Rapid Profiling of the Volatilome of Cooked Meat by PTR-ToF-MS: Characterization of Chicken, Turkey, Pork, Veal and Beef Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
- -
- Compare the 5 most important species/categories of meat: chicken, turkey, pork, veal and beef;
- -
- Obtain simultaneously samples of all the species from each of a wide variety of retailers (10 sampling sessions: 5 supermarkets belonging to different major chains and 5 local butchers);
- -
- Account for variation between animals within retailers by sampling 2 animals per species/category per session (5 species × 10 suppliers/sessions × 2 animals = 100 animals sampled, 20 per species/category);
- -
- Compare two very different cooking methods for each animal sampled: rapid grilling on a high-temperature surface, and slow cooking at a moderate temperature;
- -
- Account for variation among meat samples within animal and cooking method by preparing two burgers per animal and cooking method (100 animals × 2 cooking methods × 2 burgers/method = 400 burgers).No live animals were used for the research and therefore no ethical authorization was needed.
2.2. Meat Sampling
2.3. Raw Meat Processing and Cooking
2.4. Analyses of the Characteristics of Fresh and Cooked Meats
2.5. Analysis of the Volatile Organic Compounds
2.6. Data Editing and Univariate Statistical Analysis of the Sources of Variation in VOCs
- -
- Comparison of classes: poultry (C + T) vs. mammals (P + V + B);
- -
- Comparison of species within poultry: C vs. T;
- -
- Comparison of species within mammals: P vs. (V + B);
- -
- Comparison of categories within cattle species: V vs. B.
2.7. Multivariate Analyses of Latent Explanatory Factors of VOCs
- -
- LEF-1, explaining 35.1% of total variance, is based on 48 significant VOCs (having a loading >+0.5 or <−0.5) out of 129; only 2 of these were quantitatively more relevant (>10 µg/L): m/z 33.034 (tentatively identified (t.i.) as methanol), and m/z 61.035 (t.i. acetic acid, and fragment of acetate esters as butyl acetate, 2-methylbutyl acetate, isobutyl acetate);
- -
- LEF-2, explaining 20.8% of total variance, is based on 28 significant VOCs, of which the relevant ones are m/z 53.039 and m/z 69.070 (t.i. pentanal, pentenol);
- -
- LEF-3, explaining 9.5% of total variance, is based on 13 significant VOCs, of which the relevant ones are m/z 41.038 (common fragment), m/z 43.055 (common fragment), m/z 53.003, m/z 57.034 (t.i. propenal), m/z 57.070 (t.i. butanol, isobutanol) and m/z 71.085 (t.i. methyl butanol, pentanol);
- -
- LEF-4, explaining 7.6% of total variance, is based on 7 significant VOCs, of which the relevant ones are m/z 43,018 (common fragment) and m/z 89.060 (t.i. acetoin (3-hydroxy-2-butanone), ethyl acetate, butanoic acid);
- -
- LEF-5, explaining 4.7% of total variance, is based on 5 significant but quantitatively not relevant VOCs;
- -
- LEF-6, explaining 4.4% of total variance, is based on 7 significant but not relevant VOCs;
- -
- LEF-7, explaining 4.4% of total variance, is based on 5 significant VOCs, one of which is quantitatively relevant m/z 75.944;
- -
- LEF-8, explaining 3.8% of total variance, is based on 4 significant but not relevant VOCs;
- -
- LEF-9, explaining 3.5% of total variance, is based on 4 significant but not relevant VOCs;
- -
- LEF-10, explaining 3.4% of total variance, is based on 4 significant VOCs, of which m/z 31.019 (t.i. formaldehyde) and m/z 60.053 (acetone isotopolougue) are relevant;
- -
- LEF-11, explaining 3.1% of total variance, is based on 4 significant VOCs, of which the relevant ones are m/z 46.996 (t.i. thioformaldehyde), m/z 49.008 (methanethiol) and m/z 55.050 (t.i. butanal);
