Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Deep Eutectic Solvents (DES)
2.3. Extraction of Bioactive Compounds from CBS
2.4. DESs Extraction Coupled with MAE
2.5. HPLC Analysis
2.6. DPPH Scavenging Activity
2.7. Statistical Analysis
3. Results
3.1. DES Extraction of Bioactive Compounds from CBS
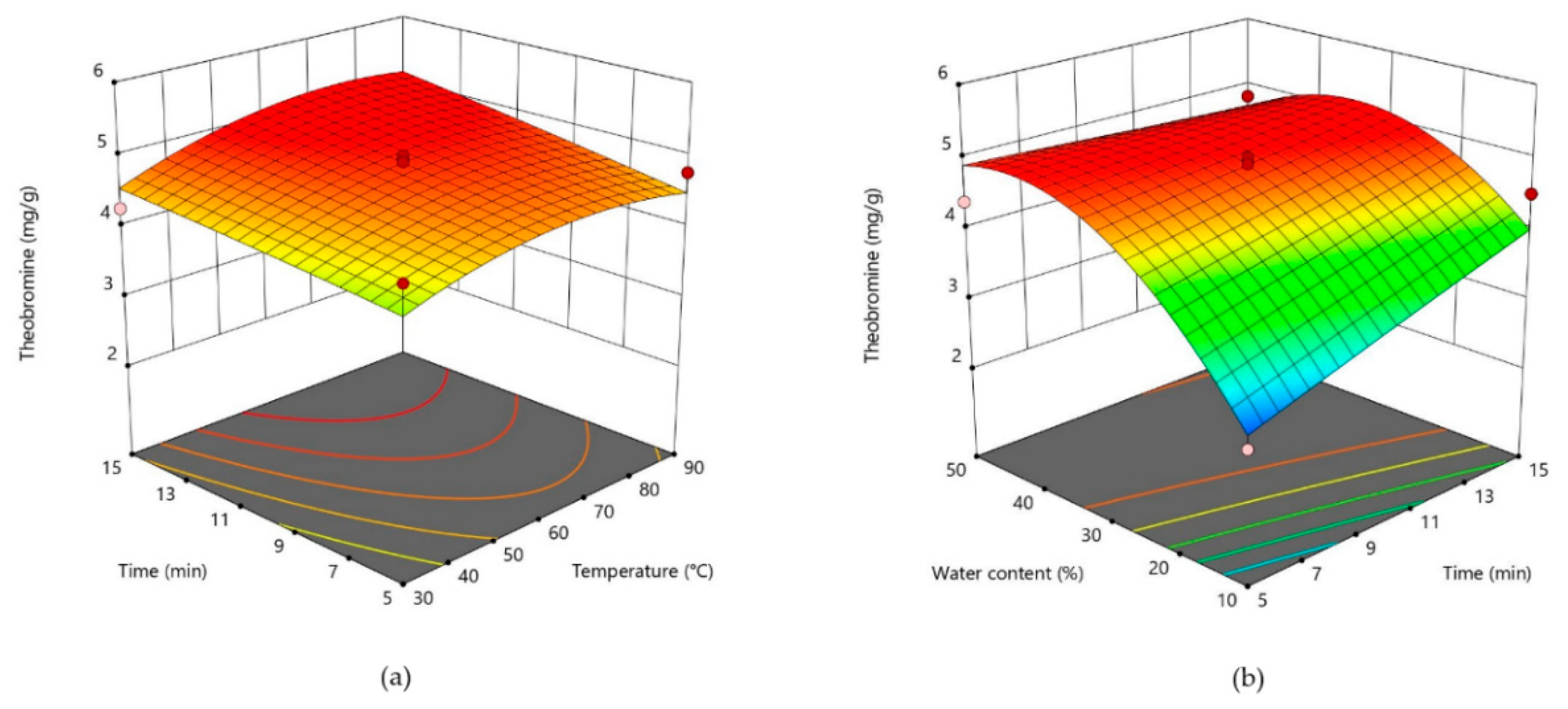
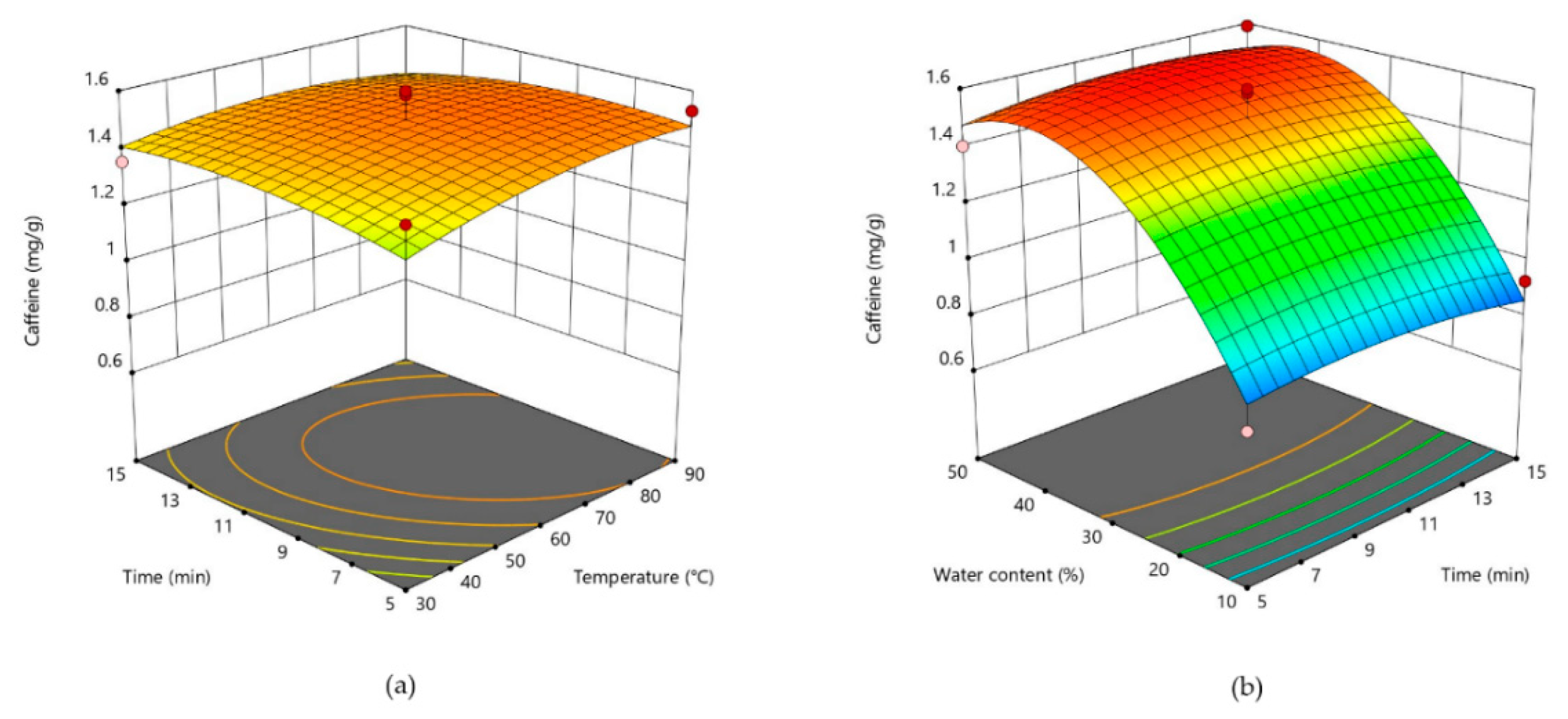
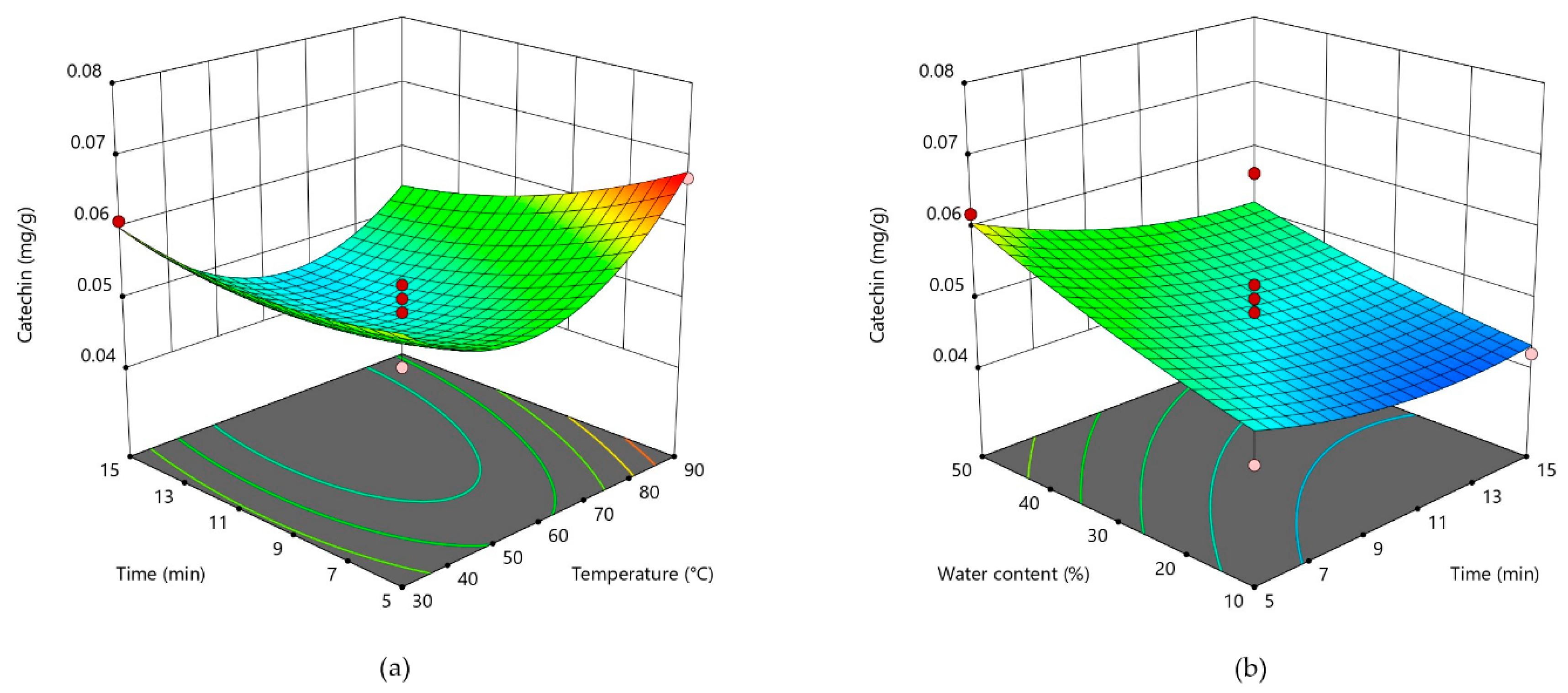
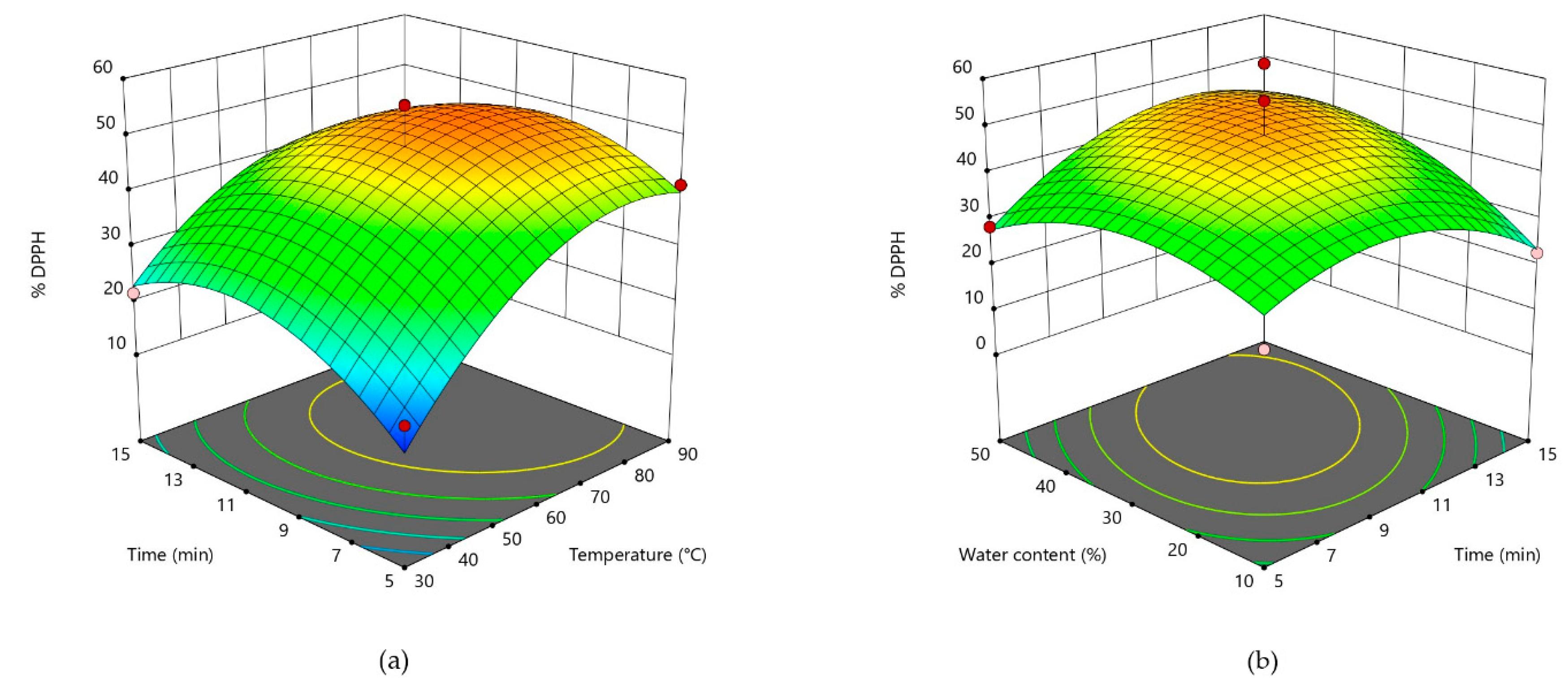
3.2. Response Surface Analysis and Process Optimization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Bolaños, J.; Bermúdez-Oria, A.; Morales, A.A.; Rodríguez-Gutiérreza, G. Cocoa bean husk: industrial source of antioxidant phenolic extract. J. Sci. Food Agric. 2019, 99, 325–333. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Tech. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Hartati, I. Hydrotropic extraction of theobromine from cocoa bean shell. Momentum 2010, 6, 17–20. [Google Scholar]
- Bentil, J.A.; Dzogbefia, V.P.; Alemawor, F. Enhancement of the nutritive value of cocoa (Theobroma cacao) bean shells for use as feed for animals through a two-stage solid state fermentation with Pleurotus ostreatus and Aspergillus niger. Int. J. Appl. Microbiol. Biotechnol. Res. 2015, 3, 20–30. [Google Scholar]
- Hamzat, R.A.; Adeola, O. Chemical Evaluation of Co-products of Cocoa and Kola as Livestock Feeding Stuffs. J. Anim. Sci. Adv. 2011, 1, 61–68. [Google Scholar]
- Adeloye, A. Efficiencies of Conversion of Some Lignocellulosic Waste Materials by Goats. Bioresour. Technol. 1992, 40, 167–169. [Google Scholar] [CrossRef]
- Adebowale, B.A.; Olubamiwa, O. Growth Response of Clarias gariepinus juvenile to Cocoa Husk Endocarp Based Diets. Agric. J. 2008, 3, 425–428. [Google Scholar]
- Falaye, A.E.; Jauncey, K. Acceptability and digestibility by tilapia Oreochromis niloticus of feeds containing cocoa husk. Aquac. Nutr. 1999, 5, 157–161. [Google Scholar] [CrossRef]
- Magistrelli, D.; Zanchi, R.; Malagutti, L.; Galassi, G.; Canzi, E.; Rosi, F. Effects of Cocoa Husk Feeding on the Composition of Swine Intestinal Microbiota. J. Agric. Food Chem. 2016, 64, 2046–2052. [Google Scholar] [CrossRef]
- Bernaert, H.; De Ruysscher, I. Methods for Extraction Cocoa. U.S. Patent US 2013/0302473 A1, 14 November 2013. [Google Scholar]
