticizers, antioxidants and reinforcement fillers from hazelnut skin and cocoa by-products: Extraction and use in PLA and PP. Polym. Degrad. Stab. 2014, 108, 297–306. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Wang, J.; Su, L.; Wang, S. Physicochemical properties of octenyl succinic anhydride-modified potato starch with different degrees of substitution. J. Sci. Food Agric. 2010, 90, 424–429. [Google Scholar] [CrossRef] [PubMed]

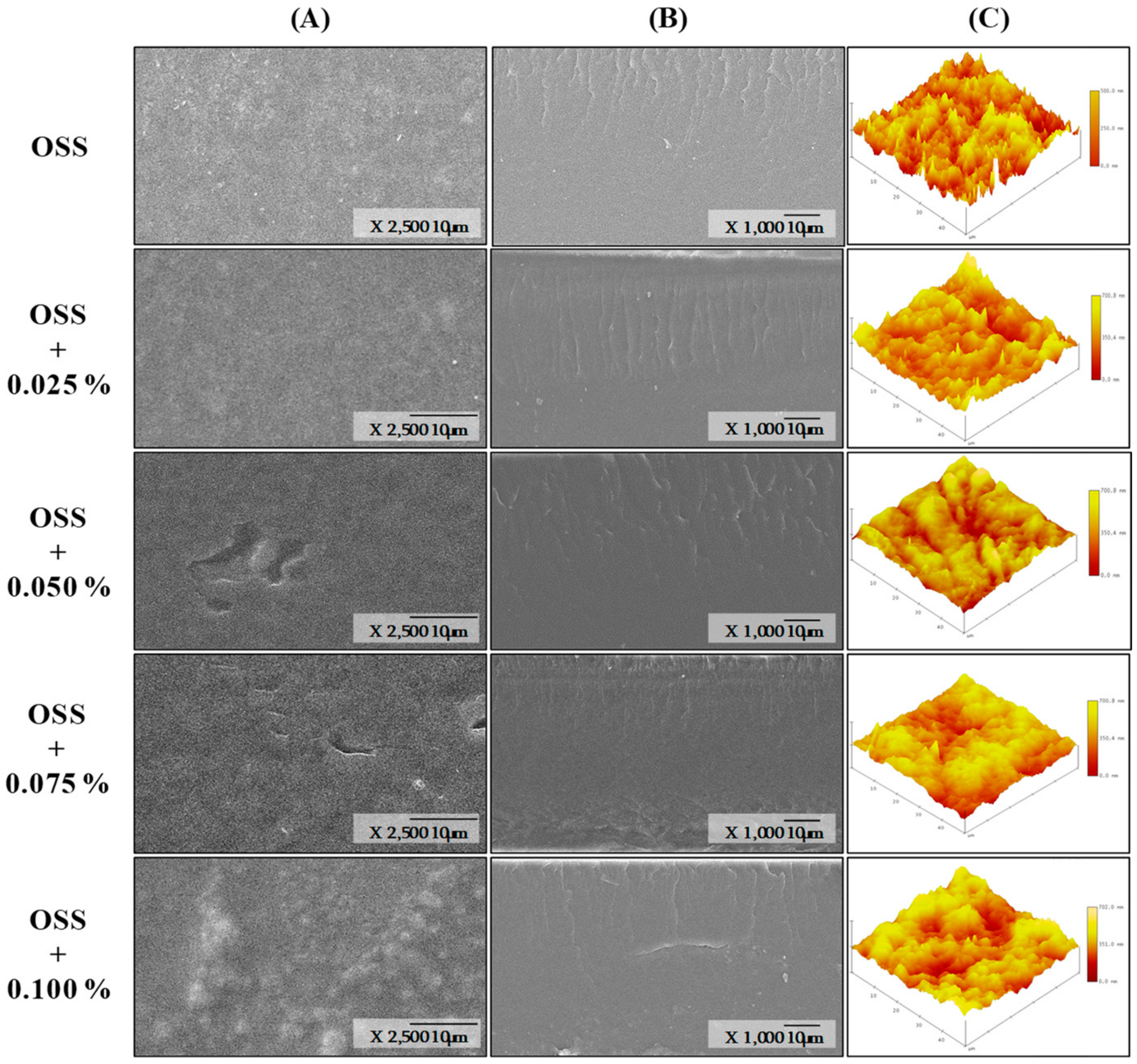
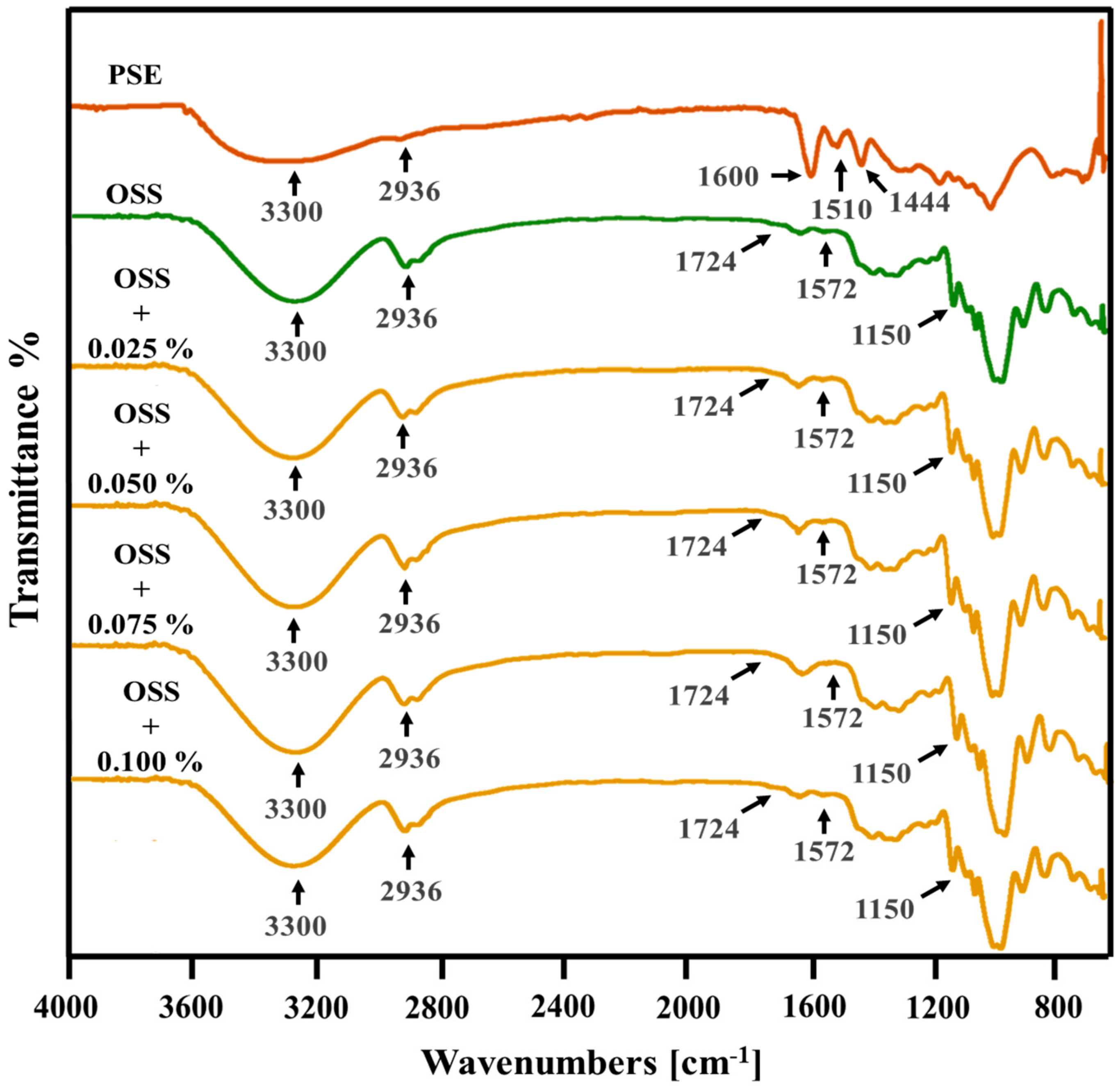
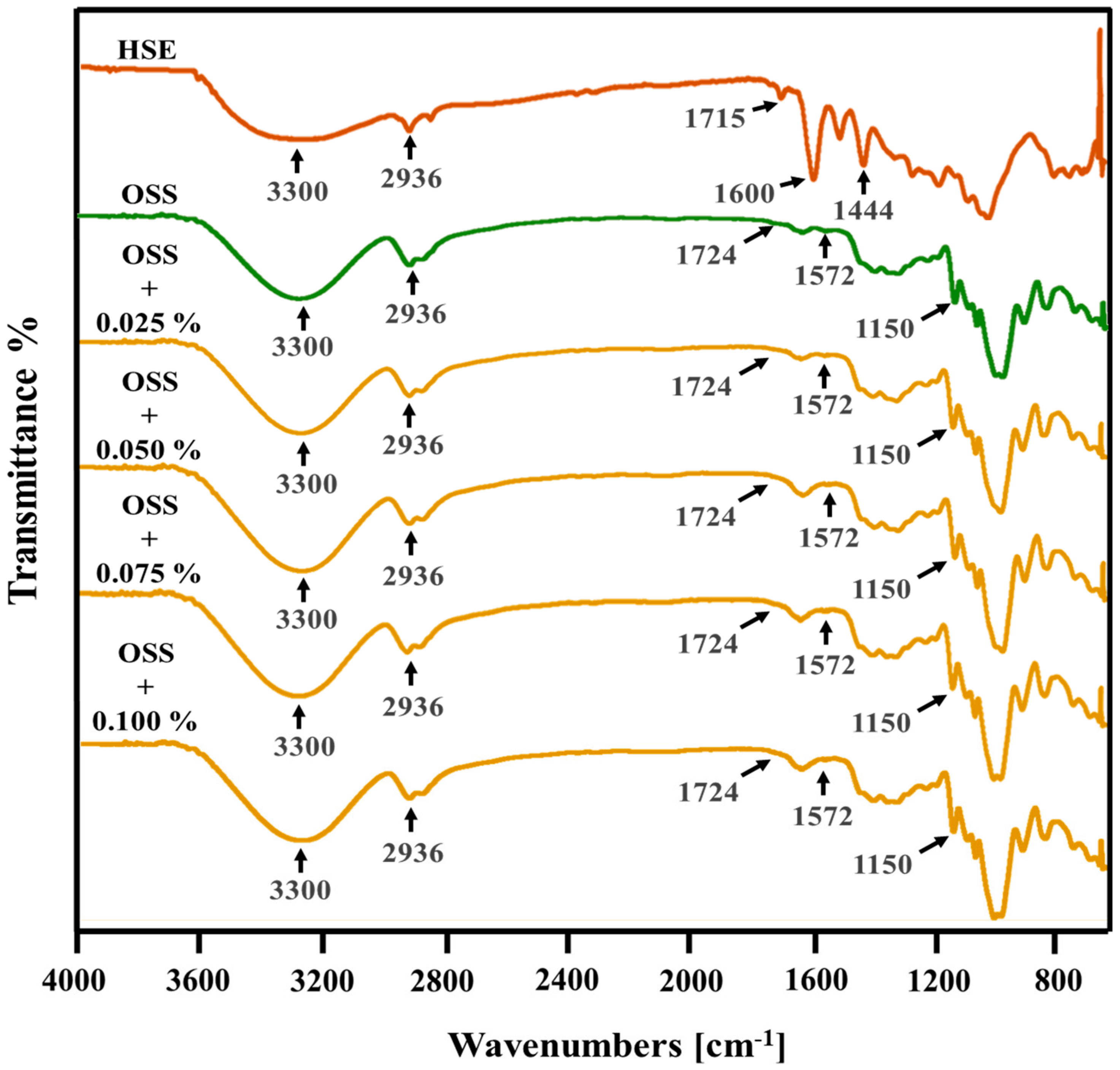
| Film Name | Film Forming Solution | Extract Solution | |||
|---|---|---|---|---|---|
| OSS (0.013) (g) | Glycerol (g) | Water (mL) | 60% Ethanol Solution (mL) | PSE or HSE (mg per 100 mL) | |
| OSS | 4 | 2 | 90 | 10 | - |
| OSS-PSE or HSE | |||||
| 0.025% | 4 | 2 | 90 | 10 | 25 |
| 0.050% | 4 | 2 | 90 | 10 | 50 |
| 0.075% | 4 | 2 | 90 | 10 | 75 |
| 0.100% | 4 | 2 | 90 | 10 | 100 |
| PSE | HSE | |
|---|---|---|
| Phenols Content (mg GAE/g) | 656.46 ± 4.91 b | 693.00 ± 5.65 a |
