Growth Performance and Nutrient Composition of Mealworms (Tenebrio Molitor) Fed on Fresh Plant Materials-Supplemented Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feeding
2.3. Growth Performance
2.4. Sample Preparation
2.5. Proximate Analysis
2.6. Ferric Reducing Power
2.7. Ferrous Chelating Activity
2.8. ABTS Radical Scavenging Activity
2.9. Statistical Analysis
3. Results and Discussion
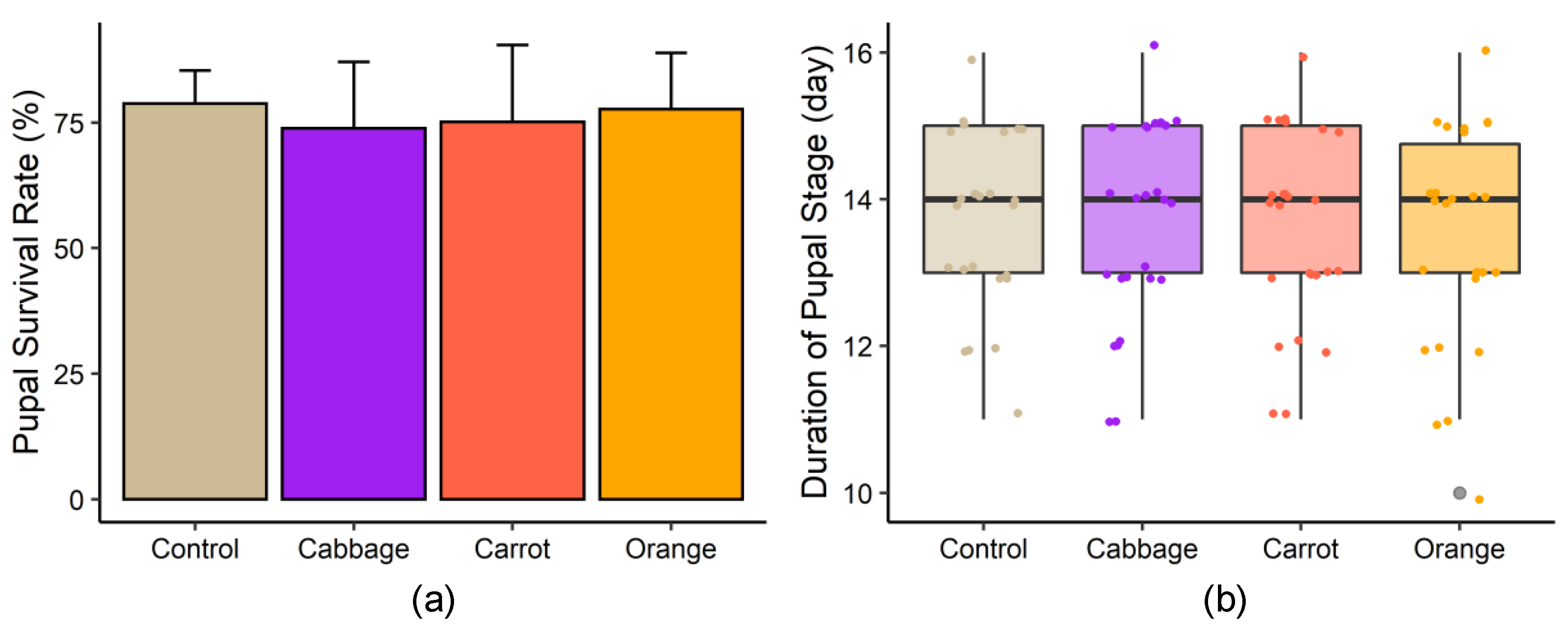
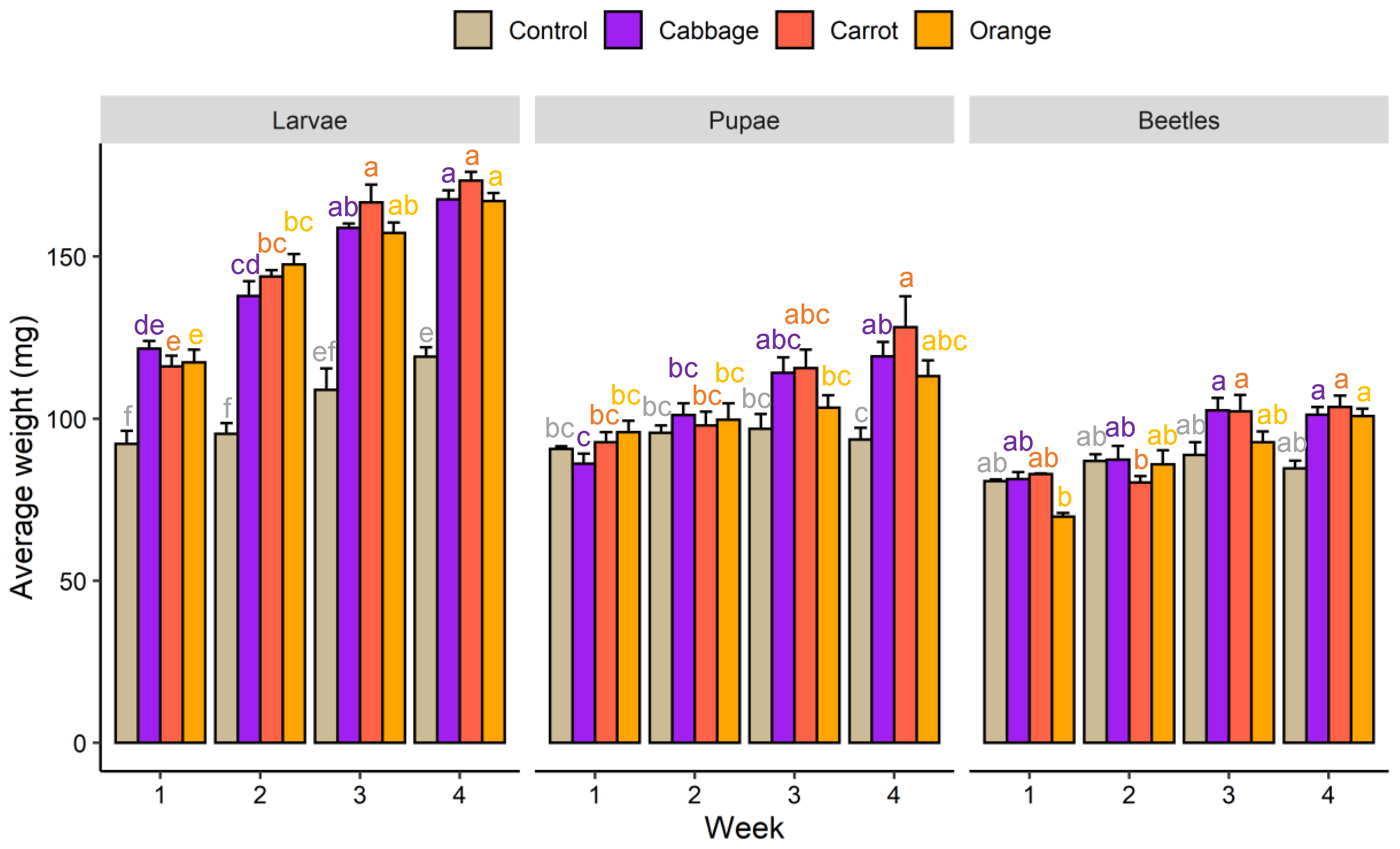
3.1. Growth Performance
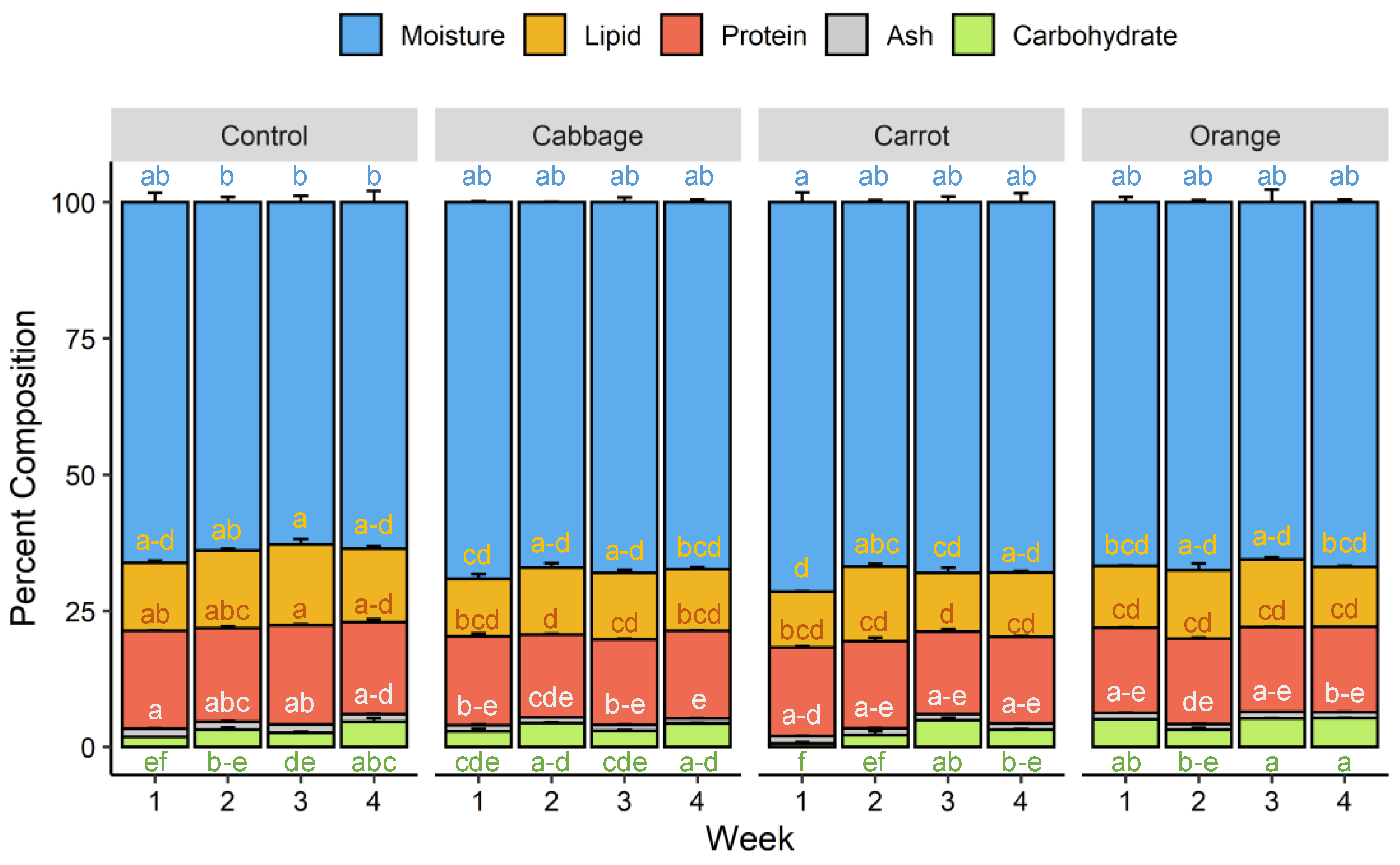
3.2. Proximate Composition
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.; Zhao, J. Insects as a Novel Food. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 428–436. [Google Scholar] [CrossRef]
- Hurd, K.J.; Shertukde, S.; Toia, T.; Trujillo, A.; Pérez, R.L.; Larom, D.L.; Love, J.J.; Liu, C. The cultural importance of edible insects in Oaxaca, Mexico. Ann. Entomol. Soc. Am. 2019, 112, 552–559. [Google Scholar] [CrossRef]
- Woolf, E.; Zhu, Y.; Emory, K.; Zhao, J.; Liu, C. Willingness to consume insect-containing foods: A survey in the United States. LWT Food Sci. Technol. 2019, 102, 100–105. [Google Scholar] [CrossRef]
- van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Dossey, A.T.; Morales-Ramos, J.A.; Rojas, M.G. Insects as Sustainable Food Ingredients: Production, Processing and food Applications; Elsevier: San Diego, CA, USA, 2016. [Google Scholar]
- Milosavljević, I.; El-Shafie, H.A.; Faleiro, J.R.; Hoddle, C.D.; Lewis, M.; Hoddle, M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest Sci. 2019, 92, 143–156. [Google Scholar] [CrossRef]
- Cortes Ortiz, J.; Ruiz, A.T.; Morales-Ramos, J.; Thomas, M.; Rojas, M.; Tomberlin, J.; Yi, L.; Han, R.; Giroud, L.; Jullien, R. Insect mass production technologies. In Insects as Sustainable Food Ingredients; Elsevier: San Diego, CA, USA, 2016; pp. 153–201. [Google Scholar]