- Jozinović, A.; Panak Balentić, J.; Ačkar, Đ.; Babić, J.; Pajin, B.; Miličević, B.; Guberac, S.; Šubarić, D. Cocoa husk application in enrichment of extruded snack products. In Proceedings of the Fourth International Congress on Cocoa Coffee and Tea, Torino, Italy, 25–28 June 2017; Elsevier: Torino, Elsevier; p. 68. [Google Scholar]
- Sanchez Mundo, M.L.; Jaramillo Flores, M.E.; Espinosa Solis, V.; Chávez-Reyes, Y.; Díaz Ramírez, M.; Salgado Cruz, M.P.; Calderón Domínguez, G. Muffins enriched with cocoa shell fiber. In Proceedings of the Fourth International Congress on Cocoa Coffee and Tea, Torino, Italy, 25–28 June 2017; Elsevier: Torino, Elsevier; p. 124. [Google Scholar]
- Sanchez Mundo, M.L.; Martínez Mendez, D.; Chávez-Reyes, Y.; Espinosa-Solis, V.; Jaramillo-Flores, M.-E.; Torruco-Uco, J.G. Chemical and nutritional characteristics of biscuits added with cocoa shell powder. In Proceedings of the Fourth International Congress on Cocoa Coffee and Tea, Torino, Italy, 25–28 June 2017; Elsevier: Torino, Elsevier; p. 124. [Google Scholar]
- Ooshima, T.; Osaka, Y.; Sasaki, H.; Osawa, K.; Yasuda, H.; Matsumura, M.; Sobue, S.; Matsumoto, M. Caries inhibitory activity of cacao bean husk extract in in-vitro and animal experiments. Arch. Oral Biol. 2000, 45, 639–645. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tsuji, M.; Okuda, J.; Sasaki, H.; Nakano, K.; Osawa, K.; Shimura, S.; Ooshima, T. Inhibitory effects of cacao bean husk extract on plaque formation in vitro and in vivo. Eur. J. Oral Sci. 2004, 112, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, G.; Bulló, M.; Anguera, A.; Escribano, J.; Salas-Salvadó, J. A controlled, randomized, double-blind trial to evaluate the effect of a supplement of cocoa husk that is rich in dietary fiber on colonic transit in constipated pediatric patients. Pediatrics 2006, 118, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa shell: A by-product with great potential for wide application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, Ž.; Šubarić, D.; Škerget, M. Separation of active compounds from food by-product (cocoa shell) using subcritical water extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Karim, A.A.; Azlan, A.; Ismail, A.; Hashim, P. Antioxidant