| Flavonoids Content (mg CE/ g) | 235.35 ± 1.50 b | 427.77 ± 1.97 a |
| Film | Thickness (µm) | Moisture Content (%) | Water Solubility (%) | Contact Angle (°) | Water Vapor Permeability (g mm/ h m2 KPa) |
|---|---|---|---|---|---|
| OSS | 87 ± 2 b | 29.54 ± 0.28 a | 20.34 ± 0.44 a | 49.25 ± 0.27 g | 2.46 ± 0.07 a |
| PSE (%) | |||||
| 0.025 | 90 ± 0 ab | 29.22 ± 0.54 a | 20.23 ± 0.30 a | 55.80 ± 1.79 f | 2.47 ± 0.05 a |
| 0.050 | 90 ± 1 ab | 29.29 ± 0.34 a | 19.39 ± 0.55 ab | 72.35 ± 0.34 e | 2.46 ± 0.06 a |
| 0.075 | 91 ± 1 a | 29.44 ± 0.78 a | 17.70 ± 0.56 c | 82.62 ± 0.88 c | 2.47 ± 0.08 a |
| 0.100 | 91 ± 1 a | 29.62 ± 0.83 a | 17.14 ± 0.21 c | 96.80 ± 0.21 a | 2.57 ± 0.08 a |
| HSE (%) | |||||
| 0.025 | 90 ± 1 ab | 29.83 ± 0.35 a | 20.72 ± 0.77 a | 70.38 ± 0.89 e | 2.59 ± 0.03 a |
| 0.050 | 90 ± 1 ab | 29.74 ± 0.40 a | 18.05 ± 0.72 bc | 77.45 ± 2.91 d | 2.61 ± 0.03 a |
| 0.075 | 91 ± 1 a | 30.03 ± 0.41 a | 17.78 ± 0.49 c | 83.96 ± 1.26 c | 2.59 ± 0.06 a |
| 0.100 | 91 ± 1 a | 29.90 ± 0.56 a | 17.08 ± 0.68 c | 92.54 ± 1.28 b | 2.62 ± 0.03 a |