- -
- Another 6 minor LEFs (LEF-12 to LEF-17) explain between 2.9 and 1.6% of total variance: one (LEF-13) is based on 2 VOCs, one of which is relevant (m/z 63.026, t.i. dimethyl sulfide, ethanthiol), 4 (LEF-12, LEF-14, LEF-15, and LEF-16) are based on one significant VOC and one (LEF-17) had no VOCs reaching the threshold.
3. Results
4. Discussion
4.1. Flavor Profiles of Cooked Meat from Avian vs. Mammal Species
4.2. Flavor Profiles of Chicken vs. Turkey Cooked Meat
4.3. Flavor Profile of Pork vs. Bovine Cooked Meat
4.4. Flavor Profile of Pork vs. Bovine Cooked Meat
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Holm, E.S.; Adamsen, A.P.S.; Feilberg, A.; Schäfer, A.; Løkke, M.M.; Petersen, M.A. Quality changes during storage of cooked and sliced meat products measured with PTR-MS and HS-GC–MS. Meat Sci. 2013, 95, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Kosowska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. (Camp.) 2017, 37. [Google Scholar] [CrossRef]
- Lytou, A.E.; Nychas, G.J.E.; Panagou, E.Z. Effect of pomegranate based marinades on the microbiological, chemical and sensory quality of chicken meat: A metabolomics approach. Int. J. Food Microbiol. 2018, 267, 42–53. [Google Scholar] [CrossRef]
- Zhou, R.; Grant, J.; Goldberg, E.M.; Ryland, D.; Aliani, M. Investigation of low molecular weight peptides (<1 kDa) in chicken meat and their contribution to meat flavor formation. J. Sci. Food Agric. 2019, 99, 1728–1739. [Google Scholar] [CrossRef]
- Saraiva, C.; Oliveira, I.; Silva, J.A.; Martins, C.; Ventanas, J.; García, C. Implementation of multivariate techniques for the selection of volatile compounds as indicators of sensory quality of raw beef. J. Food Sci. Technol. 2015, 52, 3887–3898. [Google Scholar] [CrossRef]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef]
- Serrano, E.; Cornu, A.; Kondjoyan, N.; Agabriel, J.; Micol, D. Traceability of grass feeding in beef: Terpenes, 2,3-octanedione and skatole accumulation in adipose tissue of young bulls. Animal 2011, 5, 641–649. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.J.; Lee, H.C.; Yoo, S.S.; Shim, J.H.; Chin, K.B. Effects of pork meat cut and packaging type on lipid oxidation and oxidative products during refrigerated storage (8 C). J. Food Sci. 2008, 73, C127–C134. [Google Scholar] [CrossRef]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J.; Durcan, R. A comparison of solid-phase microextraction (SPME) fibres for measurement of hexanal and pentanal in cooked turkey. Food Chem. 2000, 68, 339–345. [Google Scholar] [CrossRef]
- Mielnik, M.B.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT Food Sci. Technol. 2006, 39, 191–198. [Google Scholar] [CrossRef]
- Gkarane, V.; Brunton, N.P.; Harrison, S.M.; Gravador, R.S.; Allen, P.A.; Claffey, N.A.; Diskin, M.G.; Fahey, A.G.; Farmer, L.J.; Moloney, A.P.; et al. Volatile profile of grilled lamb as affected by castration and age at slaughter in two breeds. J. Food Sci. 2018, 83, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.; Krings, U.; Berger, R.G.; Orlien, V. Aroma development in high pressure treated beef and chicken meat compared to raw and heat treated. Meat Sci. 2010, 86, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Bittante, G.; Ni, Q.; Khomenko, I.; Gallo, L.; Biasioli, F. Rapid profiling of the volatilome of cooked meat by PTR-ToF-MS: Underlying latent explanatory factors. Foods 2020, 9, 1738. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat, 2nd ed.; Version 1.02; American Meat Science Association: Chicago, IL, USA, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Bergamaschi, M.; Biasioli, F.; Cappellin, L.; Cecchinato, A.; Cipolat-Gotet, C.; Cornu, A.; Gasperi, F.; Martin, B.; Bittante, G. Proton transfer reaction time-of-flight mass spectrometry: A high-throughput and innovative method to study the influence of dairy system and cow characteristics on the volatile compound fingerprint of cheeses. J. Dairy Sci. 2015, 98, 8414–8427. [Google Scholar] [CrossRef]