properties of cocoa pods and shells. Malaysian Cocoa J. 2014, 8, 49–56. [Google Scholar]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilotscale cavitational reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.H. Proximate composition, extraction, and purification of theobromine from cacao pod husk (Theobroma Cacao, L.). Technologies 2017, 5, 14. [Google Scholar] [CrossRef]
- Arlorio, M.; Coïsson, J.D.; Travaglia, F.; Varsaldi, F.; Miglio, G.; Lombardi, G.; Martelli, A. Antioxidant and biological activity of phenolic pigments from Theobroma cacao hulls extracted with supercritical CO2. Food Res. Int. 2005, 38, 1009–1014. [Google Scholar] [CrossRef]
- Pavlović, N.; Jakovljević, M.; Miškulin, M.; Molnar, N.; Ačkar, Đ.; Jokić, S. Green extraction techniques of bioactive components from cocoa shell. Croat. J. Food Sci. Technol. 2019, 11, 11–20. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojćić Redovniković, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Fraige, K.; Arrua, R.D.; Sutton, A.T.; Soleo Funari, C.; Cavalheiro, A.J.; Hilder, E.F.; da Silva Bolzani, V. Using natural deep eutectic solvents for the extraction of metabolites in Byrsonima. J. Sep. Sci. 2018, 42, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Molnar, M.; Jakovljević, M.; Jokić, S. Optimization of the process conditions for the extraction of rutin from Ruta graveolens L. by choline chloride based deep eutectic solvents. Solvent Extr. Res. Dev. 2018, 25, 109–116. [Google Scholar] [CrossRef]
- Jiang, Z.-M.; Wang, L.-J.; Gao, Z.; Zhuang, B.; Yin, Q.; Liu, E.-H. Green and efficient extraction of different types of bioactive alkaloids using deep eutectic solvents. Microchem. J. 2019, 145, 345–353. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.-G.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwaveassisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Tech. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Jokić, S.; Šafranko, S.; Jakovljević, M.; Cikoš, A.-M.; Kajić, N.; Kolarević, F.; Babić, J.; Molnar, M. Sustainable Green Procedure for Extraction of Hesperidin from Selected Croatian Mandarin Peels. Processes 2019, 7, 469. [Google Scholar] [CrossRef]