| Film | L | a* | b* | ΔE | *Color |
| OSS | 95.09 ± 0.11 a | −0.96 ± 0.02 f | 2.29 ± 0.04 g | - |  |
| PSE % | |||||
| 0.025 | 91.50 ± 0.35 b | 2.07 ± 0.29 e | 6.70 ± 0.52 f | 6.50 ± 0.68 e |  |
| 0.050 | 88.66 ± 0.05 c | 4.22 ± 0.10 d | 9.51 ± 0.38 de | 10.97 ± 0.30 d |  |
| 0.075 | 87.65 ± 0.52 c | 4.95 ± 0.39 d | 10.85 ± 0.37 d | 12.79 ± 0.72 d |  |
| 0.100 | 85.92 ± 0.52 d | 6.09 ± 0.60 c | 12.69 ± 0.74 c | 15.56 ± 1.01 c |  |
| HSE % | |||||
| 0.025 | 88.38 ± 0.34 c | 4.25 ± 0.29 d | 9.21 ± 0.49 e | 10.96 ± 0.66 d |  |
| 0.050 | 85.16 ± 0.61 d | 6.55 ± 0.48 c | 12.55 ± 0.69 c | 16.15 ± 1.00 c |  |
| 0.075 | 81.45 ± 0.41 e | 9.30 ± 0.31 b | 15.79 ± 0.51 b | 21.77 ± 0.65 b |  |
| 0.100 | 78.11 ± 0.71 f | 11.29 ± 0.55 a | 18.70 ± 0.60 a | 26.60 ± 0.96 a |  |
| Film | T280 nm (%) | T600 nm (%) |
|---|---|---|
| OSS | 71.24 ± 0.26 a | 86.17 ± 0.30 a |
| PSE % | ||
| 0.025 | 22.76 ± 0.16 b | 84.32 ± 0.32 b |
| 0.050 | 11.08 ± 0.20 d | 83.81 ± 0.14 b |
| 0.075 | 3.07 ± 0.14 f | 82.75 ± 0.12 c |