- Biasioli, F.; Gasperi, F.; Yeretzian, C.; Märk, T.D. PTR–MS monitoring of VOCs and BVOCs in food science and technology. Trends Anal. Chem. 2011, 30, 968–977. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Ahn, D.U.; Nam, K.C.; Jo, C. Flavour chemistry of chicken meat: A review. Asian Australas. J. Anim. Sci. 2013, 26, 732. [Google Scholar] [CrossRef]
- Franke, C.; Beauchamp, J. Real-time detection of volatiles released during meat spoilage: A case study of modified atmosphere-packaged chicken breast fillets inoculated with Br. thermosphacta. Food Anal. Methods 2017, 10, 310–319. [Google Scholar] [CrossRef]
- Du, M.; Ahn, D.U.; Nam, K.C.; Sell, J.L. Influence of dietary conjugated linoleic acid on volatile profiles, color and lipid oxidation of irradiated raw chicken meat. Meat Sci. 2000, 56, 387–395. [Google Scholar] [CrossRef]
- Silvis, I.C.J.; Luning, P.A.; Klose, N.; Jansen, M.; van-Ruth, S.M. Similarities and differences of the volatile profiles of six spices explored by Proton Transfer Reaction Mass Spectrometry. Food Chem. 2019, 271, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Rajamäki, T.; Alakomi, H.L.; Ritvanen, T.; Skyttä, E.; Smolander, M.; Ahvenainen, R. Application of an electronic nose for quality assessment of modified atmosphere packaged poultry meat. Food Control 2006, 17, 5–13. [Google Scholar] [CrossRef]
- Keupp, C.; Höll, L.; Beauchamp, J.; Langowski, H.C. Online monitoring of volatile freshness indicators from modified atmosphere packaged chicken meat using PTR-MS. In Proceedings of the 27th IAPRI Symposium on Packaging, Valencia, Spain, 8–11 June 2015; ISBN 978-84-943398-4-4. [Google Scholar]
- del-Pulgar, J.S.; Soukoulis, C.; Biasioli, F.; Cappellin, L.; García, C.; Gasperi, F.; Granitto, P.; Märk, T.D.; Piasentier, E.; Schuhfried, E. Rapid characterization of dry cured ham produced following different PDOs by proton transfer reaction time of flight mass spectrometry (PTR-ToF-MS). Talanta 2011, 85, 386–393. [Google Scholar] [CrossRef]
- Cano-García, L.; Rivera-Jiménez, S.; Belloch, C.; Flores, M. Generation of aroma compounds in a fermented sausage meat model system by Debaryomyces hansenii strains. Food Chem. 2014, 151, 364–373. [Google Scholar] [CrossRef]
- Lustig, S.; Schuetz, J.M. Meat Product Package Containing an Inner Layer of Starch/Ethylene Vinyl Acetate. U.S. Patent 4,784,863, 15 November 1988. [Google Scholar]
- Elmore, J.S.; Campo, M.M.; Enser, M.; Mottram, D.S. Effect of lipid composition on meat-like model systems containing cysteine, ribose and polyunsaturated fatty acids. J. Agric. Food Chem. 2002, 50, 1126–1132. [Google Scholar] [CrossRef]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Relationship between odour-active compounds and flavour perception in meat from lambs fed different diets. Meat Sci. 2010, 85, 700–706. [Google Scholar] [CrossRef]
- Macleod, G.; Seyyedain-Ardebili, M.; Chang, S.S. Natural and simulated meat flavors (with particular reference to beef). Crit. Rev. Food Sci. Nutr. 1981, 14, 309–437. [Google Scholar] [CrossRef]

| Items: | Chicken | Turkey | Pork | Veal | Beef |
|---|---|---|---|---|---|
| Raw patty color: | |||||
| - lightness, L* | 49.1 ± 1.8 | 47.7 ± 2.1 | 48.1 ± 3.4 | 49.9 ± 4.5 | 37.1 ± 4.6 |