- Seo, M.; Goo, T.-W.; Chung, M.; Baek, M.; Hwang, J.-S.; Kim, M.; Yun, E.-Y. Tenebrio molitor larvae inhibit adipogenesis through AMPK and MAPKs signaling in 3T3-L1 adipocytes and obesity in high-fat diet-induced obese mice. Int. J. Mol. Sci. 2017, 18, 518. [Google Scholar] [CrossRef]
- Gessner, D.K.; Schwarz, A.; Meyer, S.; Wen, G.; Most, E.; Zorn, H.; Ringseis, R.; Eder, K. Insect meal as alternative protein source exerts pronounced lipid-lowering effects in hyperlipidemic obese Zucker rats. J. Nutr. 2019, 149, 566–577. [Google Scholar] [CrossRef]
- De Carvalho, N.M.; Teixeira, F.; Silva, S.; Madureira, A.R.; Pintado, M.E. Potential prebiotic activity of Tenebrio molitor insect flour using an optimized in vitro gut microbiota model. Food Funct. 2019, 10, 3909–3922. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Oonincx, D.G.; de Boer, I.J. Environmental impact of the production of mealworms as a protein source for humans–a life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- Miglietta, P.; De Leo, F.; Ruberti, M.; Massari, S. Mealworms for food: A water footprint perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Oonincx, D.G.; van Huis, A.; van Loon, J.J. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ramos, J.; Rojas, M.; Shapiro-Ilan, D.; Tedders, W. Developmental plasticity in Tenebrio molitor (Coleoptera: Tenebrionidae): Analysis of instar variation in number and development time under different diets. J. Entomol. Sci. 2010, 45, 75–90. [Google Scholar] [CrossRef]
