Impact of a Pitanga Leaf Extract to Prevent Lipid Oxidation Processes during Shelf Life of Packaged Pork Burgers: An Untargeted Metabolomic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pork Burgers
2.2. Untargeted Metabolomics-Based Analysis of PLE and Pork Burgers during Storage
2.3. Statistical Analyses
3. Results and Discussion
3.1. UHPLC-QTOF-MS Characterization of Pitanga Leaf Extract (PLE)
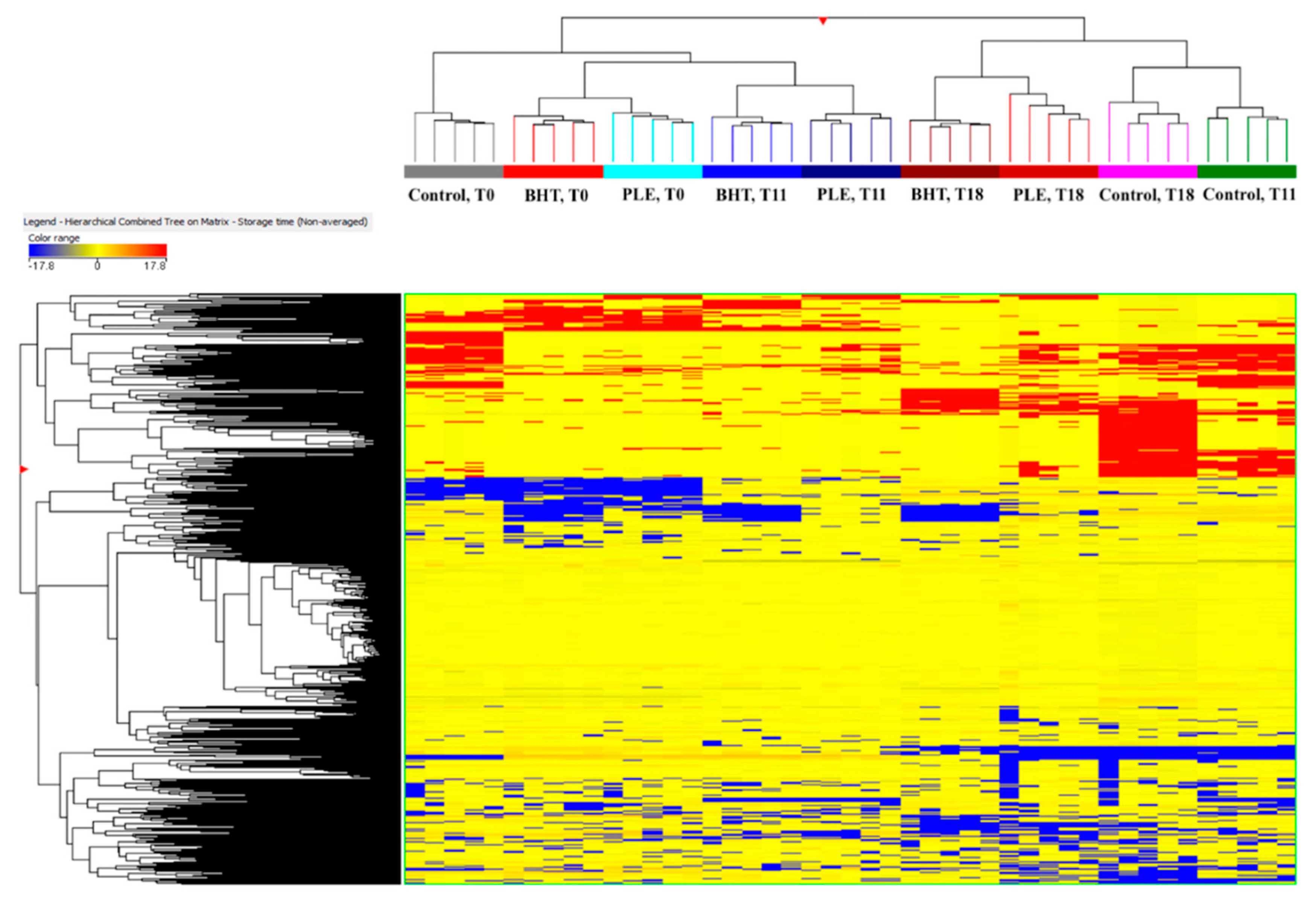
3.2. Untargeted Profile and Multivariate Statistical Discrimination of Pork Burgers during Storage
3.3. pH and Lipid Oxidation in Pork Burgers during Storage
3.4. Correlation between Metabolomic Data and MDA Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, K.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.; Hopkins, D.L.; Fahri, F.T.; Ponnampalam, E.N. Oxidative processes in muscle systems and fresh meat: Sources, markers and remedies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 565–597. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burgers during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Barba, F.J.; Gavahian, M.; Franco, D.; Khaneghah, A.M.; Carballo, J.; Ferreira, I.C.F.R.; da Silva Barretto, A.C.; Lorenzo, J.M. Fucus vesiculosus extracts as natural antioxidants for improvement of physicochemical properties and shelf life of pork patties formulated with oleogels. J. Sci. Food. Agric. 2019, 99, 4561–4570. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Anjos, O.; Fernandes, R.; Cardoso, S.M.; Delgado, T.; Farinha, N.; Paula, V.; Estevinho, L.M.; Carpes, S.T. Bee pollen as a natural antioxidant source to prevent lipid oxidation in black pudding. LWT 2019, 111, 869–875. [Google Scholar] [CrossRef]
- Sadeghinejad, N.; Sarteshnizi, R.A.; Gavlighi, H.A.; Barzegar, M. Pistachio green hull extract as a natural antioxidant in beef patties: Effect on lipid and protein oxidation, color deterioration, and microbial stability during chilled storage. LWT 2019, 102, 393–402. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Gomez, B.; Barba, F.J.; Mora, L.; Perez-Santaescolastica, C.; Toldra, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- De Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purrinow, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef] [PubMed]
- Atta, E.M.; Mohamed, N.; Abdelgawad, A.A.M. Antioxidants: An overview on the natural and synthetic types. Eur. Chem. Bull. 2017, 6, 365. [Google Scholar] [CrossRef]
- Vargas, F.C.; Arantes-Pereira, L.; Costa, P.A.D.; Melo, M.P.D.; Sobral, P.J.D.A. Rosemary and pitanga aqueous leaf extracts on beef patties stability under cold storage. Braz. Arch. Biol. Technol. 2016, 59, e16160139. [Google Scholar] [CrossRef]