| - redness, a* | 2.4 ± 1.1 | 1.1 ± 1.1 | 3.3 ± 1.8 | 5.9 ± 2.4 | 16.0 ± 2.9 |
| - yellowness, b* | 18.7 ± 4.0 | 9.1 ± 1.2 | 13.4 ± 1.4 | 14.6 ± 1.9 | 17.6 ± 2.4 |
| Raw patty composition (g/100 g): | |||||
| - moisture | 75.3 ± 0.9 | 73.7 ± 0.8 | 72.4 ± 0.9 | 75.2 ± 1.8 | 73.7 ± 1.0 |
| - protein | 21.9 ± 0.7 | 23.8 ± 0.8 | 22.5 ± 0.8 | 20.4 ± 0.9 | 22.0 ± 0.6 |
| - lipids | 1.3 ± 0.4 | 0.7 ± 0.3 | 3.3 ± 1.3 | 2.7 ± 2.4 | 2.6 ± 1.2 |
| - ash | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Raw patty pH: | 6.02 ± 0.08 | 5.89 ± 0.24 | 5.68 ± 0.06 | 5.64 ± 0.10 | 5.60 ± 0.09 |
| Cooked patty color: | |||||
| - lightness, L* | 75.8 ± 5.0 | 71.6 ± 7.4 | 64.8 ± 8.3 | 60.2 ± 8.6 | 50.6 ± 10.4 |
| - redness, a* | 3.4 ± 2.3 | 3.8 ± 3.4 | 5.4 ± 4.3 | 5.6 ± 3.5 | 7.2 ± 3.1 |
| - yellowness, b* | 22.2 ± 6.3 | 19.1 ± 6.5 | 20.4 ± 6.5 | 21.2 ± 4.6 | 20.4 ± 3.6 |
| Cooked patty shear force, N/g | 14.6 ± 2.1 | 19.5 ± 2.8 | 21.6 ± 3.7 | 18.5 ± 4.0 | 25.2 ± 5.6 |
| m/z | Raw Formula | Tentative Identification | Least Squares Means 1 | Orthogonal Contrasts (p Value) | RMSE 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | T | P | V | B | C + T vs. P + V + B | C vs. T | P vs. V + B | V vs. B | % | |||
| 26.016 | C2H2+ | Common fragment | 0.730 | 1.011 | 1.010 | 0.881 | 0.895 | - | <0.001 | <0.001 | - | 0.162 |
| 28.032 | C2H4+ | 0.104 | 0.094 | 0.077 | 0.068 | 0.084 | <0.001 | - | - | <0.001 | 0.020 | |
| 29.039 | C2H5+ | Common fragment | 0.361 | 0.228 | 0.322 | 0.247 | 0.244 | 0.03 | <0.0001 | <0.0001 | - | 0.072 |
| 29.060 | 0.028 | 0.032 | 0.035 | 0.029 | 0.028 | - | 0.01 | <0.0001 | - | 0.004 | ||
| 31.019 | CH2OH+ | Formaldehyde | 1.577 | 1.703 | 1.202 | 1.574 | 1.800 | - | - | <0.0001 | 0.02 | 0.301 |
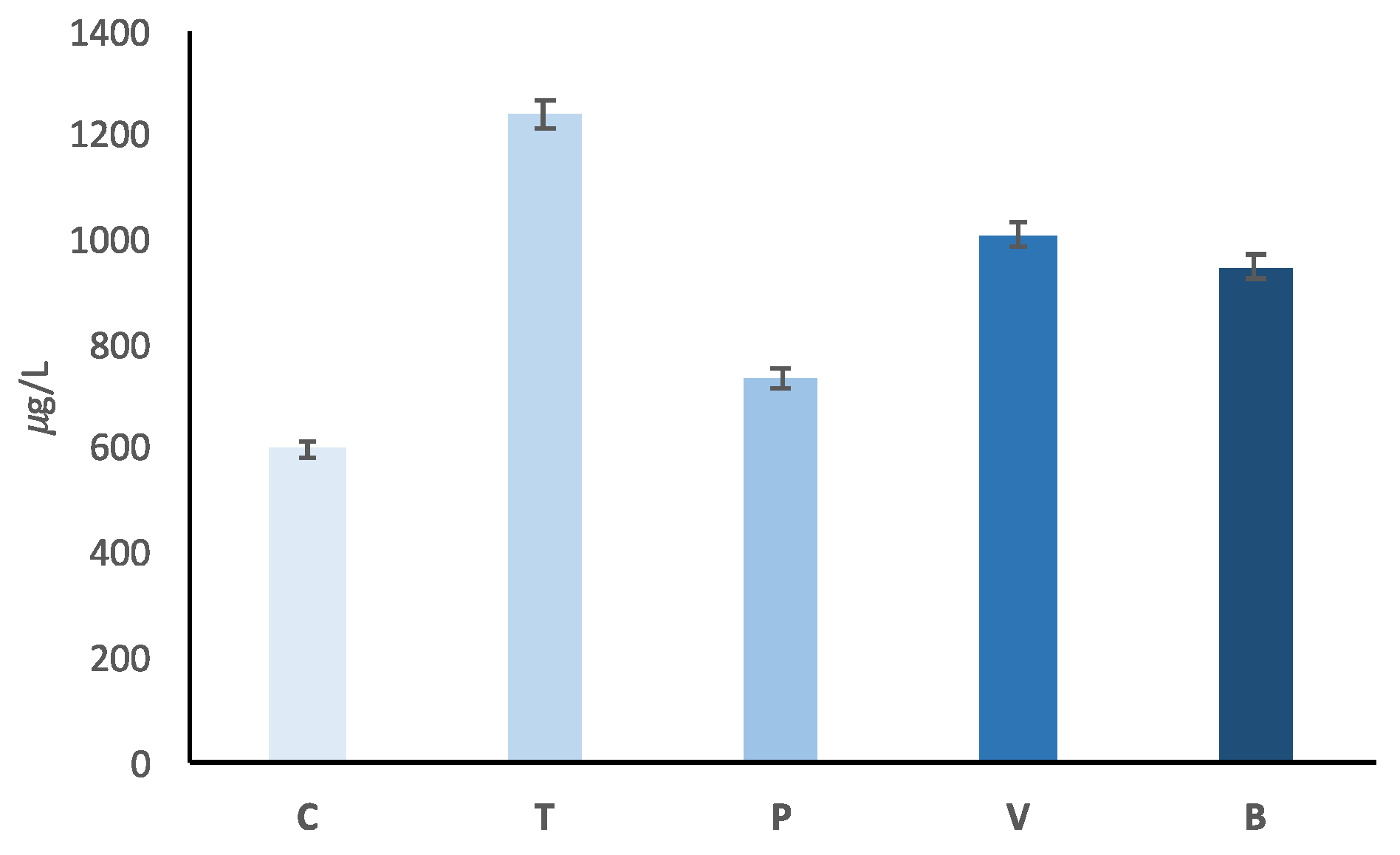
| 33.034 | CH4OH+ | Methanol | 3.279 | 2.244 | 4.217 | 3.146 | 4.230 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.697 |
| 34.996 | H2SH+ | Hydrogen sulfide | 3.870 | 0.521 | 0.887 | 0.635 | 0.704 | <0.0001 | <0.0001 | - | - | 0.603 |
| 38.018 | 0.040 | 0.047 | 0.050 | 0.044 | 0.046 | - | 0.02 | 0.04 | - | 0.012 | ||
| 41.038 | C3H5+ | Common fragment | 7.874 | 8.255 | 9.126 | 8.174 | 8.919 | 0.05 | - | - | - | 1.632 |
| 42.011 | 0.905 | 0.965 | 1.143 | 0.915 | 0.966 | 0.02 | - | <0.0001 | - | 0.198 | ||
| 42.034 | C2H3NH+ | Acetonitrile | 2.290 | 1.261 | 1.995 | 1.430 | 1.678 | - | <0.0001 | 0.01 | - | 0.740 |
| 43.018 | C2H3O+ | Common fragment | 7.092 | 5.559 | 8.824 | 12.882 | 11.225 | <0.0001 | <0.0001 | <0.0001 | <0.001 | 1.899 |
| 43.055 | C3H7+ | Common fragment | 2.605 | 1.974 | 2.902 | 2.827 | 2.736 | <0.001 | 0.03 | - | - | 0.816 |
| 46.034 | C13CH4OH+ | 0.570 | 0.672 | 0.764 | 0.735 | 0.709 | <0.0001 | <0.0001 | 0.03 | - | 0.085 | |
| 46.996 | CH2SH+ | Thioformaldehyde | 1.739 | 2.002 | 0.586 | 0.896 | 0.851 | <0.0001 | - | - | - | 0.991 |
| 47.049 | C2H6OH+ | Ethanol | 1.571 | 0.833 | 1.165 | 1.066 | 0.933 | - | <0.001 | - | - | 0.507 |
| 49.008 | CH4SH+ | Methanethiol | 7.738 | 14.756 | 1.846 | 4.875 | 4.100 | <0.0001 | <0.0001 | <0.001 | - | 4.640 |
| 52.028 | 0.006 | 0.007 | 0.006 | 0.005 | 0.005 | <0.0001 | - | <0.001 | - | 0.002 | ||
| 53.003 | 1.374 | 1.628 | 1.708 | 1.514 | 1.596 | 0.05 | <0.001 | 0.03 | - | 0.259 | ||
| 53.039 | C4H5+ | 1.454 | 2.902 | 2.601 | 2.262 | 2.075 | - | <0.0001 | 0.01 | - | 0.595 | |
| 55.050 | C4H7+ | Butanal | 14.363 | 21.819 | 24.769 | 19.182 | 17.166 | 0.01 | <0.0001 | <0.0001 | - | 5.483 |
| 57.034 | C3H4OH+ | Propenal, or common fragment | 1.596 | 1.940 | 2.074 | 1.732 | 1.838 | 0.04 | <0.0001 | <0.001 | - | 0.318 |