- de Oliveira Nascimento, D.A.; Raffin, R.P.; de Oliveira Fogaça, A.; de Moraes, C.M.B.; Boligon, A.; Ferreira Ourique, A. Evaluation of the antioxidant activity by the DPPH radical scavenging method of free and liposome-associated cocoa extracts. Disciplinarum Scientia 2016, 17, 375–386. [Google Scholar]
- Jokić, S.; Bijuk, M.; Aladić, K.; Bilić, M.; Molnar, M. Optimisation of supercritical CO2 extraction of grape seed oil using response surface methodology. Int. J. Food Sci. Tech. 2016, 51, 403–410. [Google Scholar] [CrossRef]
- de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in Organic Synthesis. Thermal and Non-Thermal Microwave Effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Adamafio, N.A. Theobromine toxicity and remediation of cocoa by-products: An overview. J. Biol. Sci. 2013, 13, 570–576. [Google Scholar] [CrossRef]
- Arlorio, M.; Coisson, J.D.; Restani, P.; Matelli, A. Characterization of pectins and some secondary compounds from Theobroma cacao hulls. J. Food Sci. 2001, 66, 653–656. [Google Scholar] [CrossRef]
- Sotelo, A.; Alvarez, R.G. Chemical composition of wild Theobroma species and their comparison to the cacao bean. J. Agric. Food Chem. 1991, 39, 1940–1943. [Google Scholar] [CrossRef]
- Nair, K.P.P. The agronomy and economy of important tree crops of the developing world, 1st ed.; Elsevier Science: London, Great Britain, 2010; pp. 131–180. [Google Scholar] [CrossRef]
- Cai, C.; Li, F.; Liu, L.; Tan, Z. Deep eutectic solvents used as the green media for the efficient extraction of caffeine from Chinese dark tea. Sep. Purif. Technol. 2019, 227, 115723. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar]
| HBD | DES | Mole Ratio HBA:HBD | % H2O | Galic Acid (mg/g) | Theobro-Mine (mg/g) | Catechin (mg/g) | Caffeine (mg/g) | Caffeic Acid (mg/g) | Epicatechin (mg/g) | % DPPH |
|---|---|---|---|---|---|---|---|---|---|---|
| AA | ChCl:AA | 1:2 | 10 | 0.009 | 3.020 | 0.052 | 0.775 | 0.024 | 0.031 | 33.582 |
| 50 | 0.016 | 3.003 | 0.062 | 0.776 | 0.029 | 0.029 | 44.741 | |||
| BD | ChCl:BD | 1:2 | 10 | 0.000 | 2.282 | 0.000 | 0.571 | 0.023 | 0.051 | 34.280 |
| 50 | 0.000 | 3.639 | 0.063 | 0.868 | 0.034 | 0.028 | 53.401 | |||
| EG | ChCl:EG | 1:1 | 10 | 0.008 | 3.019 | 0.057 | 0.766 | 0.025 | 0.019 | 50.521 |
| 50 | 0.002 | 3.133 | 0.055 | 0.715 | 0.028 | 0.022 | 52.944 | |||