- Tambara, A.L.; de Los Santos Moraes, L.; Dal Forno, A.H.; Boldori, J.R.; Soares, A.T.G.; Rodrigues, C.d.; Mariutti, L.R.B.; Mercadante, A.Z.; de Ávila, D.S.; Denardin, C. Purple pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caernorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018, 120, 639–650. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Lourenco, R.V.; Balestra, F.; Bittante, A.M.Q.B.; do Amaral Sobral, P.J.; Dalla Rosa, M. Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, R.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef]
- Tao, L. Oxidation of polyunsaturated fatty acids and its impact on food quality and human health. Adv. Food Technol. Nutr. Sci. 2015, 1, 134–141. [Google Scholar] [CrossRef]
- Capozzi, F.; Trimigno, A.; Ferranti, P. Chapter 9—Proteomics and metabolomics in relation to meat quality. In Poultry Quality Evaluation: Quality Attributes and Consumer Values; Woodhead Publishing: Cambridge, UK, 2017; pp. 221–245. [Google Scholar]
- Pinu, F.R. Metabolomics: Applications to food safety and quality research. In Microbial Metabolomics; Beale, D., Kouremenos, K., Palombo, E., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Pateiro, M.; Lorenzo, J.M.; Díaz, S.; Gende, J.A.; Fernández, M.; González, J.; García, L.; Rial, F.J.; Franco, D. Meat quality of veal: Discriminatory ability of weaning status. Span. J. Agric. Res. 2013, 11, 1044–1056. [Google Scholar] [CrossRef]
- Rocchetti, G.; Bhumireddy, S.R.; Giuberti, G.; Mandal, R.; Lucini, L.; Wishart, D.S. Edible nuts deliver polyphenols and their transformation products to the large intestine: An in vitro fermentation model combining targeted/untargeted metabolomics. Food Res. Int. 2019, 116, 786–794. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Giuberti, G.; Bhumireddy, S.R.; Mandal, R.; Trevisan, M.; Wishart, D.S. Transformation of polyphenols found in pigmented gluten-free flours during in vitro large intestinal fermentation. Food Chem. 2019, 298, 125068. [Google Scholar] [CrossRef] [PubMed]
- Senizza, B.; Rocchetti, G.; Ghisoni, S.; Busconi, M.; De Los Mozos Pascual, G.; Fernandez, J.A.; Lucini, L.; Trevisan, M. Identification of phenolic markers for saffron authenticity and origin: An untargeted metabolomics approach. Food Res. Int. 2019, 129, 108584. [Google Scholar] [CrossRef] [PubMed]
- Fortes, G.A.C.; Carvalho, A.G.; Ramalho, R.R.F.; da Silva, A.J.R.; Ferri, P.H.; Santos, S.C. Antioxidant activities of hydrolysable tannins and flavonoid glycosides isolated from Eugenia uniflora L. Rec. Nat. Prod. 2015, 9, 251–256. [Google Scholar]