- Morales-Ramos, J.; Rojas, M.; Shapiro-Ilan, D.; Tedders, W. Self-selection of two diet components by Tenebrio molitor (Coleoptera: Tenebrionidae) larvae and its impact on fitness. Environ. Entomol. 2011, 40, 1285–1294. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-llan, D.I.; Tedders, W.L. Use of nutrient self-selection as a diet refining tool in Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2013, 48, 206–221. [Google Scholar] [CrossRef]
- Davis, G.; Sosulski, F. Nutritional quality of oilseed protein isolates as determined with larvae of the yellow mealworm, Tenebrio molitor L. J. Nutr. 1974, 104, 1172–1177. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- AOAC. Official methods of analysis of AOAC International, 20 ed.; AOAC International: Arlington, TX, USA, 2016. [Google Scholar]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Sansone, K.; Kern, M.; Hong, M.Y.; Liu, C.; Hooshmand, S. Acute effects of dried apple consumption on metabolic and cognitive responses in healthy individuals. J. Med. Food 2018, 21, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xiong, Y.L.; McNear, D.H. Changes in structural characteristics of antioxidative soy protein hydrolysates resulting from scavenging of hydroxyl radicals. J. Food Sci. 2013, 78, C152–C159. [Google Scholar] [CrossRef] [PubMed]
- USDA, Agricultural Research Service. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 9 January 2013).
- Veenenbos, M.; Oonincx, D. Carrot supplementation does not affect house cricket performance (Acheta domesticus). J. Insects Food Feed 2017, 3, 217–221. [Google Scholar] [CrossRef]
- Hansen, L.L.; Ramløv, H.; Westh, P. Metabolic activity and water vapour absorption in the mealworm Tenebrio molitor L. (Coleoptera, Tenebrionidae): Real-time measurements by two-channel microcalorimetry. J. Exp. Biol. 2004, 207, 545–552. [Google Scholar] [CrossRef]
- Kramer, K.J.; Seib, P.A. Ascorbic acid and the growth and development of insects. Adv. Chem. 1982, 200, 275–291. [Google Scholar]