| Fru | ChCl:Fru | 1:1 | 10 | 0.000 | 2.062 | 0.051 | 0.560 | 0.026 | 0.039 | 48.911 |
| 50 | 0.000 | 3.414 | 0.095 | 0.752 | 0.037 | 0.031 | 53.067 | |||
| Gly | ChCl:Gly | 1:2 | 10 | 0.000 | 2.786 | 0.064 | 0.734 | 0.031 | 0.027 | 30.414 |
| 50 | 0.000 | 3.380 | 0.062 | 0.763 | 0.036 | 0.033 | 55.004 | |||
| Glu | ChCl:Glu | 1:1 | 10 | 0.00 | 2.086 | 0.048 | 0.546 | 0.024 | 0.034 | 27.308 |
| 50 | 0.009 | 2.413 | 0.000 | 0.657 | 0.031 | 0.039 | 52.499 | |||
| MA | ChCl:MA | 1:1 | 10 | 0.000 | 1.017 | 0.00 | 0.207 | 0.011 | 0.000 | 41.997 |
| 50 | 0.000 | 2.655 | 0.00 | 0.639 | 0.030 | 0.066 | 50.318 | |||
| Xy | ChCl:Xy | 1:1 | 10 | 0.000 | 1.362 | 0.000 | 0.326 | 0.016 | 0.033 | 23.632 |
| 50 | 0.000 | 3.052 | 0.074 | 0.684 | 0.029 | 0.029 | 24.076 | |||
| Lev | ChCl:Lev | 1:1 | 10 | 0.000 | 3.382 | 0.000 | 0.916 | 0.034 | 0.057 | 43.438 |
| CA | ChCl:CA | 1:1 | 50 | 0.000 | 3.032 | 0.000 | 0.706 | 0.027 | 0.071 | 45.176 |
| Mal | ChCl:Mal | 1:1 | 10 | 0.000 | 2.773 | 0.000 | 0.701 | 0.023 | 0.055 | 42.647 |
| 50 | 0.000 | 3.600 | 0.000 | 0.865 | 0.031 | 0.114 | 48.040 | |||
| LA | ChCl:LA | 1:1 | 10 | 0.000 | 3.145 | 0.000 | 0.790 | 0.036 | 0.059 | 39.654 |
| 50 | 0.000 | 2.854 | 0.000 | 0.665 | 0.026 | 0.063 | 51.524 | |||
| OA | ChCl:OA | 1:1 | 10 | 0.000 | 0.620 | 0.000 | 0.156 | 0.000 | 0.000 | 64.322 |
| 50 | 0.000 | 3.605 | 0.000 | 0.909 | 0.019 | 0.104 | 66.307 | |||
| Sor | ChCl:Sor | 1:1 | 50 | 0.000 | 3.008 | 0.062 | 0.635 | 0.040 | 0.110 | 32.153 |
| U | ChCl:U | 1:2 | 10 | 0.000 | 2.329 | 0.000 | 0.665 | 0.028 | 0.022 | 31.148 |
| 50 | 0.000 | 3.613 | 0.069 | 0.848 | 0.037 | 0.011 | 50.462 | |||
| TA | ChCl:TA | 1:1 | 10 | 0.000 | 2.056 | 0.000 | 0.642 | 0.026 | 0.030 | 46.298 |
| RUN | T (°C) | t (min) | % H2O | Galic Acid (mg/g) | Theobromine (mg/g) | Catechin (mg/g) | Caffeine (mg/g) | Caffeic Acid (mg/g) | Epicatechin (mg/g) | % DPPH |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 90 | 15 | 30 | 0.000 | 4.682 | 0.042 | 1.330 | 0.000 | 0.064 | 74.81 |
| 2 | 60 | 10 | 30 | traces | 4.185 | 0.056 | 1.436 | 0.000 | 0.077 | 61.09 |
| 3 | 60 | 10 | 30 | traces | 4.241 | 0.054 | 1.400 | 0.000 | 0.069 | 51.26 |
| 4 | 90 | 10 | 10 | traces | 3.102 | 0.045 | 1.389 | 0.022 | 0.034 | 55.35 |