| 0.100 | 1.58 ± 0.11 g | 80.26 ± 0.21 d |
| HSE % | ||
| 0.025 | 14.39 ± 0.07 c | 84.36 ± 0.10 b |
| 0.050 | 3.56 ± 0.25 e | 83.82 ± 0.23 b |
| 0.075 | 0.61 ± 0.01 h | 82.32 ± 0.34 c |
| 0.100 | 0.22 ± 0.01 h | 80.22 ± 0.40 d |
| Film | Puncture Resistance (N) | Tear Resistance (N) | Tensile Strength (MPa) | Elongation at Break (%) | Young´s Modulus (MPa) |
|---|---|---|---|---|---|
| OSS | 4.48 ± 0.40 a | 1.36 ± 0.04 ab | 9.60 ± 1.38 ab | 32.41 ± 4.24 a | 239.55 ± 15.39 a |
| PSE % | |||||
| 0.025 | 3.62 ± 0.21 b | 1.12 ± 0.15 ab | 7.94 ± 0.78 ab | 30.98 ± 3.12 a | 154.00 ± 7.23 c |
| 0.050 | 3.65 ± 0.14 b | 1.11 ± 0.03 ab | 7.87 ± 0.42 ab | 25.90 ± 6.20 a | 171.26 ± 21.00 c |
| 0.075 | 3.79 ± 0.17 ab | 1.14 ± 0.25 ab | 7.56 ± 1.16 b | 23.99 ± 1.17 a | 159.06 ± 17.61 c |
| 0.100 | 3.58 ± 0.16 b | 1.00 ± 0.15 b | 8.62 ± 1.89 ab | 27.28 ± 6.93 a | 176.38 ± 19.83 bc |
| HSE % | |||||
| 250 | 4.01 ± 0.29 ab | 1.29± 0.07 ab | 9.36 ± 0.99 ab | 29.88 ± 1.89 a | 215.00 ± 5.30 ab |
| 500 | 3.79 ± 0.38 ab | 1.42 ± 0.18 ab | 10.58 ± 0.52 a | 29.80 ± 3.15 a | 224.85 ± 19.30 a |
| 750 | 3.79 ± 0.07 ab | 1.33 ± 0.06 ab | 8.35 ± 0.95 ab | 31.65 ± 4.75 a | 161.44 ± 11.58 c |
| 1000 | 4.04± 0.33 ab | 1.54 ± 0.26 a | 7.91± 0.93 ab | 29.58 ± 4.91 a | 161.20 ± 13.43 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leon-Bejarano, M.; Durmus, Y.; Ovando-Martínez, M.; Simsek, S. Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts. Foods 2020, 9, 226. https://doi.org/10.3390/foods9020226
Leon-Bejarano M, Durmus Y, Ovando-Martínez M, Simsek S. Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts. Foods. 2020; 9(2):226. https://doi.org/10.3390/foods9020226
Chicago/Turabian StyleLeon-Bejarano, Marcos, Yusuf Durmus, Maribel Ovando-Martínez, and Senay Simsek. 2020. "Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts" Foods 9, no. 2: 226. https://doi.org/10.3390/foods9020226
APA StyleLeon-Bejarano, M., Durmus, Y., Ovando-Martínez, M., & Simsek, S. (2020). Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts. Foods, 9(2), 226. https://doi.org/10.3390/foods9020226