Abstract
The main goal of the present study was evaluating the effect of enriching meat products (cooked (C-SAU) and dry-cured sausages (D-SAU)) with monolayered (Mo) and multilayered (Mu) fish oil microcapsules on the profile of volatile compounds, with special interest in lipid oxidation markers. For that, Solid-Phase Microextraction (SPME) and Gas Chromatography-Mass Spectrometry (GC-MS) were used. Significant differences were found in the volatile compound profile between Mo and Mu, which was been reflected in the meat samples. Thus, in general, volatile compounds from lipid oxidation have shown higher abundance in Mo and C-SAU and D-SAU enriched with this type of microcapsule, indicating that the wall of Mu (chitosan-maltodextrine) might protect the encapsulated bioactive compounds more effectively than that of Mo (maltodextrine). However, this finding is not reflected in the results of previous studies evaluating the sensory perception and oxidation stability of C-SAU and D-SAU, but it should be considered since unhealthy oxidation products can be formed in the enriched meat products with Mo. Thus, the addition of Mu as an omega-3 vehicle for enriching meat products may be indicated.
1. Introduction
Numerous epidemiological studies suggest that a diet rich in omega-3 polyunsaturated fatty acids (ω-3 PUFAs), mainly eicosapentaenoic acid (EPA; C20:5 ω-3) and docosahexaenoic acid (DHA; C22:6 ω-3), has a marked influence in the avoidance and therapy of a series of chronic disorders [1], particularly coronary heart disease [2,3,4,5]. However, Western diets are poor in long chain ω-3 PUFAs and the liking of fish and seafood products (main sources of EPA and DHA) is currently static or declining [6]. Therefore, to improve the welfare state of the population, different professional organizations and health agencies have recommended the consumption of around 0.25 g EPA + DHA per person and day [7,8,9,10]. There also is a European Union law that sets up the minimum quantities of EPA + DHA (40 and 80 mg per 100 g and per 100 kcal) to label a food as a “source of ω-3 fatty acids” and “high in ω-3 fatty acids”, respectively [11].
Nowadays, meat products are highly appreciated, with a habitual consumption of around 3–4 times per week [12], which is principally ascribed to the modern standards of life and the demand of “ready-to-eat” products. The content of high biological value proteins in meat products is valued; however, the percentage saturated fatty acids (SFA) and the ratio of ω-6/ω-3 PUFA is not so desirable in some of these foods [13]. Hence, industries are focused on the developing of meat products with a healthier lipid profile [14], there being some investigations on the possibility of incorporating fish oils, as bulk or emulsified, in different foods [15,16,17]. However, the presence of numerous double bonds in ω-3 PUFAs causes a rapid oxidation in contact with oxidant promoters, like iron, light, oxygen and high temperatures [18,19] that accelerate the formation of primary oxidation products, such as hydroperoxides, which easily isomerize and degrade to volatile compounds. Some of them impart undesirable off-fishy and rancid odors and flavors such as, 4-heptenal, 3,5-octadiene or 2-ethylfuran [20,21,22].
In this context, several studies have investigated the possibility of producing stable foods enriched with ω-3 PUFA microcapsules [15,23,24,25]. The microencapsulation technique aims to form a wall around the active compounds, reducing the perception of off-flavors [26] and the contact with oxidant promoters [18,19]. Moreover, this technique is of easy application in the food industry, economic and scalable [27]. Recent studies have pointed out the possibility of adding fish oil microcapsules to enrich different meat products, which have been recently reviewed [28,29].
Most studies on enrichment of meat products with ω-3 PUFAs microcapsules have focused on the evaluation of the proximal composition, oxidative stability, fatty acid profile and sensory attributes of the enriched foods [24,25,30,31], without taking into account the influence of the different processing conditions and microcapsules addition on the volatile compounds profile of these meat derivatives, except for a previous study in chicken nuggets [32].
Authors of the present study have developed two types of fish oil microcapsules (from homogenized monolayered (Mo) and multilayered (Mu) fish oil emulsions) with high quality characteristics in terms of yield, microencapsulation efficiency and oxidative stability [29]. Results on meat products have shown similar quality characteristics in control and enriched batches with Mo and Mu fish oil microcapsules [28].
The main objective of the present study was to evaluate the profile of volatile compounds of cooked and dry cured meat products enriched with monolayered and multilayered fish oil microcapsules, paying special attention to those from oxidation processes. The profile of volatile compounds of the fish oil microcapsules was also investigated.
2. Materials and Methods
2.1. Experimental Design
This study was carried out with cooked (C-SAU) and dry-cured sausages (D-SAU). For each meat product, three batches were prepared: added with monolayered (Mo) (C-SAU-Mo and D-SAU-Mo) and multilayered (Mu) microcapsules (C-SAU-Mu and D-SAU-Mu), and a control batch (without enriching) (C-SAU-Co, D-SAU-Co). In the added bathes, the batter was modified by the addition of 2.75% (w/w) of Mo and 5.26% (w/w) of Mu. These are 3 and 5 g of Mo and Mu, respectively, per 100 g of dough. The different quantities of Mo and Mu added are due to the differences between Mo and Mu in the efficiency of fish oil encapsulation (87.39 and 56.43%, respectively) and consequently, in the quantity of EPA and DHA (2.75 and 5.26 g EPA + DHA per 100 g of microcapsule, respectively). The quantities of Mo and Mu were deliberately added in order to give 40 mg of EPA + DHA per 100 g of meat products and labelling with “source of ω-3 fatty acids”.
The profile of volatile compounds was analyzed in the three batches of C-SAU and D-SAU, and also in Mo and Mu.
2.2. Preparation of Omega-3 Sources
The source of EPA and DHA was fish oil from cod liver, supplied by Biomega Nutrition (Silkeborg, Spain) and containing 5.96% EPA and 25.83% DHA. Mo and Mu microcapsules were prepared following the procedure described by [33], with slight modifications. Firstly, Mo and Mu emulsions were prepared, by mixing fish oil (20 g) and soybean lecithin (6 g), provided by Across Organics (Madrid, Spain), with a magnetic stirring overnight. Water was added until the mixture reached a total weight of 200 g. The mixture was homogenized (20,000 rpm, 10 min) using an Ultraturrax T-18 basic (IKA, Königswinter, Germany), with a primary emulsion that was homogenized at high-pressure (SPX, model APV-200a, Silkeborg, Denmark). The high-pressure homogenization conditions have been previously optimized: 1200 Ba and 3 passes for Mo and 1100 and 2 passes for Mu [29]. The next step was different for each type of emulsion: water (200 g) was blended with Mo, while 200 g of 1% of chitosan (w/w) with 95% of deacetylation (Chitoclear FG 95, kindly provided by Trades, Murcia, Spain) in acetic acid 1% were added to Mu. The mixtures were slowly agitated with a magnetic stirrer for 15 min. Finally, in Mo and Mu, 400 g of maltodextrin solution (120 g maltodextrin + 280 g water) with a dextrose equivalent of 12% (Glucidex 12, kindly provided Roquette, Lestrem, France) were added. Thus, feed emulsions (800 g) of Mo and Mu were obtained.