| 5 | 60 | 15 | 50 | traces | 4.537 | 0.046 | 1.524 | 0.040 | 0.033 | 42.02 |
| 6 | 90 | 5 | 30 | traces | 4.564 | 0.051 | 1.397 | 0.058 | 0.027 | 48.25 |
| 7 | 60 | 5 | 10 | traces | 2.145 | 0.035 | 0.681 | 0.056 | 0.030 | 46.79 |
| 8 | 60 | 10 | 30 | traces | 3.943 | 0.053 | 1.243 | 0.047 | 0.067 | 37.55 |
| 9 | 60 | 10 | 30 | traces | 4.010 | 0.056 | 1.208 | 0.040 | 0.066 | 33.46 |
| 10 | 60 | 10 | 30 | traces | 4.119 | 0.053 | 1.182 | 0.031 | 0.061 | 40.18 |
| 11 | 60 | 5 | 50 | traces | 4.024 | 0.051 | 1.252 | 0.064 | 0.017 | 24.03 |
| 12 | 60 | 15 | 10 | traces | 4.010 | 0.032 | 0.871 | 0.098 | 0.015 | 31.13 |
| 13 | 30 | 10 | 10 | traces | 2.324 | 0.031 | 0.710 | 0.045 | 0.011 | 37.16 |
| 14 | 90 | 10 | 50 | traces | 4.285 | 0.034 | 1.289 | 0.104 | 0.005 | 31.42 |
| 15 | 30 | 15 | 30 | traces | 2.630 | 0.051 | 1.185 | 0.073 | 0.000 | 24.22 |
| 16 | 30 | 5 | 30 | 0.000 | 3.195 | 0.054 | 1.084 | 0.043 | 0.000 | 30.35 |
| 17 | 30 | 10 | 50 | 0.000 | 2.877 | 0.053 | 1.032 | 0.047 | 0.000 | 35.80 |
| RUN | T (°C) | t (min) | % H2O | Galic Acid (mg/g) | Theobromine (mg/g) | Catechin (mg/g) | Caffeine (mg/g) | Caffeic Acid (mg/g) | Epicatechin (mg/g) | % DPPH |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 90 | 15 | 30 | 0.463 | 4.754 | 0.050 | 1.311 | 0.120 | 0.059 | 32.315 |
| 2 | 60 | 10 | 30 | 0.103 | 5.004 | 0.047 | 1.599 | 0.103 | 0.000 | 55.444 |
| 3 | 60 | 10 | 30 | 0.101 | 4.912 | 0.040 | 1.412 | 0.083 | 0.000 | 55.315 |
| 4 | 90 | 10 | 10 | 0.000 | 3.348 | 0.066 | 0.969 | 0.098 | 0.000 | 48.238 |
| 5 | 60 | 15 | 50 | 0.221 | 4.823 | 0.056 | 1.590 | 0.128 | 0.017 | 48.597 |
| 6 | 90 | 5 | 30 | 0.304 | 4.765 | 0.067 | 1.531 | 0.067 | 0.137 | 41.290 |
| 7 | 60 | 5 | 10 | 0.043 | 2.502 | 0.043 | 0.792 | 0.047 | 0.000 | 24.392 |
| 8 | 60 | 10 | 30 | 0.103 | 4.941 | 0.050 | 1.585 | 0.096 | 0.000 | 43.803 |
| 9 | 60 | 10 | 30 | 0.110 | 4.769 | 0.048 | 1.490 | 0.079 | 0.000 | 42.162 |
| 10 | 60 | 10 | 30 | 0.096 | 4.875 | 0.052 | 1.417 | 0.085 | 0.000 | 43.956 |
| 11 | 60 | 5 | 50 | 0.191 | 4.386 | 0.062 | 1.403 | 0.132 | 0.085 | 28.469 |
| 12 | 60 | 15 | 10 | 0.211 | 4.500 | 0.042 | 0.927 | 0.102 | 0.000 | 22.777 |
| 13 | 30 | 10 | 10 | 0.019 | 2.465 | 0.047 | 0.778 | 0.054 | 0.014 | 11.751 |
| 14 | 90 | 10 | 50 | 0.201 | 4.804 | 0.058 | 1.509 | 0.116 | 0.036 | 35.521 |