- Samy, M.N.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Kamel, M.S. Taxiphyllin 6′-O-gallate, actinidioionoside 6′-O-gallate and myricetrin 2″-O-sulfate from the leaves of Syzygium samarangense and their biological activities. Chem. Pharm. Bull. 2014, 62, 1013–1018. [Google Scholar]
- Bakr, R.O.; Mohamed, S.A.; Waly, N.E. Phytochemical and biological investigation of Eugenia uniflora L. cultivated in Egypt. J. Pharmacognosy Phytother. 2017, 9, 57–66. [Google Scholar]
- Falcão, T.R.; de Araújo, A.A.; Lira Soares, L.A.; de Moraes Ramos, R.T.; Ferraz Bezerra, I.C.; Assunçäo Ferreira, M.R.; de Souza Neto, M.A.; Nunes Melo, M.C.; de Araújo, R.F.J.; de Aguiar Guerra, A.C.V.; et al. Crude extract and fractions from Eugenia uniflora Linn leaves showed anti-inflammatory, antioxidant, and antibacterial activities. BMC Complement. Altern. Med. 2018, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.C.; Gómez, B.; Khaneghah, A.M.; Strozzi, I.; Gavahian, M.; Barba, F.J.; do Amaral Sobral, P.J.; Lorenzo, J.M. Assessment of the suitability of pitanga leaf extract as a natural antioxidant for enhancing canola oil stability: Monitoring lipid oxidation parameters. Eur. J. Lipid Sci. Technol. 2019, 121, 1800447. [Google Scholar] [CrossRef]
- Bezerra, I.C.F.; de Ramos, R.T.M.; Ferreira, M.R.A.; Soares, L.A.L. Chromatographic profiles of extractives from leaves of Eugenia uniflora. Rev. Bras. Farmacogn. 2018, 28, 92–101. [Google Scholar] [CrossRef]
- Amorim, A.C.L.; Lima, C.K.F.; Hovell, A.M.C.; Miranda, A.L.P.; Rezende, C.M. Antinociceptive and hypothermic evaluation of the leaf essential oil and isolated terpenoids from Eugenia uniflora L. (Brazilian Pitanga). Phytomedicine 2009, 16, 923–928. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Jongberg, S.; Racanicci, A.M.C.; Skibsted, L.H. Mate extract is superior to green tea extract in the protection against chicken meat protein thiol oxidation. Food Chem. 2019, 300, 125134. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic analysis in food science: A review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Meynier, A.; Genot, C.; Gandemer, G. Volatile compounds of oxidized pork phospholipids. J. Am. Oil Chem. Soc. 1998, 75, 1–7. [Google Scholar] [CrossRef]
- Pegg, A.E.; Michael, A.J. Spermine synthase. Cell. Mol. Life Sci. 2011, 67, 113. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Li, H.-J.; He, Z.-F.; Wang, T.; Qin, G. Study on the flavor contribution of phospholipids and triglycerides to pork. Food Sci. Biotechnol. 2010, 19, 1267–1276. [Google Scholar] [CrossRef]
- Spickett, C.M. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol. 2013, 1, 145–152. [Google Scholar] [CrossRef]