A laboratory-scale spray drier equipped with a 0.5-mm nozzle atomizer (Mini spray-dryer B-290, Buchi, Switzerland) was used to dry the feed emulsions, applying the following parameters (80% aspirator rate = 80%, feed rate = 1 L/h, inlet temperature = 180 °C, outlet temperature = 85–90 °C). During the spray-drying process, the emulsions were agitated in a magnetic stirrer at room temperature. The dried powders collected were refrigerated (4 °C) until they were added to the meat products. Quality characteristics of Mo and Mu emulsions and microcapsules have been previously analyzed [29].
2.3. Elaboration of Meat Products
The ingredients for the elaboration of C-SAU were meat mechanically separated from chicken (60%), water (20%), pork fat (12%), salt (14g/kg), pork plasma (8%), stabilizer (E-450), aromas, vegetable fiber, spices, spice extracts, smoke flavor, antioxidant (E-316) and preservative (E-250). Moreover, the enriched batches were added with the corresponding microcapsules in the knead phase. The mincer Asgo MVZ 600 (Oporto, Portugal) was used for mixing. All C-SAU batches followed the same processing: stuffing in 18 mm diameter cellulose casings, using a Stuffer Vernag HPE (Barcelona, Spain), heating at 85 °C for 30 min in a cooking pot Gaser MCA 200 (Girona, Spain), cooling at 7 °C for 1 h, and finally being vacuum packed. Samples were maintained in refrigeration (0–5 °C for 7–9 days), heated (90 °C, 3 min) and analyzed.
In the case of D-SAU, the ingredients were Iberian pork meat (80%) and fat (15%), salt (20 g/kg), dextrose, soy protein, spices, aromas, stabilizers (E-451 and E-450), antioxidant (E-301), preservatives (E-252 and E-250), flavor enhancer (E-621), coloring (E-120) and the corresponding microcapsules in the case of the enriched batches. The meat and the fat were minced (Sheydelman AU 200, Aalen, Germany), through a 6 mm diameter mincing plate, mixed with the rest of ingredients (Asgo MVZ 600, Porto, Portugal). No starter culture was added. Collagen casings (40 cm length, 60 mm diameter) and a Stuffer Vernag HPE (Barcelona, Spain) were used to stuff the dough. Once stuffing, the products were dry-cured processed in three consecutive stages: (i) 4 °C, 82% of relative humidity, 3 days; (ii) 8 °C, 80% of relative humidity, 21 days; (iii) 5 °C, 85% relative humidity, 14 days. At the end of this process, the percentage of loss was 38–40%. The dry-cured sausages were analyzed after storing at ambient temperature (18–20 °C for 7–9 days).
C-SAU and D-SAU batches were formulated and manufactured in a meat industry (remain anonymous).
2.4. Analysis of Volatile Compounds
Microcapsules and minced meat products were sampled (1 g) into a 10 mL glass flask (Hewlett–Packard, Palo Alto, CA, USA) sealed with an aluminum cap and polytetrafluoroethylene (PTFE) butyl septum (Perkin-Elmer, Foster City, CA, USA). A solid-phase microextraction (SPME) method [34] was first applied for the volatile compound extraction. A cross-linked carboxen/polydimethylsiloxane fiber (10 mm long, 100 µm thick; Supelco, Bellefonte, PA, USA) was used. It was conditioned (220 °C, 50 min) in the gas chromatograph (GC) injection port before use. For the absorption of the volatile compounds of samples, the fiber was introduced in the sealed vial, being placed in a water bath a 40 °C, for 30 min. After that, SPME fiber was moved to the injection port and maintained for 30 min for desorption. Analyses were performed using a Hewlett–Packard 6890 series II GC coupled to a mass selective detector (HP 5973) (Hewlett–Packard, Wilmington, DE, USA). A 5% phenyl–95% polydimethylsiloxane column (30 m × 0.32 mm ID, 1.05 μm film thickness, Hewlett–Packard) was used, operating at 40 °C, with a column head pressure of 6 psi of and a flow of 1.3 mL min−1. The mode of the injection port was splitless. The following temperature program was applied: isothermal for 15 min at 35 °C, increased to 150 °C at 4 °C min−1, and then to 250 °C at 20 °C min−1. Temperature of the transfer line to the mass spectrometer was 280 °C. The mass spectra were obtained using a mass selective detector by electronic impact at 70 eV, a multiplier voltage of 1756 V and collecting data at a rate of one scan over the m/z range of 30–550 u.m.a. The linear retention indexes (LRIs) of the volatile compounds were calculated by means of N-alkanes (Sigma R-8769), which were analyzed under the same conditions. Identification of volatile compounds of samples was done by comparison of mass spectra with databases (National Institute of Standards and Technology (NIST) and Wiley libraries) and by comparison of their LRI with those available in the literature [20,21,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Results from volatile analyses are provided in area units (AU).
2.5. Sampling Replication and Statistical Analysis
Replicate experimental samples (n = 3) of Mo and Mu microcapsules and of the three batches (Co, Mo and Mu) of meat products (C-SAU and D-SAU) were analyzed in triplicate. One-way analysis of variance (ANOVA) was applied to evaluate the addition of different types of fish oil microcapsules and the differences between microcapsules. In the case of being significant effects (p < 0.05), paired comparisons between means were conducted using Tukey’s test. Furthermore, all volatile compounds that showed significant differences in the ANOVA analysis were included into a principal component analysis (PCA). The statistics were run using the program IBM SPSS Statistics v.22 (IBM, Armonk, NY, USA).