- Vanderzant, E.S.; Richardson, C.D. Ascorbic acid in the nutrition of plant-feeding insects. Science 1963, 140, 989–991. [Google Scholar] [CrossRef]
- Vigneron, A.; Jehan, C.; Rigaud, T.; Moret, Y. Immune defenses of a beneficial pest: The mealworm beetle, Tenebrio molitor. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Dhinaut, J.; Balourdet, A.; Teixeira, M.; Chogne, M.; Moret, Y. A dietary carotenoid reduces immunopathology and enhances longevity through an immune depressive effect in an insect model. Sci. Rep. 2017, 7, 12429. [Google Scholar] [CrossRef]
- Després, L.; David, J.-P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef]
- Pracros, P.; Couranjou, C.; Moreau, R. Effects on growth and respiration due to the ingestion of the rapeseed meal glucosinolates in young larvae of Tenebrio molitor. Comp. Biochem. Physiol. A Comp. Physiol. 1992, 103, 391–395. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, J. Tenebrio molitor larvae are a better food option than adults. J. Insects Food Feed 2019, 5, 241–242. [Google Scholar] [CrossRef]
- Behmer, S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Et Biophys. Acta (Bba) Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef]
- Duffey, S.S. Sequestration of plant natural products by insects. Annu. Rev. Entomol. 1980, 25, 447–477. [Google Scholar] [CrossRef]

| Diet | Week | Ferric Reducing Power (μmol Ascorbic Acid Equivalent/g Dry Mass) | Ferrous Chelating Activity (μmol EDTA Equivalent/g Dry Mass) | ABTS Radical Scavenging Activity (μmol Trolox Equivalent/g Dry Mass) |
|---|---|---|---|---|
| Control | 1 | 13.10 ± 0.71 a | 36.41 ± 0.06 d,e | 55.05 ± 1.10 a,b |
| 2 | 11.86 ± 0.19 a,b,c | 41.18 ± 0.47 c | 46.99 ± 0.88 a,b | |
| 3 | 12.97 ± 0.81 a,b | 36.47 ± 0.22 d,e | 45.06 ± 2.50 a,b | |