| 57.070 | C4H9+ | Butanol, isobutanol | 1.877 | 1.267 | 1.729 | 1.300 | 1.500 | - | <0.0001 | 0.01 | - | 0.560 |
| 59.967 | 0.275 | 0.156 | 0.198 | 0.122 | 0.153 | <0.0001 | <0.0001 | <0.001 | - | 0.061 | ||
| 60.053 | C213CH7+ | Isotope of Acetone, Propan-2-one | 2.247 | 1.169 | 0.990 | 1.550 | 1.710 | <0.0001 | <0.0001 | <0.0001 | - | 0.308 |
| 61.035 | C2H4O2H+ | Acetic acid, fragment of Butyl acetate, 2-Methylbutyl acetate, Isobutyl acetate | 5.513 | 2.705 | 5.084 | 3.793 | 4.187 | - | <0.0001 | <0.0001 | - | 1.615 |
| 62.023 | 0.077 | 0.025 | 0.024 | 0.053 | 0.052 | - | <0.0001 | <0.0001 | - | 0.016 | ||
| 63.026 | C2H6SH+ | Dimethyl sulfide, ethanthiol | 6.067 | 1.778 | 0.390 | 3.648 | 3.837 | 0.01 | <0.0001 | <0.0001 | - | 1.617 |
| 63.947 | 0.003 | 0.001 | 0.001 | 0.001 | 0.001 | <0.0001 | <0.0001 | - | - | 0.001 | ||
| 63.986 | 0.078 | 0.029 | 0.049 | 0.037 | 0.039 | <0.0001 | <0.0001 | <0.0001 | - | 0.013 | ||
| 67.021 | 0.011 | 0.017 | 0.008 | 0.009 | 0.010 | <0.0001 | <0.0001 | - | - | 0.005 | ||
| 67.055 | C5H7+ | Pentenal or common fragment | 0.273 | 0.435 | 0.419 | 0.341 | 0.345 | - | <0.0001 | <0.001 | - | 0.067 |
| 67.992 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | - | 0.02 | 0.03 | - | 0.001 | ||
| 69.034 | C4H4OH+ | Furan | 0.058 | 0.058 | 0.083 | 0.060 | 0.066 | 0.02 | - | <0.001 | - | 0.049 |
| 69.070 | C5H9+ | Isoprene or common fragment of aldehydes, alcohols and terpenes) | 2.006 | 3.543 | 3.179 | 2.389 | 2.720 | - | <0.0001 | <0.001 | - | 0.694 |
| 70.004 | 0.004 | 0.003 | 0.004 | 0.003 | 0.004 | - | <0.001 | <0.001 | - | 0.001 | ||
| 71.015 | 0.031 | 0.027 | 0.033 | 0.035 | 0.036 | <0.0001 | 0.01 | - | - | 0.006 | ||
| 71.049 | C4H6OH+ | 2-Butenal, Methyl vinyl ketone | 0.271 | 0.217 | 0.421 | 1.381 | 0.984 | <0.0001 | - | <0.0001 | <0.0001 | 0.384 |
| 71.085 | C5H11+ | Methyl butanol, Pentanol | 2.062 | 1.342 | 2.013 | 1.822 | 2.033 | 0.04 | <0.001 | - | - | 0.711 |
| 73.065 | C4H8OH+ | 2-Butanone, Butanal | 4.364 | 2.350 | 2.385 | 2.176 | 4.196 | - | <0.0001 | 0.05 | <0.0001 | 0.970 |
| 75.028 | C3H6SH+ | Allyl mercaptan | 0.096 | 0.049 | 0.081 | 0.063 | 0.067 | - | <0.0001 | <0.0001 | - | 0.021 |
| 75.044 | C3H6O2H+ | Propanoic acid, Methyl acetate? | 0.374 | 0.251 | 0.415 | 0.381 | 0.382 | <0.0001 | <0.0001 | 0.02 | - | 0.081 |
| 75.081 | C4H10OH+ | 0.059 | 0.035 | 0.056 | 0.038 | 0.042 | - | <0.0001 | <0.001 | - | 0.030 | |
| 75.944 | 3.676 | 0.912 | 1.308 | 1.023 | 1.110 | <0.0001 | <0.0001 | - | - | 1.424 | ||
| 77.016 | 0.027 | 0.016 | 0.022 | 0.018 | 0.020 | 0.03 | <0.0001 | <0.001 | 0.02 | 0.005 | ||
| 77.059 | C3H8O2H+ | Propylene Glycol | 0.892 | 0.723 | 0.474 | 0.742 | 0.854 | <0.0001 | 0.01 | <0.0001 | - | 0.156 |
| 77.976 | 0.054 | 0.026 | 0.030 | 0.023 | 0.028 | <0.0001 | <0.0001 | - | - | 0.012 | ||
| 78.979 | 0.006 | 0.007 | 0.006 | 0.005 | 0.006 | <0.001 | 0.02 | 0.02 | - | 0.004 | ||
| 79.039 | C2H6O3H+ | Adduct of water and C2H4O2H+ | 0.149 | 0.072 | 0.161 | 0.118 | 0.124 | <0.0001 | <0.0001 | <0.0001 | - | 0.051 |
| 79.055 | C6H7+ | Benzene, Aromatic fragment | 0.536 | 0.447 | 0.659 | 0.355 | 0.477 | - | - | 0.01 | - | 0.643 |
| 79.938 | 0.009 | 0.002 | 0.003 | 0.003 | 0.003 | <0.0001 | <0.0001 | - | - | 0.003 | ||
| 80.041 | 0.016 | 0.008 | 0.015 | 0.011 | 0.012 | - | <0.0001 | <0.0001 | - | 0.003 | ||
| 81.038 | C5H4OH+ | 0.063 | 0.038 | 0.054 | 0.043 | 0.049 | - | <0.0001 | <0.0001 | 0.00 | 0.009 | |
| 81.071 | C6H9+ | Hexenal, common fragment | 0.249 | 0.433 | 0.389 | 0.340 | 0.349 | - | <0.0001 | 0.04 | - | 0.085 |
| 82.047 | 0.008 | 0.006 | 0.007 | 0.005 | 0.006 | <0.0001 | <0.0001 | <0.001 | 0.03 | 0.002 | ||