3. Results and Discussion
3.1. Volatile Compounds in Fish Oil Microcapsules
A total of 40 volatile compounds were identified in the Mo and Mu fish oil microcapsules, which were grouped in the following chemical families: aliphatic hydrocarbons, alcohols, aldehydes, ketones, furans and acids. Figure 1 shows the area percentage of each chemical family in Mo and Mu. In both types of fish oil microcapsules, aliphatic hydrocarbon was the major chemical family, followed in decreasing order by aldehydes, ketones, alcohols, furans and acids. This profile of volatile compounds is quite according to a previous study with double and multilayered fish oil microcapsules [35]. The percentage of aliphatic hydrocarbons has been used as an indicator of quality and stability of different commercial fish oils, from salmon, tuna, sardines and shrimp. Considering the relationship between the decrease in the percentage of this family of volatile compounds with an increase in lipid oxidation [51], the high percentage of aliphatic hydrocarbons in Mo and Mu may support the protective effect of the wall materials of these types of microcapsules, minimizing the contact and reactivity of fish oil with oxidizing promoters. Significant differences were detected in the percentage of the most chemical families of volatile compounds between the Mo and Mu of this study, showing Mo higher percentages of aldehydes (8.43% vs. 4.78%), ketones (3.82% vs. 2.65%), alcohols (1.97% vs. 1.10%) and lower of aliphatic hydrocarbon (87.89 vs. 90.43%) and acids (n.d. vs. 0.32%) than Mu. Accordingly, [59] also found significant differences in the percentage of chemical families of volatile compounds between different types of fish oil microcapsules.
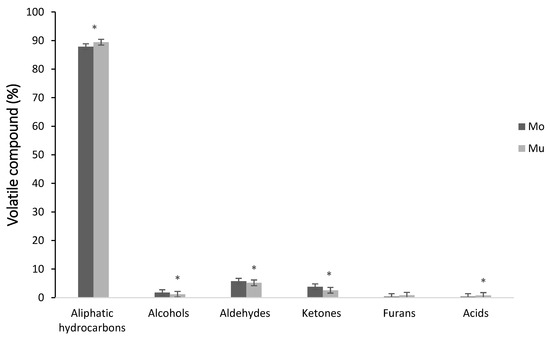
Figure 1.
Volatile compounds of monolayered (Mo) and multilayered (Mu) fish oil microcapsules classified according to chemical families. Values are expressed as the average percentage of each family. *: significant differences (p < 0.05).
Table 1 lists the individual volatile compounds of Mo and Mu, being expressed as AU × 106. Hexane was the major volatile compound in Mo and Mu, followed by pentane, hexanal and 3-hydroxy-2-butanone, and the rest of individual volatile compounds showed less than 1 AU × 106. This agrees with results described for double and multilayered fish oil microcapsules [35]. From the 40 individual volatile compounds identified in the fish oil microcapsules of the present study, 9 of them were found in Mu but not in Mo (tridecane, 1,2,4-butanetriol, phenylethyl alcohol, 2-heptenal, 2-octenal, 2-nonanone, 2-butyltetrahydrofuran, heptanoic and sorbic acid) and 7 were only detected in Mo (1-heptene, heptane, decane, 2-propanol, 4-hexen-1-ol, 1-heptanol and 2-hexenal).

Table 1.
Volatile compounds from monolayered (Mo) and multilayered (Mu) fish oil microcapsules. Values are expressed as peak area × 106.
Significant differences were found in most individual volatile compounds between Mo and Mu (Table 1). Regarding the aldehydes, which have been described as the most important indicators of fish oil oxidation [51], Mo showed significant higher levels of propanal, pentanal, hexanal, 2-hexenal, heptanal, octanal and nonanal compared to Mu. It has been described that propanal comes from the lipid hydroperoxides derived from ω-3 PUFA while hydroperoxides derived from ω-6 PUFA mainly generate hexanal, as consequence of the breakdown of the first double bond of the n position of the ω-3 and ω-6 fatty acids, respectively [60]. In addition, hexanal has been used in previous studies as a marker to measure the quality and oxidative stability of fish oil microcapsules [20]. On the other hand, Mu had a significant higher AU of 2-pentenal and 2-octenal than Mo, but these two volatile compounds have not been related to the oxidation of ω-3 PUFAs. In addition, other relevant indicators of fish oil oxidation, such as 2,4-heptadienal and 2,4-decadienal, which have been associated to the perception of rancid flavor [20,48,60], or other aldehyde volatile compounds related to rancid odors, such as decanal or 2-nonenal [20,48,61], have not been identified in Mo or Mu.
Most individual alcohols and ketones have shown significant differences between Mo and Mu, with higher AU in Mo in comparison to Mu in most cases. However, as is our knowledge, either of the ketones detected in Mo and Mu are associated with the lipid oxidation process. The most important ketones from lipid autoxidation reactions are 3,5-octadien-2-one and 1-octen-3-one [49]. In fact, they have been detected in mayonnaise and milk enriched with fish oil, being strongly correlated with the strength of the oxidation process [48,50]. However, these ketones were not detected in Mo or Mu. Regarding the alcohols, 1-penten-3-ol and 2-penten-1-ol have been described as one of the most characteristics oxidation markers for PUFA [48,49]. 1-penten-3-ol was detected in both types of microcapsules, with higher AU in Mo than in Mu, while 2-penten-1-ol was not found in these fish oil microcapsules. Another common oxidation product of ω-3 PUFA is 2-ethylfuran, which can be generated from the 12-hydroperoxide of EPA and 16-hydroperoxide of DHA [35,62]. This volatile compound has been identified in both types of microcapsules, Mo having significantly higher AU than Mu. Two volatiles compounds, heptanoic and sorbic acid, were found in Mu, but not in Mo. Heptanoic acid at high concentrations imparts unpleasant rancid odor, but the AU of this compound in Mu are very low.
The higher AU in some individual volatile compounds related to lipid oxidation found in Mo in comparison to Mu could be explained by the different wall material of these fish oil microcapsules, being of maltodextrine and of chitosan plus maltodextrine, respectively. In fact, it has been described that chitosan increases the electrostatic force and viscosity of the layers [63], avoids the oxidative damage and could act as a free scavenger [64]. Thus, the Mu coating may be more effective than Mo to protect fish oil from oxidative damage. This aspect can be marked in the case of volatile compounds such as hexanal, 1-penten-3-ol and 2-ethylfuran, with low odor thresholds (4.5, 1 and 2.2 µg kg−1 oil, respectively) and associated with sensory defects [65,66].
Nevertheless, in comparison to the profile of volatile compounds in bulk fish oil, Mo and Mu have not shown to be polyunsaturated lipid oxidation products with rancid taste perceptions, such as 2,4-heptadienal, 2,4-decadienal, 2-nonenal, 3,5-octadien-2-one and 1-octen-3-one [51,67], which points out the effectiveness of the Mo and Mu microcapsules of the present study to minimize the contact and reactivity of fish oil encapsulated with oxidizing promoters.