- Choi, Y.S.; Choi, J.H.; Han, D.J.; Kim, H.Y.; Lee, M.A.; Jeong, J.Y.; Chung, H.-J.; Kim, C.J. Effects of replacing pork back fat with vegetable oils and rice bran fiber on the quality of reduced-fat frankfurters. Meat Sci. 2010, 84, 557–563. [Google Scholar] [CrossRef]
- Ceribeli, C.; Zawadzki, A.D.; Racanicci, A.; Colnago, L.A.; Skibsted, L.H.; Cardoso, D.R. Maté as dietary supplement for broiler chickens: Effect on the metabolic profile and redox chemistry of meat. J. Brazil. Chem. Soc. 2018, 29, 2266–2277. [Google Scholar] [CrossRef]

| Classification | Discriminant Compounds | VIP Scores | LogFC (Control vs. PLE)T18 | Accumulation |
|---|---|---|---|---|
| Fatty acyls | Cetyl alcohol | 1.37 ± 0.59 | −1.77 | Down |
| Isobutyryl-L-carnitine | 1.34 ± 0.44 | −0.25 | Down | |
| Avocadene 2-acetate | 1.29 ± 0.72 | −1.37 | Down | |
| 7,8-Dihydrovomifoliol 9-(apiosyl-(1->6)-glucoside) | 1.15 ± 0.84 | −0.47 | Down | |
| Docosenic acid | 1.10 ± 0.90 | −0.93 | Down | |
| L-Acetylcarnitine | 1.09 ± 0.65 | −1.10 | Down | |
| Sodium oleate | 1.06 ± 0.47 | −0.34 | Down | |
| Stearoylcarnitine | 1.01 ± 1.08 | −0.18 | Down | |
| Methyl 2E,4Z-hexadecadienoate | 1.36 ± 0.62 | 0.14 | Up | |
| 2-Hexenyl octanoate | 1.02 ± 0.90 | 0.18 | Up | |
| 2-Octenoylcarnitine | 1.41 ± 0.77 | 0.57 | Up | |
| Prostaglandin F1a | 1.05 ± 0.95 | 0.67 | Up | |
| Sorbitan palmitate | 1.49 ± 0.14 | 0.67 | Up | |
| Hept-2-en-1-yl isovalerate | 1.29 ± 0.65 | 0.92 | Up | |
| 18-hydroxyoleate | 1.42 ± 0.47 | 0.93 | Up | |
| 3,4-Dimethyl-5-pentyl-2-furanheptanoic acid | 1.32 ± 0.60 | 1.24 | Up | |
| MG(0:0/18:2(9Z,12Z)/0:0) | 1.43 ± 0.34 | 1.37 | Up | |
| (9Z)-12-oxo-dodec-9-enoate | 1.49 ± 0.26 | 1.50 | Up | |
| (Z)-15-Oxo-11-eicosenoic acid | 1.50 ± 0.26 | 1.56 | Up | |
| 16-Hydroxy-hexadecanoic acid | 1.52 ± 0.28 | 2.54 | Up | |
| (9S,10E,12S,13S)-9,12,13-Trihydroxy-10-octadecenoic acid | 1.51 ± 0.33 | 2.67 | Up | |
| 4-Hydroxy-2-nonenal | 1.03 ± 0.85 | 11.1 | Up | |
| 6-Hydroxypentadecanedioic acid | 1.55 ± 0.22 | 18.46 | Up | |
| 20-Hydroxy-PGF2a | 1.49 ± 0.24 | 20.11 | Up | |
| Sativic acid (9,10,12,13-tetrahydroxyoctadecanoic acid) | 1.60 ± 0.41 | 20.79 | Up | |
| Hexanoylcarnitine | 1.52 ± 0.18 | 21.52 | Up | |
| Glycerophospholipids | PS(14:0/14:1(9Z)) | 1.14 ± 1.34 | −1.66 | Down |
| LysoPC(22:5(7Z,10Z,13Z,16Z,19Z)) | 1.02 ± 0.96 | −0.25 | Down | |
| LysoPC(20:4(5Z,8Z,11Z,14Z)) | 1.40 ± 0.46 | 0.09 | Up | |
| LysoPC(18:2(9Z,12Z)) | 1.47 ± 0.29 | 0.32 | Up | |
| PGP(16:0/16:0) | 1.42 ± 0.57 | 0.54 | Up | |
| LysoPC(P-16:0) | 1.51 ± 0.42 | 0.56 | Up | |
| LysoPE(16:0/0:0) | 1.54 ± 0.26 | 0.86 | Up | |
| LysoPC(20:2(11Z,14Z)) | 1.48 ± 0.31 | 0.87 | Up | |
| LysoPE(0:0/18:2(9Z,12Z)) | 1.54 ± 0.13 | 1.30 | Up | |
| LysoPC(16:0) | 1.55 ± 0.15 | 1.31 | Up | |