| 15 | 30 | 15 | 30 | 0.148 | 4.263 | 0.061 | 1.356 | 0.116 | 0.071 | 21.521 |
| 16 | 30 | 5 | 30 | 0.117 | 4.591 | 0.064 | 1.451 | 0.123 | 0.074 | 17.726 |
| 17 | 30 | 10 | 50 | 0.200 | 4.505 | 0.065 | 1.443 | 0.137 | 0.107 | 24.667 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9.16 | 9 | 1.02 | 5.77 | 0.0153 |
| X1-Temperature | 0.4264 | 1 | 0.4264 | 2.42 | 0.1637 |
| X2-Time | 0.5492 | 1 | 0.5492 | 3.12 | 0.1208 |
| X3-Water content | 4.07 | 1 | 4.07 | 23.07 | 0.0020 |
| X1 × 2 | 0.0251 | 1 | 0.0251 | 0.1426 | 0.7169 |
| X1 × 3 | 0.0853 | 1 | 0.0853 | 0.4839 | 0.5091 |
| X2X3 | 0.6092 | 1 | 0.6092 | 3.46 | 0.1053 |
| X12 | 0.3531 | 1 | 0.3531 | 2.00 | 0.1998 |
| X22 | 0.0013 | 1 | 0.0013 | 0.0072 | 0.9348 |
| X32 | 2.90 | 1 | 2.90 | 16.47 | 0.0048 |
| Residual | 1.23 | 7 | 0.1762 | ||
| Lack of Fit | 1.20 | 3 | 0.4010 | 52.72 | 0.0011 |
| Pure Error | 0.0304 | 4 | 0.0076 | ||
| Cor Total | 10.39 | 16 | |||
| R2 | 0.8813 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.0011 | 9 | 0.0001 | 3.80 | 0.0461 |
| X1-Temperature | 2.000 × 10−6 | 1 | 2.000 × 10−6 | 0.0635 | 0.8083 |
| X2-Time | 0.0001 | 1 | 0.0001 | 2.89 | 0.1327 |
| X3-Water content | 0.0002 | 1 | 0.0002 | 7.34 | 0.0302 |
| X1X2 | 0.0000 | 1 | 0.0000 | 1.56 | 0.2524 |
| X1X3 | 0.0002 | 1 | 0.0002 | 5.37 | 0.0537 |
| X2X3 | 6.250 × 10−6 | 1 | 6.250 × 10−6 | 0.1985 | 0.6694 |
| X12 | 0.0005 | 1 | 0.0005 | 15.24 | 0.0059 |
| X22 | 0.0000 | 1 | 0.0000 | 0.7862 | 0.4047 |
| X32 | 3.603 × 10−6 | 1 | 3.603 × 10−6 | 0.1144 | 0.7451 |
| Residual | 0.0002 | 7 | 0.0000 | ||
| Lack of Fit | 0.0001 | 3 | 0.0000 | 2.20 | 0.2306 |
| Pure Error | 0.0001 | 4 | 0.0000 | ||
| Cor Total | 0.0013 | 16 | |||
| R2 | 0.8302 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.15 | 9 | 0.1276 | 10.01 | 0.0031 |
| X1-Temperature | 0.0107 | 1 | 0.0107 | 0.8361 | 0.3909 |
| X2-Time | 6.125 × 10−6 | 1 | 6.125 × 10−6 | 0.0005 | 0.9831 |
| X3-Water content | 0.7682 | 1 | 0.7682 | 60.26 | 0.0001 |
| X1X2 | 0.0039 | 1 | 0.0039 | 0.3064 | 0.5971 |
| X1X3 | 0.0039 | 1 | 0.0039 | 0.3064 | 0.5971 |
| X2X3 | 0.0007 | 1 | 0.0007 | 0.0530 | 0.8245 |
| X12 | 0.0088 | 1 | 0.0088 | 0.6928 | 0.4327 |
| X22 | 0.0076 | 1 | 0.0076 | 0.5980 | 0.4646 |
| X32 | 0.3302 | 1 | 0.3302 | 25.90 | 0.0014 |