3.2. Volatile Compounds in Dry-Cured and Cooked Sausages Enriched with Fish Oil Microcapsules
A total of 53 and 60 volatile compounds were identified in D-SAU and C-SAU of the present study, respectively, which were grouped in the following chemical families: aliphatic hydrocarbons, alcohols, aldehydes, furans, ketones, terpenes, acids, esters, aromatics, cyclic hydrocarbons and pyrazines. Figure 2A,B show the percentages of these chemical families of volatile compounds in D-SAU and C-SAU as affected by type of fish oil microcapsule addition, respectively. The most abundant families in all batches of D-SAU were acids and aldehydes, followed far behind by terpenes and esters. Minor percentages were found for aliphatic hydrocarbons, aromatics, ketones, cyclic hydrocarbons, alcohols and furans. This profile is quite in concordance with previous studies in fermented sausages [53,58]. Moreover, significant differences were detected in the chemical families of volatile compounds between the D-SAU batches of this study, with Mo showing higher percentages of aldehydes and terpenes (16.03 and 8.2) than Co and Mu batches, and Mu having a higher percentage of acids (69.63) and lower percentages of esters (5.58) than Co and Mo.
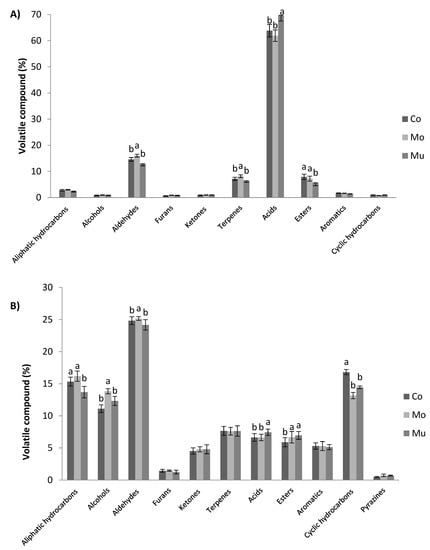
Figure 2.
Volatile compounds of dry-cured (A) and cooked (B) sausages as affected by enrichment with omega-3 polyunsaturated fatty acids (ω-3 PUFA) (control: dark gray; enriched with multilayered fish oil microcapsules: medium gray; enriched monolayered fish oil microcapsules: light gray) classified according to chemical families. Values are expressed as the average percentage of each family. Bars with different letters (a, b) within the same formulation show significant differences (p < 0.05) due to enrichment effect (Co vs. Mo vs. Mu).
In C-SAU, the major family of volatile compounds was aldehydes, followed by aliphatic hydrocarbons, cyclic hydrocarbons and alcohols, while minor abundance was detected for terpenes, acids, esters, aromatics, ketones, furans and pyrazines. This agrees with the profile of volatile compounds previously reported in other studies in cooked sausages [42,46]. Moreover, significant differences were found in the chemical families of volatile compounds between the C-SAU batches of this study, showing in C-SAU-Mo higher percentages of aldehydes and alcohols than in C-SAU-Co and C-SAU-Mu; C-SAU-Mu obtained a higher percentage of acids and esters and a lower percentage of aliphatic hydrocarbons than C-SAU-Co and C-SAU-Mo, and the percentage of cyclic hydrocarbons was higher in C-SAU-Co than in the enriched batches. Thus, at first, considering the results on the percentage of volatile compounds, the differences between microcapsules are reflected in the bathes of meat products. Taking a step forward, the individual volatile compounds of the control and enriched batches of the meat products of the present study are analyzed in the following sections.
Table 2 lists the individual volatile compounds of D-SAU as affected by the type of fish oil microcapsule added. Acetic acid was the major volatile compound in all batches, followed by hexanal, methyl hexanoate, β-myrcene, pentanoic acid, butanoic acid and heptanal, the rest of the individual volatile compounds showing less than 10 AU × 106. This profile is quite in concordance with previous studies in dry fermented sausages [53,55]. The high content of acetic acid would be related to microbial fermentation of carbohydrates [55,68]. Others compounds also typical of carbohydrate fermentation, such as 3-hydroxy-2-butanone, were also detected in D-SAU batches [57]. The high AU of β-myrcene in these samples is also noted, which may be ascribed to the addition of species [69].

Table 2.
Volatile compounds (arbitrary area units × 106) on dry-cured sausages (D-SAU) as affected by enrichment with ω-3 PUFA (p) *.
The enrichment effect with Mo and Mu fish oil microcapsules significantly influence the volatile compounds of most chemical groups (Table 2), excluding esters, aromatics and cyclic hydrocarbons. Only 13 in 53 volatile compounds identified in D-SAU showed significant differences among batches: C-SAU-Mo showed higher AU in six volatile compounds (1-propanone, 1-penten-3-ol, 1-octen-3-ol, pentanal, 3,5-octadien-2-one and acetic acid) and lower in one (butanal) in comparison to C-SAU-Co and C-SAU-Mu; C-SAU-Mu obtained higher abundance in two volatile compounds (heptane and β-mycene) and lower in one (3-hydroxy-2-butanone) than in C-SAU-Co and C-SAU-Mo, and C-SAU-Co showed higher AU in one volatile compound (2-penthyl-furan) and lower in two (1-propanol and 2-ethylfuran) in comparison to the enriched batches. Figure 3a represents the score plots of the PCA of volatile compounds data from the D-SAU samples. The first principal component (PC1) comprised 55.48% of the total variance, and the second principal component (PC2) accounted for 29.47%. The score plot indicates a clear differentiation of samples as affected by the addition of fish oil microcapsules: those with high positive PC1 scores (D-SAU-Mo), those with high positive PC2 scores (D-SAU-Mu) and those with high negative PC2 scores (D-SAU-Co). Several volatile compounds (3,5-octadien-2-one, 1-propanol, pentanal, 1-octen-3-ol, 1-penten-3-ol, acetic acid, 2-ethylfuran, 1-propene, allyl sulphide) are located in the right quadrants (upper and lower), which correspond to high positive charges in PC1, associated with D-SAU-Mo batch. On the other hand, there were a few volatile compounds allocated in the PC2: heptane, β-myrcene, and butanal in the left upper quadrant, and 2-pentyl-furan and 3-hydroxy-2-butanone in the left lower quadrant, related to D-SAU-Mu and D-SAU-Co, respectively. Thus, in comparison to C-SAU-Co and C-SAU-Mu, C-SAU-Mo are more related to typical volatiles compounds of fatty acid oxidation, such as 1-propanol, 1-octen-3-ol and pentanal [45], and to characteristic oxidation markers for PUFA oxidation, such as 1-penten-3-ol, 2-ethyl-furan and 3,5-octadien-2-one, which have been previously observed in mayonnaise and chicken nuggets enriched with fish oil [23,48], and are correlated with the strength of the oxidation process [48,50]. The low odor thresholds some of these volatile compounds, such as 1-octen-3-ol and 3,5-octadien-2-one (1 and 0.45 µg kg−1 of oil, respectively) [70,71], would lead to the perception of anomalous odor and/or flavor, which may have a negative impact in the products enriched with Mo. Nevertheless, in a previous study carried out with the D-SAU samples of the present work [28], no significant differences in acceptability were found among Co, Mo and Mu samples. So, the influence of the fish oil microcapsules addition on the profile of volatile compounds does not seem to be reflected in the consumer’s perception of D-SAU.