| 84.044 | C513CH11+ | Hexanal, Hexenol | 0.019 | 0.014 | 0.021 | 0.014 | 0.015 | - | <0.0001 | <0.0001 | - | 0.006 |
| 85.014 | C4H4SH+ | Thiophene | 0.050 | 0.027 | 0.026 | 0.016 | 0.022 | <0.001 | <0.001 | - | - | 0.020 |
| 85.073 | 0.052 | 0.055 | 0.059 | 0.051 | 0.052 | - | - | <0.0001 | - | 0.009 | ||
| 85.101 | C6H13+ | Hexanol | 0.248 | 0.170 | 0.255 | 0.191 | 0.218 | - | <0.001 | 0.03 | - | 0.109 |
| 86.022 | 0.012 | 0.006 | 0.009 | 0.006 | 0.008 | <0.001 | <0.0001 | <0.0001 | 0.04 | 0.003 | ||
| 86.970 | 0.008 | 0.005 | 0.011 | 0.010 | 0.010 | <0.0001 | <0.0001 | - | - | 0.004 | ||
| 87.044 | C4H6O2H+ | 2,3-Butanedione, diacetyl | 0.315 | 0.243 | 0.394 | 0.616 | 0.581 | <0.0001 | - | <0.0001 | - | 0.150 |
| 87.080 | C5H10OH+ | Pentanal, Pentanone | 0.432 | 0.756 | 0.810 | 0.673 | 0.741 | <0.0001 | <0.0001 | 0.01 | - | 0.230 |
| 88.960 | 0.007 | 0.003 | 0.007 | 0.010 | 0.008 | <0.0001 | <0.0001 | <0.001 | 0.01 | 0.002 | ||
| 89.060 | C4H8O2H+ | Acetoin (3-Hydroxy-2-butanone),Ethyl acetate, Butanoic acid | 0.393 | 0.235 | 0.997 | 3.609 | 2.302 | <0.0001 | - | <0.0001 | <0.0001 | 0.726 |
| 91.059 | C4H10SH+ | Diethyl sulfide | 0.143 | 0.096 | 0.143 | 0.116 | 0.121 | - | <0.001 | 0.02 | - | 0.073 |
| 93.069 | C7H9+ | Toluene | 0.369 | 0.207 | 0.273 | 0.198 | 0.214 | <0.001 | <0.0001 | 0.01 | - | 0.176 |
| 95.019 | C2H6O2SH+ | Dimethyl sulfone | 0.410 | 0.199 | 0.359 | 0.265 | 0.253 | - | <0.0001 | <0.0001 | - | 0.139 |
| 95.053 | 0.140 | 0.086 | 0.133 | 0.092 | 0.105 | - | <0.0001 | <0.0001 | - | 0.040 | ||
| 95.088 | C7H11+ | Heptenal, common fragment | 0.062 | 0.071 | 0.072 | 0.062 | 0.065 | - | <0.001 | <0.001 | - | 0.011 |
| 97.064 | C6H8OH+ | 2,5-Dimethylfuran, Ethylfuran | 0.049 | 0.091 | 0.056 | 0.067 | 0.078 | - | <0.0001 | <0.001 | 0.03 | 0.021 |
| 97.101 | 0.208 | 0.176 | 0.180 | 0.167 | 0.228 | - | 0.02 | - | <0.0001 | 0.048 | ||
| 99.082 | C6H10OH+ | 2-Hexenal, Trans-2-hexenal, 2-Hexanone, Hexanone acid | 0.048 | 0.087 | 0.077 | 0.068 | 0.071 | - | <0.0001 | - | - | 0.019 |
| 101.097 | C6H12OH+ | Hexanal, Hexan-1-one, Hexan-2-one | 2.498 | 6.605 | 5.565 | 4.764 | 4.531 | - | <0.0001 | 0.02 | - | 1.724 |
| 102.026 | 0.009 | 0.005 | 0.008 | 0.006 | 0.006 | - | <0.0001 | <0.0001 | - | 0.002 | ||
| 103.048 | 0.025 | 0.013 | 0.021 | 0.016 | 0.018 | - | <0.0001 | <0.001 | 0.03 | 0.005 | ||
| 105.041 | 0.012 | 0.007 | 0.011 | 0.011 | 0.012 | <0.0001 | <0.0001 | - | - | 0.002 | ||
| 105.069 | C8H9+ | Styrene | 0.047 | 0.017 | 0.029 | 0.021 | 0.022 | - | <0.0001 | - | - | 0.019 |
| 106.079 | 0.021 | 0.015 | 0.024 | 0.014 | 0.017 | - | - | 0.01 | - | 0.022 | ||
| 107.056 | 0.088 | 0.057 | 0.088 | 0.085 | 0.094 | <0.0001 | <0.0001 | - | 0.05 | 0.018 | ||
| 107.086 | C8H11+ | Xylene | 0.638 | 0.518 | 0.854 | 0.422 | 0.580 | - | - | 0.01 | - | 0.983 |
| 109.076 | C6H8N2H+ | 2,5-Dimethylpyrazine | 0.021 | 0.016 | 0.031 | 0.021 | 0.025 | <0.001 | - | 0.01 | - | 0.017 |
| 109.103 | C8H13+ | Octenal, common fragment | 0.058 | 0.047 | 0.051 | 0.044 | 0.046 | <0.0001 | <0.0001 | <0.001 | - | 0.011 |
| 110.969 | 0.006 | 0.003 | 0.004 | 0.004 | 0.005 | - | <0.0001 | - | - | 0.001 | ||
| 111.118 | C8H15+ | Octenol, Octanal | 0.124 | 0.159 | 0.122 | 0.101 | 0.110 | <0.0001 | <0.001 | 0.03 | - | 0.028 |
| 115.079 | C6H10O2H+ | Caprolactone | 0.031 | 0.018 | 0.027 | 0.022 | 0.023 | - | <0.0001 | <0.0001 | - | 0.005 |
| 115.113 | C7H14OH+ | Heptanal, Heptan-2-one | 0.090 | 0.096 | 0.091 | 0.080 | 0.100 | - | - | - | <0.001 | 0.022 |
| 117.092 | C6H12O2H+ | Hexanoic acid, Ethyl butanoate, Methyl isovalerate and other C6 esters/acids | 0.030 | 0.017 | 0.025 | 0.023 | 0.023 | - | <0.0001 | - | - | 0.005 |