| Lysolecithin | 1.49 ± 0.31 | 1.46 | Up | |
| LysoPE(20:0/0:0) | 1.51 ± 0.31 | 1.53 | Up | |
| LysoPE(0:0/18:1(9Z)) | 1.52 ± 0.15 | 1.54 | Up | |
| LysoPC(P-18:0) | 1.18 ± 0.90 | 1.65 | Up | |
| LysoPC(16:1(9Z)) | 1.49 ± 0.26 | 1.77 | Up | |
| LysoPC(14:0) | 1.53 ± 0.05 | 2.37 | Up | |
| LysoPE(16:1(9Z)/0:0) | 1.52 ± 0.15 | 18.75 | Up | |
| LPA(18:1(9Z)/0:0) | 1.43 ± 0.39 | 19.25 | Up | |
| Prenol lipids | R1-Barrigenol | 1.56 ± 0.33 | −20.26 | Down |
| Ganodermic acid Jb | 1.46 ± 0.61 | −20.06 | Down | |
| Melleolide B | 1.14 ± 0.85 | −0.01 | Down | |
| (1beta,2alpha,3alpha)-1,2,3,24-Tetrahydroxy-12-oleanen-28-oic acid | 1.12 ± 0.79 | −0.07 | Down | |
| Geranyl benzoate | 1.09 ± 1.23 | −0.02 | Down | |
| Austroinulin | 1.08 ± 0.43 | −0.15 | Down | |
| Cichorioside M | 1.08 ± 0.73 | −0.82 | Down | |
| Prephytoene diphosphate | 1.35 ± 0.72 | −18.03 | Down | |
| (-)-Isoxanthochymol | 1.33 ± 0.63 | 0.23 | Up | |
| Madlongiside C | 1.33 ± 0.68 | 0.51 | Up | |
| Hovenidulcigenin B | 1.53 ± 0.23 | 0.67 | Up | |
| Hovenidulcigenin A | 1.53 ± 0.27 | 0.91 | Up | |
| Fasciculol C | 1.42 ± 0.92 | 1.25 | Up | |
| Hydroxysintaxanthin 5,6-epoxide | 1.19 ± 0.72 | 1.84 | Up | |
| Avenestergenin B2 | 1.54 ± 0.25 | 18.01 | Up | |
| Steroids | 3-Sulfodeoxycholic acid | 1.03 ± 0.76 | −0.09 | Down |
| 23-O-beta-D-Glucopyranosyl-25-methyldolichosterone | 1.01 ± 1.31 | −1.42 | Down | |
| (24R)-5b,8b-Epidioxyergosta-6,22E-dien-3b-ol 3-glucoside | 1.41 ± 0.55 | 0.34 | Up | |
| (3alpha,5beta,7alpha)-23-Carboxy-7-hydroxy-24-norcholan-3-yl-beta-D-glucopyranosiduronic acid | 1.23 ± 0.70 | 0.42 | Up | |
| Notoginsenoside R10 | 1.48 ± 0.29 | 1.87 | Up | |
| 28-Homobrassinolide | 1.53 ± 0.22 | 2.02 | Up | |
| Lithocholate 3-O-glucuronide | 1.50 ± 0.27 | 2.44 | Up | |
| (3beta,22E,24R)-Ergosta-4,6,8(14),22-tetraen-3-ol | 1.53 ± 0.20 | 18.83 | Up | |
| Lipids and lipid-like molecules | PE(P-16:0e/0:0) | 1.22 ± 0.66 | −0.01 | Down |
| 2-Arachidonylglycerol | 1.27 ± 0.49 | 0.25 | Up | |
| LysoPE(20:4(5Z,8Z,11Z,14Z)/0:0) | 1.48 ± 0.36 | 0.58 | Up | |
| 10-Nitrolinoleic acid | 1.53 ± 0.32 | 1.26 | Up | |
| 8R-Hydroperoxylinoleic acid | 1.37 ± 0.57 | 1.41 | Up | |
| Organooxygen compounds | 2-Hexadecanone | 1.53 ± 0.43 | 2.77 | Up |
| Dodecanal dimethyl acetal | 1.44 ± 0.62 | 3.23 | Up | |
| Lupulone | 1.48 ± 0.58 | 18.90 | Up | |
| Dihydrojasmone | 1.53 ± 0.14 | 19.32 | Up | |
| Zingiberol | 1.49 ± 0.20 | 19.40 | Up | |
| Colupulone | 1.55 ± 0.20 | 21.35 | Up | |
| Carboxylic acid and derivatives | Racemethionine | 1.10 ± 0.79 | −0.77 | Down |
| N-gamma-L-Glutamyl-L-isoleucine | 1.19 ± 0.83 | 0.19 | Up | |
| Desmosine | 1.52 ± 0.20 | 1.78 | Up | |