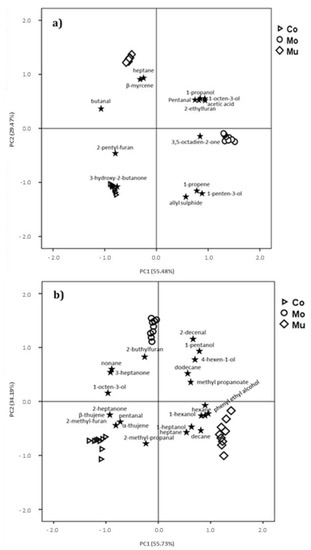
Figure 3.
Principal component analysis (PCA) of the significant volatile compounds in dry-cured (a) and cooked (b) sausages. The plots represent, for the two first principal components, the loading of each volatile compound and the average scores of each one of batches. Control (▷); enriched with monolayered fish oil microcapsules (○); enriched with multilayered fish oil microcapsules (◊).
Table 3 showed the individual volatile compounds of C-SAU as affected by the type of fish oil microcapsule added. α-thujene was the major volatile compound in all batches (around 6.07 AU × 106), followed by pentanal (around 4.84 AU × 106), β-thujene (around 3.52 AU × 106), hexanal (around 3.5 AU × 106), 1-octen-3-ol (around 3.40 AU × 106), gamma-terpinene (around 3.20 AU × 106) and heptanal (around 3.15 AU × 106), and the rest individual volatile compounds showed less than 3 AU × 106. In previous studies in cooked sausages, hexanal has been identified as the most abundant volatile compound, followed by heptanal, pentanal and volatiles compounds from the chemical families of alcohols (1-pentanol and 1-octen-3-ol) and terpenes (limonene, β-myrcene, and gamma-terpinene) [39,40,42], which is quite in agreement with the findings of the present study. However, in these previous works α-thujene and β-thujene were identified but with lower AU than in the present work. These compounds are associated with spicy flavor and have been found in a wide variety of medicinal herbs, essential oils, flavorings and spices such as nutmeg [72]; therefore, its abundance in the present study could be related to the addition of spices in the meat product formulation.

Table 3.
Volatile compounds (arbitrary area units × 106) on cooked sausages (C-SAU) as affected by enrichment with fish oil microcapsules (p) *.
In C-SAU, the addition of Mo and Mu fish oil microcapsules significantly influenced the volatile compounds of most chemical families (Table 3), excluding terpenes and pyrazines, finding significant differences in 27 volatile compounds. Higher abundance was found in two volatile compounds in C-SAU-Mo (1-pentanol and 2-decenal) than in C-SAU-Co and C-SAU-Mu. A total of nine volatile compounds (hexane, heptane, decane, tridecane, 1-hexanol, phenyl ethyl alcohol, acetic acid, nonanoic acid, methyl propanoate and methyl propanoate) obtained higher AU in C-SAU-Mu than in C-SAU-Co and C-SAU-Mo. On the contrary, C-SAU-Mu showed lower abundance for 2-octene, nonane, 1-octen-3-ol, 2-methyl-propanal, 3-heptanone, 2-buthyl-furan and octanoic acid than C-SAU-Co and C-SAU-Mo. In comparison to the enriched batches, C-SAU-Co showed higher AU for six volatile compounds (pentanal, 2-heptanone, 2-methyl-furan, pentanoic acid, β-thujene and α-thujene) and lower for three (dodecane, 4-hexen-1-ol and 1-heptanol). A score plot of PCA of volatile compounds data from the C-SAU samples is shown in Figure 3b. The PC1 accounted for 54.78% of the total variance, and the PC2 comprised 34.19%. The score plot allowed a clear separation of the samples: those with high positive PC1 scores (C-SAU-Mu), those with high positive PC2 scores (C-SAU-Mo) and those with negative PC2 scores (C-SAU-Co). 2-decenal, 2-buthylfuran, 3-heptanone, 1-pentanol, 4-hexen-1-ol, 1-octen-3-ol, dodecane, nonane and methyl-propanoate were grouped and allocated in the upper quadrants (left and right), which correspond to the C-SAU-Mo batch, while 2-heptanone, pentanal, 2-methylpropanal, β-thujene, α-thujene, 2-methylfuran and hexane, heptane, decane, 1-hexanol, 1-heptanol, phenyl-ethyl-alcohol were in the lower left and right quadrants, related to C-SAU-Co and C-SAU-Mu, respectively. Thus, in C-SAU, Mo enriched batches also showed a close relation with volatile compounds from lipid oxidation, such us 2-decenal, a characteristic volatile compound of ω-3 PUFA oxidation, and in 1-pentanol, 4-hexen-1-ol and 1-octen-3-ol, described as typical lipid oxidation products [42,46]. 2-decenal has been related in previous studies to rancid odors in fish oil enriched mayonnaise [48] and fish oil microcapsules [20]. These volatile compounds have a fatty and fishy aroma [71] with a low odor threshold, around 10 µg kg−1 oil [73], which may be detrimental the acceptability of the meat products added with Mo. However, as occurred in D-SAU, similar scores in the acceptability analysis have been found by [28] in the three batches of C-SAU, the differences in the profile volatile compounds not being reflected by the sensory results.
It is worth noting that the differences found in the present study in the profile volatile compounds depend on the type of fish oil microcapsules added. Anyway, a major protection of the microencapsulated fish oil against lipid oxidation when Mu are added could be indicated. This can be ascribed to the different wall material in Mo (maltodextrine) and Mu (chitosan plus maltodextrine). Thus, the multilayer structured of chitosan-maltodextrine may protect the encapsulated material more effectively than the simple coating of maltodextrine. Indeed, chitosan improves the emulsion stability, increasing the electrostatic force and viscosity of the layers, and can also act as an antioxidant [63,64]. Moreover, a high oxidative stability has been found in microcapsules with chitosan [33]. So, although no marked effects on sensory or oxidation stability have been previously found [28], differences in the volatile compounds should be considered since they could release to unhealthy oxidized products [74]. This can be the case of furans, such us 2-ethylfuran, 2-butylfuran, 2-acetylfuran, 2-pentylfuran, 2-furfural and furfural alcohol, which have been found in different fish products [75,76], and have revealed toxicity in animals and humans [77,78]. In fact, 2-ethylfuran and 2-buthylfuran were closely related to the D-SAU-Mo and C-SAU-Mo samples in this study. Considering this aspect, more studies should be conducted in this sense, for evaluating the formation of contaminants in different omega-3 enriched meat products.