| 118.056 | 0.002 | 0.001 | 0.002 | 0.003 | 0.003 | <0.0001 | <0.0001 | <0.0001 | 0.01 | 0.001 | ||
| 119.105 | C6H14O2H+ | 0.126 | 0.258 | 0.239 | 0.197 | 0.182 | - | <0.0001 | <0.001 | - | 0.058 | |
| 121.066 | C8H8OH+ | Acetophenone, 4-Methyl-benzaldehyde | 0.037 | 0.018 | 0.030 | 0.024 | 0.026 | - | <0.0001 | 0.01 | - | 0.010 |
| 121.105 | C9H13+ | Trimethylbenzene | 0.010 | 0.008 | 0.011 | 0.008 | 0.008 | - | <0.0001 | <0.0001 | - | 0.003 |
| 123.050 | C4H10O2SH+ | 0.019 | 0.010 | 0.017 | 0.013 | 0.014 | - | <0.0001 | <0.0001 | - | 0.004 | |
| 123.114 | C9H15+ | Nonenal | 0.015 | 0.012 | 0.014 | 0.011 | 0.013 | - | <0.001 | <0.001 | 0.02 | 0.003 |
| 125.024 | C6H4O3H+ | Hydroxy-benzoquinone | 0.009 | 0.005 | 0.007 | 0.006 | 0.006 | - | <0.0001 | <0.0001 | - | 0.002 |
| 125.067 | 0.009 | 0.008 | 0.010 | 0.008 | 0.009 | - | <0.001 | <0.0001 | - | 0.002 | ||
| 125.097 | C8H12OH+ | Octadienone | 0.057 | 0.156 | 0.127 | 0.130 | 0.125 | 0.02 | <0.0001 | - | - | 0.044 |
| 125.132 | C9H17+ | Nonanal, Nonenol | 0.027 | 0.031 | 0.027 | 0.022 | 0.026 | 0.01 | - | - | - | 0.007 |
| 127.081 | 0.006 | 0.004 | 0.006 | 0.005 | 0.005 | - | <0.0001 | <0.0001 | - | 0.001 | ||
| 127.113 | C8H14OH+ | Octenal, 1-Octen-3-one | 0.022 | 0.022 | 0.024 | 0.021 | 0.020 | - | - | <0.001 | - | 0.004 |
| 128.973 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | <0.001 | 0.000 | ||
| 129.093 | C7H12O2H+ | Butyl propenoate, Allyl butyrate | 0.014 | 0.008 | 0.012 | 0.010 | 0.011 | - | <0.0001 | <0.001 | - | 0.002 |
| 129.128 | C8H16OH+ | Octanal, Octanone | 0.071 | 0.073 | 0.054 | 0.044 | 0.057 | <0.0001 | - | - | <0.001 | 0.013 |
| 130.041 | 0.006 | 0.003 | 0.005 | 0.004 | 0.004 | - | <0.0001 | <0.001 | - | 0.001 | ||
| 131.076 | 0.005 | 0.003 | 0.004 | 0.005 | 0.004 | <0.0001 | <0.0001 | - | 0.01 | 0.001 | ||
| 131.109 | C7H14O2H+ | Heptanoic acid, Ethyl-2-methylbutanoate, Ethyl-3-methylbutanoate, Methylbutyl acetate or other C7 esters/acids | 0.006 | 0.004 | 0.005 | 0.004 | 0.005 | - | <0.0001 | <0.001 | <0.001 | 0.001 |
| 133.112 | 0.006 | 0.005 | 0.005 | 0.005 | 0.006 | - | <0.0001 | - | <0.001 | 0.001 | ||
| 134.975 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | - | - | <0.001 | - | 0.000 | ||
| 135.043 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | - | <0.0001 | <0.001 | 0.04 | 0.000 | ||
| 135.087 | C6H14OSH+ | 3-Mercaptohexanol | 0.005 | 0.003 | 0.004 | 0.003 | 0.004 | - | <0.0001 | <0.0001 | - | 0.001 |
| 137.067 | 0.007 | 0.004 | 0.006 | 0.005 | 0.005 | - | <0.0001 | <0.0001 | - | 0.001 | ||
| 137.132 | C10H17+ | Monoterpenes | 0.028 | 0.017 | 0.023 | 0.016 | 0.017 | 0.01 | <0.0001 | <0.001 | - | 0.009 |
| 139.114 | C9H14OH+ | 2,6-Nonaienal, Isophorone, Pentylfuran | 0.021 | 0.056 | 0.034 | 0.038 | 0.031 | 0.05 | <0.0001 | - | 0.03 | 0.010 |
| 141.130 | C9H16OH+ | Nonenal, Nonenone | 0.006 | 0.008 | 0.007 | 0.006 | 0.007 | 0.01 | <0.0001 | - | - | 0.001 |
| 143.106 | C8H14O2H+ | Hexenyl acetate, | 0.138 | 0.407 | 0.358 | 0.375 | 0.359 | <0.001 | <0.0001 | - | - | 0.142 |
| 143.146 | C9H18OH+ | Methyloctanol, Nonanal, Nonan-2-one | 0.073 | 0.081 | 0.073 | 0.059 | 0.072 | 0.04 | - | - | - | 0.021 |
| 145.060 | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | <0.001 | <0.0001 | 0.01 | - | 0.000 | ||
| 147.130 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.02 | - | - | - | 0.001 | ||
| 151.120 | 0.005 | 0.003 | 0.005 | 0.003 | 0.004 | <0.001 | <0.0001 | <0.0001 | - | 0.001 | ||
| 153.131 | 0.005 | 0.004 | 0.005 | 0.004 | 0.004 | - | 0.02 | <0.0001 | - | 0.001 | ||