| Frangulanine | 1.54 ± 0.07 | 2.22 | Up | |
| Sakacin A | 1.49 ± 0.15 | 3.45 | Up | |
| Merodesmosine | 1.52 ± 0.14 | 19.65 | Up | |
| Other compounds | Subaphylline | 1.31 ± 0.60 | 0.15 | Up |
| Arenaine | 1.04 ± 1.18 | −0.02 | Down | |
| Rotundine C | 1.49 ± 0.18 | 19.24 | Up | |
| (+/-)-2-(5-Methyl-5-vinyltetrahydrofuran-2-yl)propionaldehyde | 1.47 ± 0.39 | 1.35 | Up | |
| (+)-1,2-Epoxyneomenthyl acetate | 1.52 ± 0.26 | 2.22 | Up | |
| protoporphyrin IX | 1.54 ± 0.18 | 19.20 | Up | |
| 2-Aminomuconic acid semialdehyde | 1.28 ± 0.74 | −1.15 | Down | |
| L-Tryptophan | 1.14 ± 0.90 | −0.69 | Down | |
| 3-Hydroxy-11Z-octadecenoylcarnitine | 1.35 ± 0.71 | 0.81 | Up | |
| 3-Methylbutyl 3-oxobutanoate | 1.43 ± 0.38 | 19.83 | Up | |
| Hesperaline | 1.43 ± 0.46 | 0.76 | Up | |
| Triethanolamine | 1.00 ± 1.21 | −0.71 | Down |
| VIP Discriminant Compounds | Correlations with TBARS |
|---|---|
| 18-hydroxyoleate | 0.94 ** |
| 3,4-dimethyl-5-pentyl-2-furanheptanoic acid | 0.78 ** |
| 8R-hydroperoxylinoleic acid | 0.83 ** |
| 16-hydroxy-hexadecanoic acid | 0.96 ** |
| 4-hydroxy-2-nonenal | 0.74 ** |
| 6-hydroxypentadecanedioic acid | 0.98 ** |
| 20-hydroxy-PGF2a | 0.93 ** |
| 9,10,12,13-tetrahydroxyoctadecanoic acid | 0.98 ** |
| 3-methylbutyl 3-oxobutanoate | 0.91 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchetti, G.; Bernardo, L.; Pateiro, M.; Barba, F.J.; Munekata, P.E.S.; Trevisan, M.; Lorenzo, J.M.; Lucini, L. Impact of a Pitanga Leaf Extract to Prevent Lipid Oxidation Processes during Shelf Life of Packaged Pork Burgers: An Untargeted Metabolomic Approach. Foods 2020, 9, 1668. https://doi.org/10.3390/foods9111668
Rocchetti G, Bernardo L, Pateiro M, Barba FJ, Munekata PES, Trevisan M, Lorenzo JM, Lucini L. Impact of a Pitanga Leaf Extract to Prevent Lipid Oxidation Processes during Shelf Life of Packaged Pork Burgers: An Untargeted Metabolomic Approach. Foods. 2020; 9(11):1668. https://doi.org/10.3390/foods9111668
Chicago/Turabian StyleRocchetti, Gabriele, Letizia Bernardo, Mirian Pateiro, Francisco J. Barba, Paulo E. S. Munekata, Marco Trevisan, José M. Lorenzo, and Luigi Lucini. 2020. "Impact of a Pitanga Leaf Extract to Prevent Lipid Oxidation Processes during Shelf Life of Packaged Pork Burgers: An Untargeted Metabolomic Approach" Foods 9, no. 11: 1668. https://doi.org/10.3390/foods9111668
APA StyleRocchetti, G., Bernardo, L., Pateiro, M., Barba, F. J., Munekata, P. E. S., Trevisan, M., Lorenzo, J. M., & Lucini, L. (2020). Impact of a Pitanga Leaf Extract to Prevent Lipid Oxidation Processes during Shelf Life of Packaged Pork Burgers: An Untargeted Metabolomic Approach. Foods, 9(11), 1668. https://doi.org/10.3390/foods9111668