4. Conclusions
The type of fish oil microcapsules influences its profile of volatile compounds and that of the enriched meat products. The use of multilayered microcapsules with chitosan-maltodextrine walls may be more protective to the formation of lipid oxidation products, especially from omega-3 fatty acids, than microcapsules with a maltodextrine layer. Thus, the use of multilayered fish oil emulsions to elaborate omega-3 microcapsules for enriching meat products may be indicated.
Author Contributions
Conceptualization, T.A. and T.P.-P.; Methodology, J.C.S., A.M., T.P.-P. and T.A.; Software, A.M.; Validation, J.C.S., T.P.-P. and T.A.; Formal Analysis, J.C.S.; Investigation, T.P.-P. and J.C.S.; Resources, T.P.-P.; Data Curation, J.C.S.; Writing–Original Draft Preparation, J.C.S.; Writing—Review and Editing, T.P.-P. and T.A.; Visualization, J.C.S.; Supervision, A.M., T.P.-P.; Project Administration, T.P.-P.; Funding Acquisition, T.P.-P. All authors have read and agreed to the published version of the manuscript.
Funding
Authors, especially Trinidad Perez-Palacios, acknowledge to the Agencia Estatal de Investigación (AEI) and the Fondo Europeo de Desarrollo Regional (FEDER) the funding for this study, which was supported by the project AGL2016-73260-JIN (AEI/FEDER/UE).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schunck, W.H.; Konkel, A.; Fischer, R.; Weylandt, K.H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef]
- Horrobin, D.F. Seafoods and fish oils in human health and disease. Int. J. Cardiol. 1989, 22, 409. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004, 17, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.B.; Arnesen, H.; De Caterina, R.; Rasmussen, L.H.; Kristensen, S.D. Marine n-3 polyunsaturated fatty acids and coronary heart disease: Part I. Background, epidemiology, animal data, effects on risk factors and safety. Thromb. Res. 2005, 115, 163–170. [Google Scholar] [CrossRef]
- Yashodhara, B.M.; Umakanth, S.; Pappachan, J.M.; Bhat, S.K.; Kamath, R.; Choo, B.H. Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar] [CrossRef]
- Lee, S.; Faustman, C.; Djordjevic, D.; Faraji, H.; Decker, E.A. Effect of antioxidants on stabilization of meat products fortified with n-3 fatty acids. Meat Sci. 2006, 72, 18–24. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Garg, M.L.; Wood, L.G.; Singh, H.; Moughan, P.J. Means of delivering recommended levels of long chain n-3 polyunsaturated fatty acids in human diets. J. Food Sci. 2006, 71, R66–R71. [Google Scholar] [CrossRef]
- European Food Safety Authority. Reglamento (UE) 116/2010 que Modifica al Reglamento (CE) 1924/2006 en lo Relativo a la Lista de Declaraciones Nutricionales; European Food Safety Authority: Parma, Italy, 2010. [Google Scholar]
- International Society for the Study of Fatty Acids and Lipids. In Proceedings of the 6th Congress of the International Society for the Study of Fatty Acids and Lipids, Brighton, UK, 26 June–1 July 2004; Volume 43.
- EU Commision Regulation. (EU) N°116/2010 of 9 February 2010 amending Regulation (EC) N° 1924/2006 of the European Parliament and of the Council with regard to the list of nutrition claims. Off. J. Eur. Union 2010, 37, 16–18.
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, K.; Fischer, K.; Nuernberg, G.; Kuechenmeister, U.; Klosowska, D.; Eliminowska-Wenda, G.; Fiedler, I.; Ender, K. Effects of dietary olive and linseed oil on lipid composition, meat quality, sensory characteristics and muscle structure in pigs. Meat Sci. 2005, 70, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Colmenero, F. Healthier lipid formulation approaches in meat-based functional foods. Technological options for replacement of meat fats by non-meat fats. Trends Food Sci. Technol. 2007, 18, 567–578. [Google Scholar] [CrossRef]
- Josquin, N.M.; Linssen, J.P.H.; Houben, J.H. Quality characteristics of Dutch-style fermented sausages manufactured with partial replacement of pork back-fat with pure, pre-emulsified or encapsulated fish oil. Meat Sci. 2012, 90, 81–86. [Google Scholar] [CrossRef]
- Cáceres, E.; García, M.L.; Selgas, M.D. Effect of pre-emulsified fish oil-as source of PUFA n-3-on microstructure and sensory properties of mortadella, a Spanish bologna-type sausage. Meat Sci. 2008, 80, 183–193. [Google Scholar] [CrossRef]
- Delgado-Pando, G.; Cofrades, S.; Ruiz-Capillas, C.; Solas, M.T.; Triki, M.; Jiménez-Colmenero, F. Low-fat frankfurters formulated with a healthier lipid combination as functional ingredient: Microstructure, lipid oxidation, nitrite content, microbiological changes and biogenic amine formation. Meat Sci. 2011, 89, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Uemura, M.; Hosokawa, M. Effective Prevention of Oxidative Deterioration of Fish Oil: Focus on Flavor Deterioration. Annu. Rev. Food Sci. Technol. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Onwulata, C.I. Microencapsulation and functional bioactive foods. J. Food Process. Preserv. 2013, 37, 510–532. [Google Scholar] [CrossRef]
- Jónsdóttir, R.; Bragadóttir, M.; Arnarson, G. Oxidatively Derived Volatile Compounds in Microencapsulated Fish Oil Monitored by Solid-phase Microextraction (SPME). J. Food Sci. 2005, 70, c433–c440. [Google Scholar] [CrossRef]
- Jimenez-Alvarez, D.; Giuffrida, F.; Golay, P.A.; Cotting, C.; Destaillats, F.; Dionisi, F.; Keely, B. Profiles of volatile compounds in milk containing fish oil analyzed by HS-SPME-GC/MS. Eur. J. Lipid Sci. Technol. 2008, 110, 277–283. [Google Scholar] [CrossRef]
- Yang, K.M.; Cheng, M.C.; Chen, C.W.; Tseng, C.Y.; Lin, L.Y.; Chiang, P.Y. Characterization of volatile compounds with HS-SPME from oxidized n-3 PUFA rich oils via rancimat tests. J. Oleo Sci. 2017, 66, 113–122. [Google Scholar] [CrossRef]