| 159.137 | C9H18O2H+ | Nonanoic acid, 3-Methylbutyl butanoate or other C9 easters/acids | 0.009 | 0.014 | 0.009 | 0.012 | 0.012 | - | <0.0001 | <0.0001 | - | 0.004 |
| 160.899 | 0.002 | 0.000 | 0.001 | 0.001 | 0.001 | <0.0001 | <0.0001 | <0.001 | - | 0.001 | ||
| 161.120 | 0.004 | 0.006 | 0.006 | 0.005 | 0.005 | 0.02 | <0.0001 | 0.01 | - | 0.001 | ||
| 165.161 | C12H21+ | 2-Dodecenal | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.01 | - | 0.01 | - | 0.000 |
| 173.148 | 0.002 | 0.002 | 0.002 | 0.001 | 0.002 | <0.001 | 0.02 | - | 0.01 | 0.000 | ||
| 175.122 | 0.002 | 0.003 | 0.003 | 0.002 | 0.002 | 0.01 | <0.0001 | <0.0001 | - | 0.000 | ||
| 187.169 | C11H22O2H+ | Methyl caprate, Ethyl nonanoate or other C11 esters/acids | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.04 | <0.0001 | - | - | 0.001 |
| 201.182 | C11H24O2H+ | 0.002 | 0.008 | 0.005 | 0.005 | 0.005 | - | <0.0001 | - | - | 0.003 | |
| 241.959 | 0.003 | 0.003 | 0.004 | 0.003 | 0.003 | 0.01 | <0.001 | <0.001 | - | 0.001 | ||
| Latent Factors | Least Squares Means 1 | Orthogonal Contrasts (p Value) | RMSE 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | T | P | V | B | C + T vs. P+ V + B | C vs. T | P vs. V + B | V vs. B | % | |
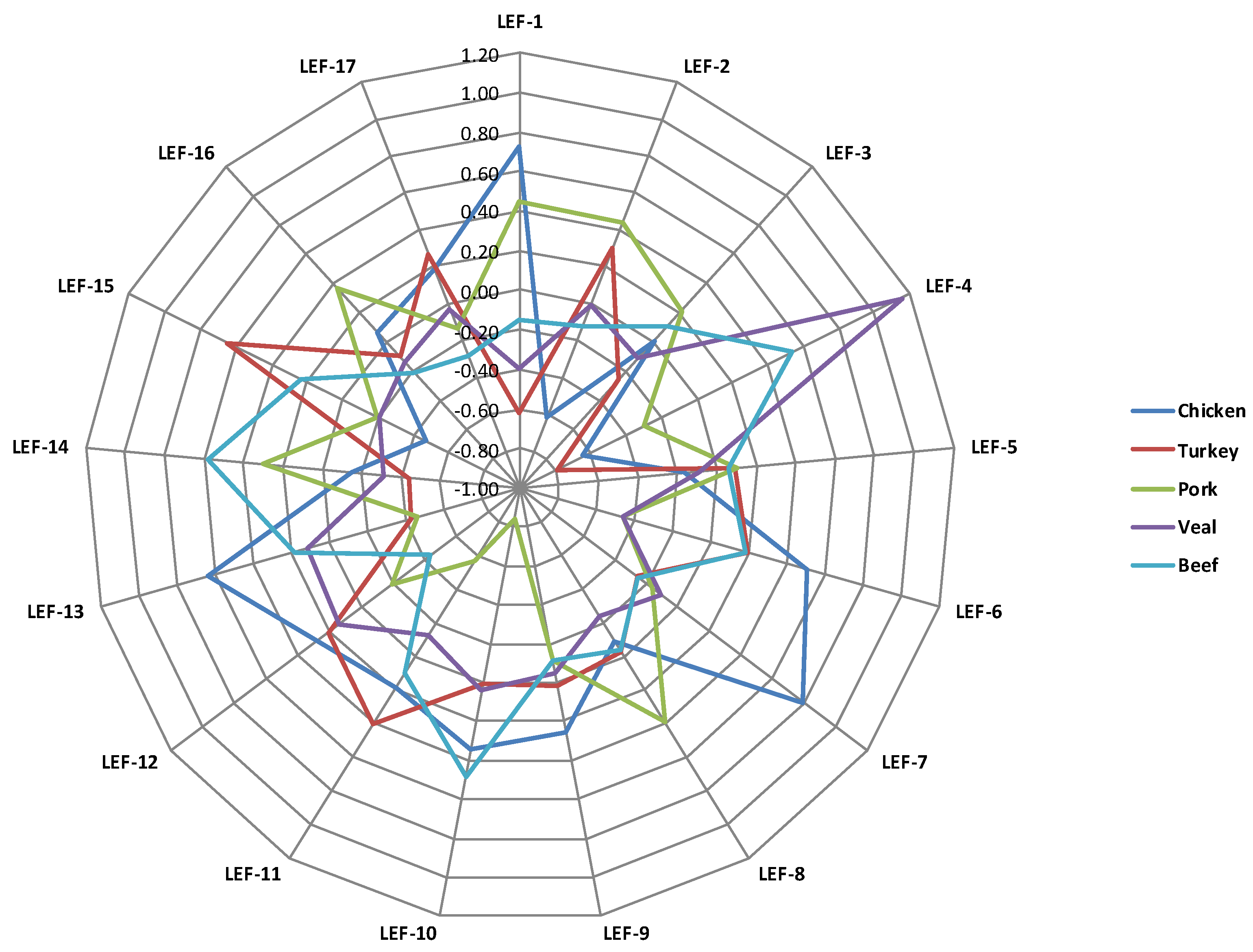
| LEF-1 | 0.72 | −0.62 | 0.44 | −0.40 | −0.15 | - | <0.001 | <0.001 | - | 0.63 |
| LEF-2 | −0.62 | 0.30 | 0.44 | −0.01 | −0.12 | 0.03 | <0.001 | 0.002 | - | 0.72 |
| LEF-3 | 0.00 | −0.26 | 0.21 | −0.11 | 0.10 | - | - | - | - | 0.60 |
| LEF-4 | −0.64 | −0.78 | −0.30 | 1.15 | 0.54 | <0.001 | - | <0.001 | <0.001 | 0.41 |
| LEF-5 | −0.16 | 0.09 | 0.10 | −0.09 | 0.07 | - | - | - | - | 0.95 |
| LEF-6 | 0.50 | 0.19 | −0.46 | −0.46 | 0.19 | <0.001 | - | - | 0.001 | 0.62 |
| LEF-7 | 0.79 | −0.26 | −0.16 | −0.10 | −0.25 | <0.001 | <0.001 | - | - | 0.74 |
| LEF-8 | −0.10 | −0.02 | 0.39 | −0.24 | −0.03 | - | - | <0.001 | - | 0.96 |
| LEF-9 | 0.25 | 0.01 | −0.11 | −0.05 | −0.11 | - | - | - | - | 0.44 |
| LEF-10 | 0.34 | 0.00 | −0.84 | 0.04 | 0.49 | 0.04 | - | <0.001 | 0.03 | 0.39 |
| LEF-11 | 0.18 | 0.40 | −0.57 | −0.13 | 0.10 | <0.001 | - | <0.001 | - | 0.69 |
| LEF-12 | 0.28 | 0.21 | −0.20 | 0.14 | −0.43 | <0.001 | - | - | 0.003 | 0.87 |
| LEF-13 | 0.63 | −0.43 | −0.47 | 0.11 | 0.18 | - | <0.001 | <0.001 | - | 0.65 |
| LEF-14 | −0.15 | −0.44 | 0.30 | −0.31 | 0.58 | <0.001 | - | - | <0.001 | 0.73 |
| LEF-15 | −0.47 | 0.65 | −0.20 | −0.20 | 0.24 | - | <0.001 | - | 0.007 | 0.69 |
| LEF-16 | 0.06 | −0.10 | 0.37 | −0.13 | −0.21 | - | - | <0.001 | - | 0.89 |
| LEF-17 | 0.19 | 0.27 | −0.13 | −0.03 | −0.28 | <0.001 | - | - | - | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Q.; Khomenko, I.; Gallo, L.; Biasioli, F.; Bittante, G. Rapid Profiling of the Volatilome of Cooked Meat by PTR-ToF-MS: Characterization of Chicken, Turkey, Pork, Veal and Beef Meat. Foods 2020, 9, 1776. https://doi.org/10.3390/foods9121776
Ni Q, Khomenko I, Gallo L, Biasioli F, Bittante G. Rapid Profiling of the Volatilome of Cooked Meat by PTR-ToF-MS: Characterization of Chicken, Turkey, Pork, Veal and Beef Meat. Foods. 2020; 9(12):1776. https://doi.org/10.3390/foods9121776
Chicago/Turabian StyleNi, Qianlin, Iuliia Khomenko, Luigi Gallo, Franco Biasioli, and Giovanni Bittante. 2020. "Rapid Profiling of the Volatilome of Cooked Meat by PTR-ToF-MS: Characterization of Chicken, Turkey, Pork, Veal and Beef Meat" Foods 9, no. 12: 1776. https://doi.org/10.3390/foods9121776
APA StyleNi, Q., Khomenko, I., Gallo, L., Biasioli, F., & Bittante, G. (2020). Rapid Profiling of the Volatilome of Cooked Meat by PTR-ToF-MS: Characterization of Chicken, Turkey, Pork, Veal and Beef Meat. Foods, 9(12), 1776. https://doi.org/10.3390/foods9121776