| Residual | 0.0892 | 7 | 0.0127 | ||
| Lack of Fit | 0.0575 | 3 | 0.0192 | 2.41 | 0.2071 |
| Pure Error | 0.0318 | 4 | 0.0079 | ||
| Cor Total | 1.24 | 16 | |||
| R2 | 0.9279 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2499.90 | 9 | 277.77 | 5.14 | 0.0211 |
| X1-Temperature | 834.36 | 1 | 834.36 | 15.44 | 0.0057 |
| X2-Time | 22.22 | 1 | 22.22 | 0.4111 | 0.5418 |
| X3-Water content | 113.21 | 1 | 113.21 | 2.09 | 0.1911 |
| X1X2 | 40.76 | 1 | 40.76 | 0.7541 | 0.4140 |
| X1X3 | 164.27 | 1 | 164.27 | 3.04 | 0.1248 |
| X2X3 | 118.20 | 1 | 118.20 | 2.19 | 0.1827 |
| X12 | 461.47 | 1 | 461.47 | 8.54 | 0.0223 |
| X22 | 376.34 | 1 | 376.34 | 6.96 | 0.0335 |
| X32 | 244.66 | 1 | 244.66 | 4.53 | 0.0709 |
| Residual | 378.38 | 7 | 54.05 | ||
| Lack of Fit | 201.49 | 3 | 67.16 | 1.52 | 0.3389 |
| Pure Error | 176.89 | 4 | 44.22 | ||
| Cor Total | 2878.28 | 16 | |||
| R2 | 0.8685 |
| Regresion Coefficient | 2nd Order Polynomial Equation |
|---|---|
| Theobromine (Y1) | 4.90 + 0.2309X1 + 0.2620X2 + 0.7129X3 − 0.2896X12 − 0.0173X22 − 0.8301X32 + 0.0793X1X2 − 0.1460X1X3 − 0.3902X2X3 |
| Caffeine (Y2) | 1.50 + 0.0365X1 + 0.0009X2 + 0.3099X3 − 0.0458X12 − 0.0426X22 − 0.2800X32 − 0.0312X1X2 − 0.0313X1X3 + 0.0130X2X3 |
| Catechin (Y3) | 0.0474 + 0.0005X1 − 0.0034X2 + 0.0054X3 + 0.0107X12 + 0.0024X22 + 0.0009X32 − 0.0035X1X2 − 0.0065X1X3 − 0.0013X2X3 |
| DPPH (Y4) | 48.14 + 10.21X1 + 1.67X2 + 3.76X3 − 10.47X12 − 9.45X22 − 7.62X32 − 3.19X1X2 − 6.41X1X3 + 5.44X2X3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlović, N.; Jokić, S.; Jakovljević, M.; Blažić, M.; Molnar, M. Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods 2020, 9, 140. https://doi.org/10.3390/foods9020140
Pavlović N, Jokić S, Jakovljević M, Blažić M, Molnar M. Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods. 2020; 9(2):140. https://doi.org/10.3390/foods9020140
Chicago/Turabian StylePavlović, Nika, Stela Jokić, Martina Jakovljević, Marijana Blažić, and Maja Molnar. 2020. "Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell" Foods 9, no. 2: 140. https://doi.org/10.3390/foods9020140
APA StylePavlović, N., Jokić, S., Jakovljević, M., Blažić, M., & Molnar, M. (2020). Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods, 9(2), 140. https://doi.org/10.3390/foods9020140