- Jiménez-Martín, E.; Pérez-Palacios, T.; Carrascal, J.R.; Rojas, T.A. Enrichment of Chicken Nuggets with Microencapsulated Omega-3 Fish Oil: Effect of Frozen Storage Time on Oxidative Stability and Sensory Quality. Food Bioprocess Technol. 2016, 9, 285–297. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Pateiro, M.; Campagnol, P.C.B.; Domínguez, R. Healthy Spanish salchichón enriched with encapsulated n−3 long chain fatty acids in konjac glucomannan matrix. Food Res. Int. 2016, 89, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, C.; Pérez-Palacios, T.; Sirtori, F.; Jiménez-Martín, E.; Antequera, T.; Franci, O.; Acciaioli, A.; Bozzi, R.; Pugliese, C. Enrichment of Cinta Senese burgers with omega-3 fatty acids. Effect of type of addition and storage conditions on quality characteristics. Grasas y Aceites 2018, 69, 235. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schwarz, K. Sensory odour profiling and lipid oxidation status of fish oil and microencapsulated fish oil. Food Chem. 2010, 123, 968–975. [Google Scholar] [CrossRef]
- Felix, P.H.C.; Birchal, V.S.; Botrel, D.A.; Marques, G.R.; Borges, S.V. Physicochemical and Thermal Stability of Microcapsules of Cinnamon Essential Oil by Spray Drying. J. Food Process. Preserv. 2017, 41, e12919. [Google Scholar] [CrossRef]
- Solomando, J.; Antequera, T.; Perez-Palacios, T. Evaluating the use of fish oil microcapsules as omega-3 vehicle in cooked and dry-cured sausages as affected by their processing, storage and cooking. Meat Sci. 2020, 162, 108031. [Google Scholar] [CrossRef] [PubMed]
- Solomando, J.C.; Antequera, T.; Ruiz-Carrascal, J.; Pérez-Palacios, T. Improvement of encapsulation and stability of EPA and DHA from monolayered and multilayered emulsions by high-pressure homogenization. J. Food Process. Preserv. 2019, 44. [Google Scholar] [CrossRef]
- Pelser, W.M.; Linssen, J.P.H.; Legger, A.; Houben, J.H. Lipid oxidation in n-3 fatty acid enriched Dutch style fermented sausages. Meat Sci. 2007, 75, 1–11. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; González-Mohíno, A.; Perez-Palacios, T. Fish oil/lycopene microcapsules as a source of eicosapentaenoic and docosahexaenoic acids: A case study on spreads. J. Sci. Food Agric. 2020, 100, 1875–1886. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Ruiz-Carrascal, J.; Jiménez-Martín, E.; Solomando, J.C.; Antequera, T. Improving the lipid profile of ready-to-cook meat products by addition of omega-3 microcapsules: Effect on oxidation and sensory analysis. J. Sci. Food Agric. 2018, 98, 5302–5312. [Google Scholar] [CrossRef]
- Jiménez-Martín, E.; Gharsallaoui, A.; Pérez-Palacios, T.; Carrascal, J.R.; Rojas, T.A. Suitability of using monolayered and multilayered emulsions for microencapsulation of ω-3 fatty acids by spray drying: Effect of storage at different temperatures. Food Bioprocess Technol. 2014, 8, 100–111. [Google Scholar] [CrossRef]
- Garcia-Esteban, M.; Ansorena, D.; Astiasarán, I.; Ruiz, J. Study of the effect of different fiber coatings and extraction conditions on dry cured ham volatile compounds extracted by solid-phase microextraction (SPME). Talanta 2004, 64, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martín, E.; Gharsallaoui, A.; Pérez-Palacios, T.; Ruiz Carrascal, J.; Antequera Rojas, T. Volatile compounds and physicochemical characteristics during storage of microcapsules from different fish oil emulsions. Food Bioprod. Process. 2015, 96, 52–64. [Google Scholar] [CrossRef]
- Pop, F. Chemical stabilization of oils rich in long-chain polyunsaturated fatty acids during storage. Food Sci. Technol. Int. 2011, 17, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.U.; Olson, D.G.; Jo, C.; Love, J.; Jin, S.K. Volatiles production and lipid oxidation in irradiated cooked sausage as related to packaging and storage. J. Food Sci. 1999, 64, 226–229. [Google Scholar] [CrossRef]
- Chevance, F.F.V.; Farmer, L.J. Identification of major volatile odor compounds in frankfurters. J. Agric. Food Chem. 1999, 47, 5151–5160. [Google Scholar] [CrossRef]
- Chevance, F.F.V.; Farmer, L.J. Release of volatile odor compounds from full-fat and reduced-fat frankfurters. J. Agric. Food Chem. 1999, 47, 5161–5168. [Google Scholar] [CrossRef]
- Chevance, F.F.V.; Farmer, L.J.; Desmond, E.M.; Novelli, E.; Troy, D.J.; Chizzolini, R. Effect of some fat replacers on the release of volatile aroma compounds from low-fat meat products. J. Agric. Food Chem. 2000, 48, 3476–3484. [Google Scholar] [CrossRef]
- Jo, C.; Ahn, D.U.; Byun, M.W. Irradiation-induced oxidative changes and production of volatile compounds in sausages prepared with vitamin E-enriched commercial soybean oil. Food Chem. 2002, 76, 299–305. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Ramírez, R.; Cava, R. Influence of the addition of rosemary essential oil on the volatiles pattern of porcine frankfurters. J. Agric. Food Chem. 2005, 53, 8317–8324. [Google Scholar] [CrossRef]
- Carrapiso, A.I. Effect of fat content on flavour release from sausages. Food Chem. 2007, 103, 396–403. [Google Scholar] [CrossRef]
- Yoo, S.S.; Kook, S.H.; Park, S.Y.; Shim, J.H.; Chin, K.B. Physicochemical characteristics, textural properties and volatile compounds in comminuted sausages as affected by various fat levels and fat replacers. Int. J. Food Sci. Technol. 2007, 42, 1114–1122. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; López-Díez, J.J.; Belloch, C.; Flores, M. Counteracting the effect of reducing nitrate/nitrite levels on dry fermented sausage aroma by Debaryomyces hansenii inoculation. Meat Sci. 2020, 164, 108103. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, Q.; Zhao, H.; Zhao, M.; Yang, B. Volatile compounds of Cantonese sausage released at different stages of processing and storage. Food Chem. 2010, 121, 319–325. [Google Scholar] [CrossRef]
- Feng, C.H.; Li, C.; García-Martín, J.F.; Malakar, P.K.; Yan, Y.; Liu, Y.W.; Wang, W.; Liu, Y.T.; Yang, Y. Physical Properties and Volatile Composition Changes of Cooked Sausages Stuffed in a New Casing Formulation Based in Surfactants and Lactic Acid During Long-Term Storage. J. Food Sci. 2017, 82, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; Hartvigsen, K.; Lund, P.; Adler-Nissen, J.; Hølmer, G.; Meyer, A.S. Oxidation in fish-oil-enriched mayonnaise. Eur. Food Res. Technol. 2000, 210, 242–257. [Google Scholar] [CrossRef]