| 4 | 11.66 ± 0.98 a,b,c | 36.64 ± 0.19 d,e | 58.80 ± 6.41 a,b | |
| Cabbage | 1 | 8.50 ± 1.20 c | 37.77 ± 0.08 d | 55.20 ± 4.53 a,b |
| 2 | 11.94 ± 1.23 a,b,c | 43.77 ± 0.23 b | 49.81 ± 1.65 a,b | |
| 3 | 10.52 ± 0.72 a,b,c | 38.35 ± 0.19 d | 46.91 ± 3.51 a,b | |
| 4 | 9.82 ± 0.40 a,b,c | 37.89 ± 0.31 d | 61.77 ± 7.06 a | |
| Carrot | 1 | 10.90 ± 0.58 a,b,c | 43.74 ± 0.11 b | 54.22 ± 3.50 a,b |
| 2 | 8.86 ± 0.85 b,c | 49.96 ± 1.22 a | 46.42 ± 3.87 a,b | |
| 3 | 11.98 ± 0.61 a,b,c | 43.93 ± 0.01 b | 47.39 ± 1.10 a,b | |
| 4 | 10.55 ± 0.79 a,b,c | 43.98 ± 0.14 b | 62.17 ± 1.91 a | |
| Orange | 1 | 8.39 ± 0.83 c | 34.77 ± 0.27 e | 53.97 ± 5.75 a,b |
| 2 | 7.99 ± 1.09 c | 40.42 ± 0.43 c | 48.65 ± 3.76 a,b | |
| 3 | 9.63 ± 1.01 a,b,c | 34.74 ± 0.21 e | 40.40 ± 1.56 b | |
| 4 | 10.80 ± 0.78 a,b,c | 35.10 ± 0.08 e | 58.34 ± 7.77 a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth Performance and Nutrient Composition of Mealworms (Tenebrio Molitor) Fed on Fresh Plant Materials-Supplemented Diets. Foods 2020, 9, 151. https://doi.org/10.3390/foods9020151
Liu C, Masri J, Perez V, Maya C, Zhao J. Growth Performance and Nutrient Composition of Mealworms (Tenebrio Molitor) Fed on Fresh Plant Materials-Supplemented Diets. Foods. 2020; 9(2):151. https://doi.org/10.3390/foods9020151
Chicago/Turabian StyleLiu, Changqi, Jasmin Masri, Violet Perez, Cassandra Maya, and Jing Zhao. 2020. "Growth Performance and Nutrient Composition of Mealworms (Tenebrio Molitor) Fed on Fresh Plant Materials-Supplemented Diets" Foods 9, no. 2: 151. https://doi.org/10.3390/foods9020151
APA StyleLiu, C., Masri, J., Perez, V., Maya, C., & Zhao, J. (2020). Growth Performance and Nutrient Composition of Mealworms (Tenebrio Molitor) Fed on Fresh Plant Materials-Supplemented Diets. Foods, 9(2), 151. https://doi.org/10.3390/foods9020151