- Roberts, D.D.; Pollien, P.; Milo, C. Solid-phase microextraction method development for headspace analysis of volatile flavor compounds. J. Agric. Food Chem. 2000, 48, 2430–2437. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Let, M.B.; Meyer, A.S.; Jacobsen, C. Chemical and Olfactometric Characterization of Volatile Flavor Compounds in a Fish Oil Enriched Milk Emulsion. J. Agric. Food Chem. 2004, 52, 311–317. [Google Scholar] [CrossRef]
- Giogios, I.; Grigorakis, K.; Nengas, I.; Papasolomontos, S.; Papaioannou, N.; Alexis, M.N. Fatty acid composition and volatile compounds of selected marine oils and meals. J. Sci. Food Agric. 2009, 89, 88–100. [Google Scholar] [CrossRef]
- Cano-García, L.; Rivera-Jiménez, S.; Belloch, C.; Flores, M. Generation of aroma compounds in a fermented sausage meat model system by Debaryomyces hansenii strains. Food Chem. 2014, 151, 364–373. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Flores, M. Effect of fat content on aroma generation during processing of dry fermented sausages. Meat Sci. 2011, 87, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.; Navarro, J.L.; Flores, M. Establishment of the contribution of volatile compounds to the aroma of fermented sausages at different stages of processing and storage. Food Chem. 2009, 115, 1464–1472. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; Montero, R.; Belloch, C.; Flores, M. Nitrate reduction in the fermentation process of salt reduced dry sausages: Impact on microbial and physicochemical parameters and aroma profile. Int. J. Food Microbiol. 2018, 282, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Corral, S.; Salvador, A.; Flores, M. Salt reduction in slow fermented sausages affects the generation of aroma active compounds. Meat Sci. 2013, 93, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.; Navarro, J.L.; Flores, M. The influence of nitrite and nitrate on microbial, chemical and sensory parameters of slow dry fermented sausage. Meat Sci. 2006, 73, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Corral, S.; Belloch, C.; López-Díez, J.J.; Flores, M. Lipolysis and aroma generation as mechanisms involved in masking boar taint in sodium reduced fermented sausages inoculated with Debaryomyces hansenii yeast. J. Sci. Food Agric. 2018, 98, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martín, E.; Rojas, T.A.; Gharsallaoui, A.; Ruiz, J.; Pérez-Palacios, T. Fatty acid composition in double and multilayered microcapsules of ω-3 as affected by storage conditions and type of emulsions. Food Chem. 2016, 194, 476–486. [Google Scholar] [CrossRef]
- Suh, J.H.; Niu, Y.S.; Hung, W.-L.; Ho, C.-T.; Wang, Y. Lipidomic analysis for carbonyl species derived from fish oil using liquid chromatography–tandem mass spectrometry. Talanta 2017, 168, 31–42. [Google Scholar] [CrossRef]
- Rørbæk, K. Oxidation and Flavours in Fish Oil; Technical University of Denmark: Copenhagen, Denmark, 1994. [Google Scholar]
- Medina, I.; Satué-Gracia, M.T.; Frankel, E.N. Static Headspace Gas Chromatographic Analyses to Determine Oxidation of Fish Muscle Lipids during Thermal Processing. JAOCS J. Am. Oil Chem. Soc. 1999, 76, 231–236. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; Decker, E.A.; McClements, D.J. Encapsulation of emulsified tuna oil in two-layered interfacial membranes prepared using electrostatic layer-by-layer deposition. Food Hydrocoll. 2005, 19, 1044–1053. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Antioxidant effects of chitin, chitosan, and their derivatives. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2014; Volume 73, pp. 15–31. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds; Oliemans, Punter & Partners BV: Utrecht, The Netherlands, 2003; ISBN 9789081089418. [Google Scholar]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Xiao, Z. Evaluation of the synergism among volatile compounds in Oolong tea infusion by odour threshold with sensory analysis and E-nose. Food Chem. 2017, 221, 1484–1490. [Google Scholar] [CrossRef]
- Karahadian, C.; Lindsay, R.C. Evaluation of compounds contributing characterizing fishy flavors in fish oils. J. Am. Oil Chem. Soc. 1989, 66, 953–960. [Google Scholar] [CrossRef]
- El Sheikha, A.F.; Bakar, J. Fermented Meat Products. In Microorganisms and Fermentation of Traditional Foods; Informa UK Limited: Colchester, UK, 2014; pp. 233–257. [Google Scholar]
- Škrlep, M.; Čandek-Potokar, M.; Lukač, N.B.; Tomažin, U.; Flores, M. Aromatic Profile, Physicochemical and Sensory Traits of Dry-Fermented Sausages Produced without Nitrites Using Pork from Krškopolje Pig Reared in Organic and Conventional Husbandry. Animals 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.; Grosch, W. Odorants of Virgin Olive Oils with Different Flavor Profiles. J. Agric. Food Chem. 1998, 46, 2754–2763. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Sirisoma, N.S.; Höld, A.K.M.; Casida, J.E. α- and β-Thujones (Herbal Medicines and Food Additives): Synthesis and Analysis of Hydroxy and Dehydro Metabolites. J. Agric. Food Chem. 2001, 49, 1915–1921. [Google Scholar] [CrossRef]
- Kalua, C.; Allen, M.; Bedgood, D.; Bishop, A.; Prenzler, P.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Taneja, A.; Zhu, X.; Singh, H. Evaluation of processed cheese fortified with fish oil emulsion. Food Res. Int. 2009, 42, 1093–1098. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Petisca, C.; Melo, A.; Ferreira, I.M.P.L.V.O. Quantification of furanic compounds in coated deep-fried products simulating normal preparation and consumption: Optimisation of HS-SPME analytical conditions by response surface methodology. Food Chem. 2012, 135, 1337–1343. [Google Scholar] [CrossRef]
- Sujatha, P. Monitoring cytotoxic potentials of furfuryl alcohol and 2-furyl methyl ketone in mice. Food Chem. Toxicol. 2008, 46, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.H.; Muijser, H.; Appel, M.J.; Kuper, C.F.; Bessems, J.G.; Woutersen, R.A. Subacute (28-day) toxicity of furfural in Fischer 344 rats: A comparison of the oral and inhalation route. Food Chem. Toxicol. 2004, 42, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).