Effect of an Olive Vegetation Water Phenolic Extract on the Physico-Chemical, Microbiological and Sensory Traits of Shrimp (Parapenaeus longirostris) during the Shelf-Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Phenolic Extract and Shrimps Sample
- tap water (control group, CTRL);
- tap water solution containing 2 g/L of phenols (phenolic extract group, PE) obtained by adding 1.92 g PEOVW/L;
- 0.5% sodium metabisulfite tap water solution (sulfites group, S);
- 0.25% sodium metabisulfite tap water solution containing 1 g/L of phenols (phenolic extract + sulfites group, PE + S) obtained by adding 3.84 g PEOVW/L.
2.2. Phenolic Extraction and HPLC Analysis
2.3. Instrumental Analysis: Color and pH
2.4. Chemical Analyses
2.5. Microbiological Analyses
2.6. Melanosis Assessment
2.7. Statistical Analyses
3. Results and Discussion
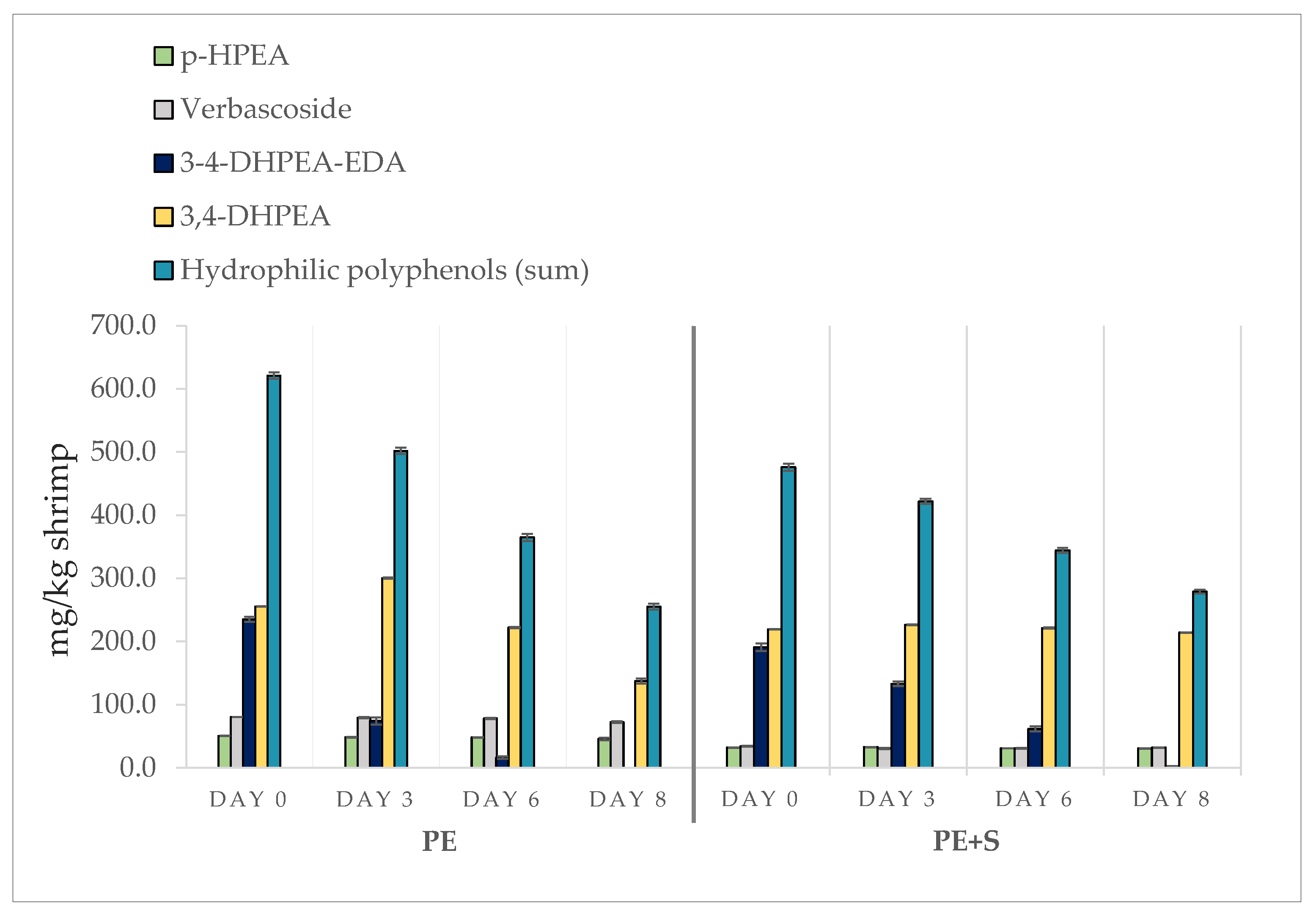
3.1. Phenolic Compound Assessment
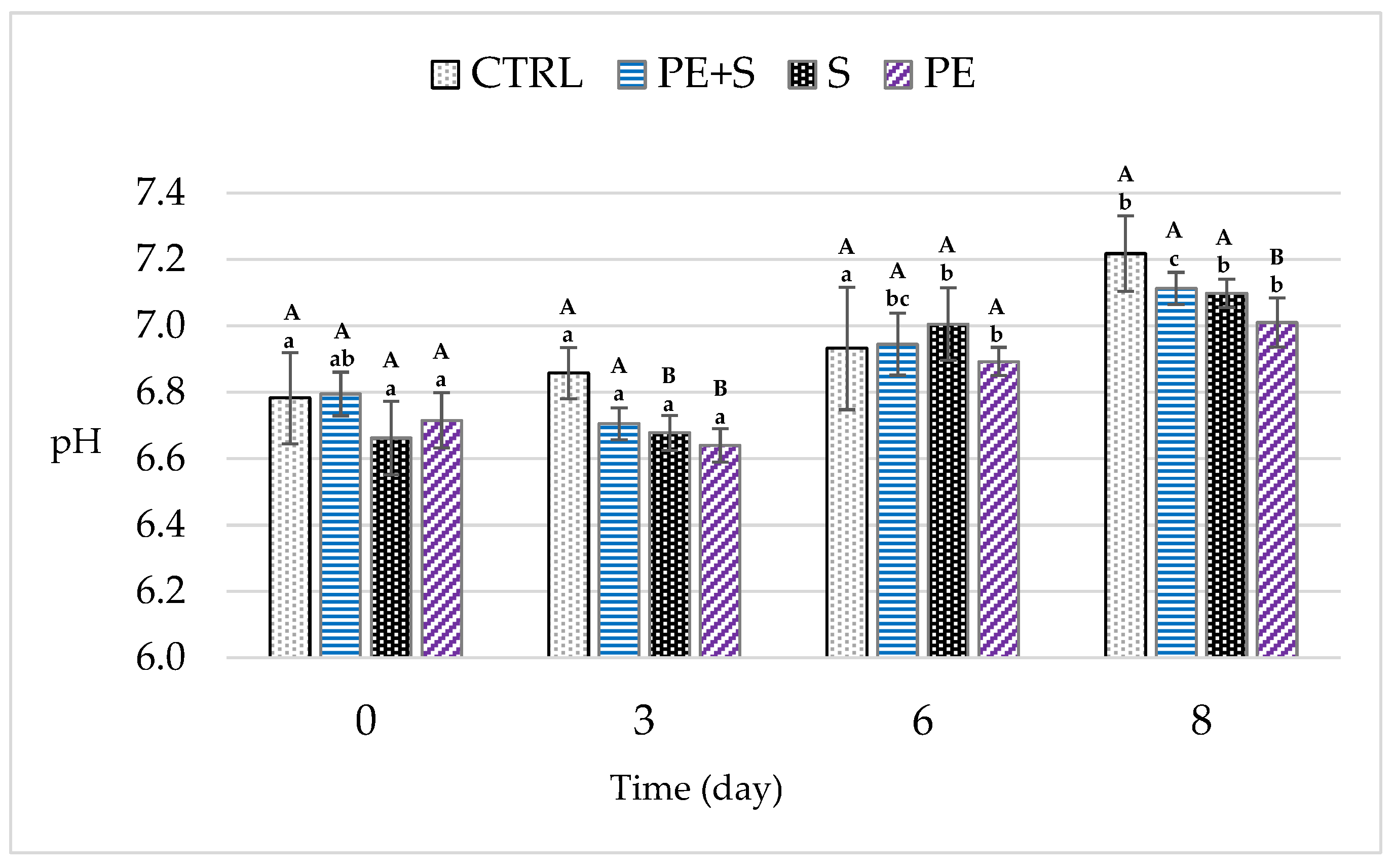
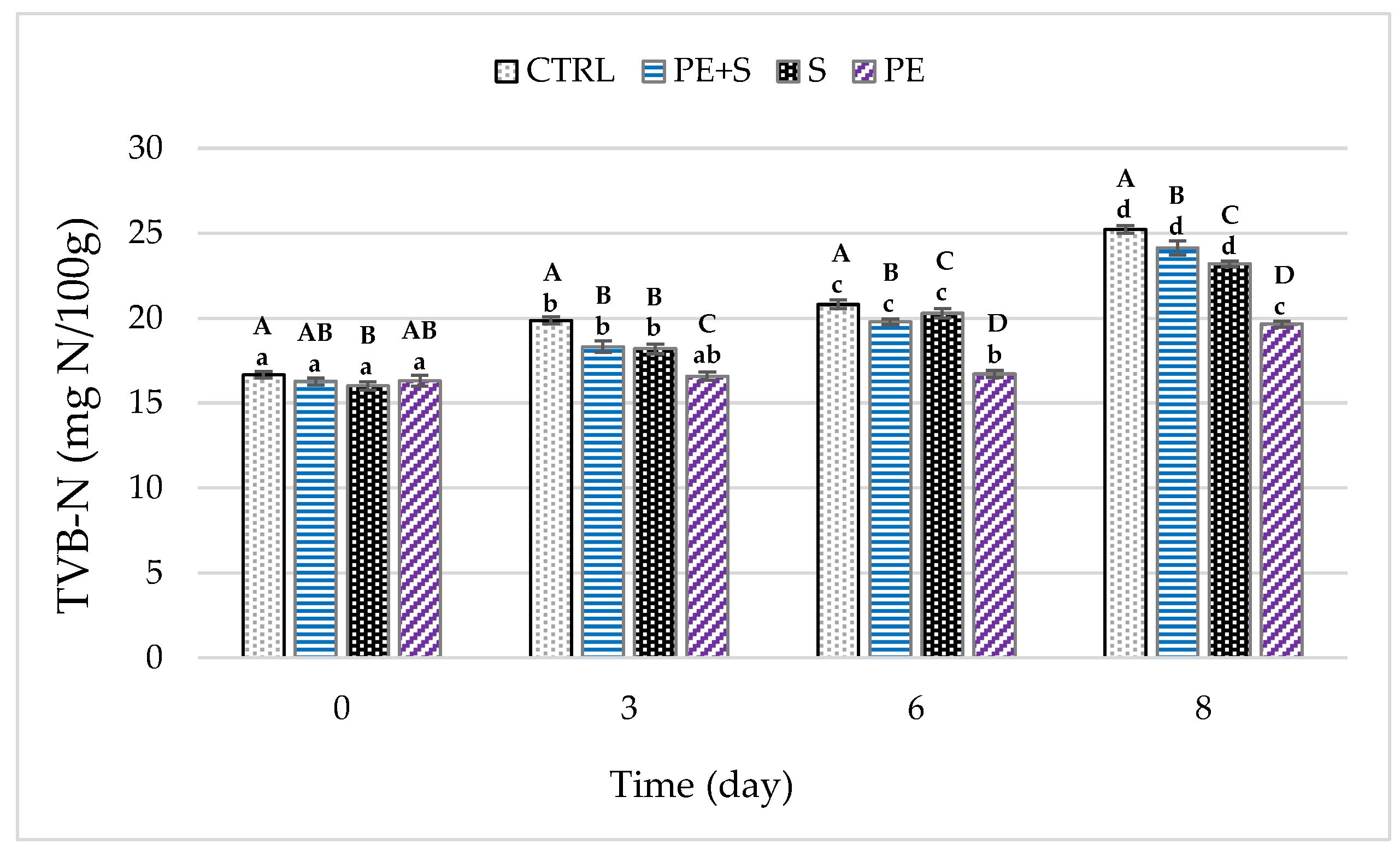
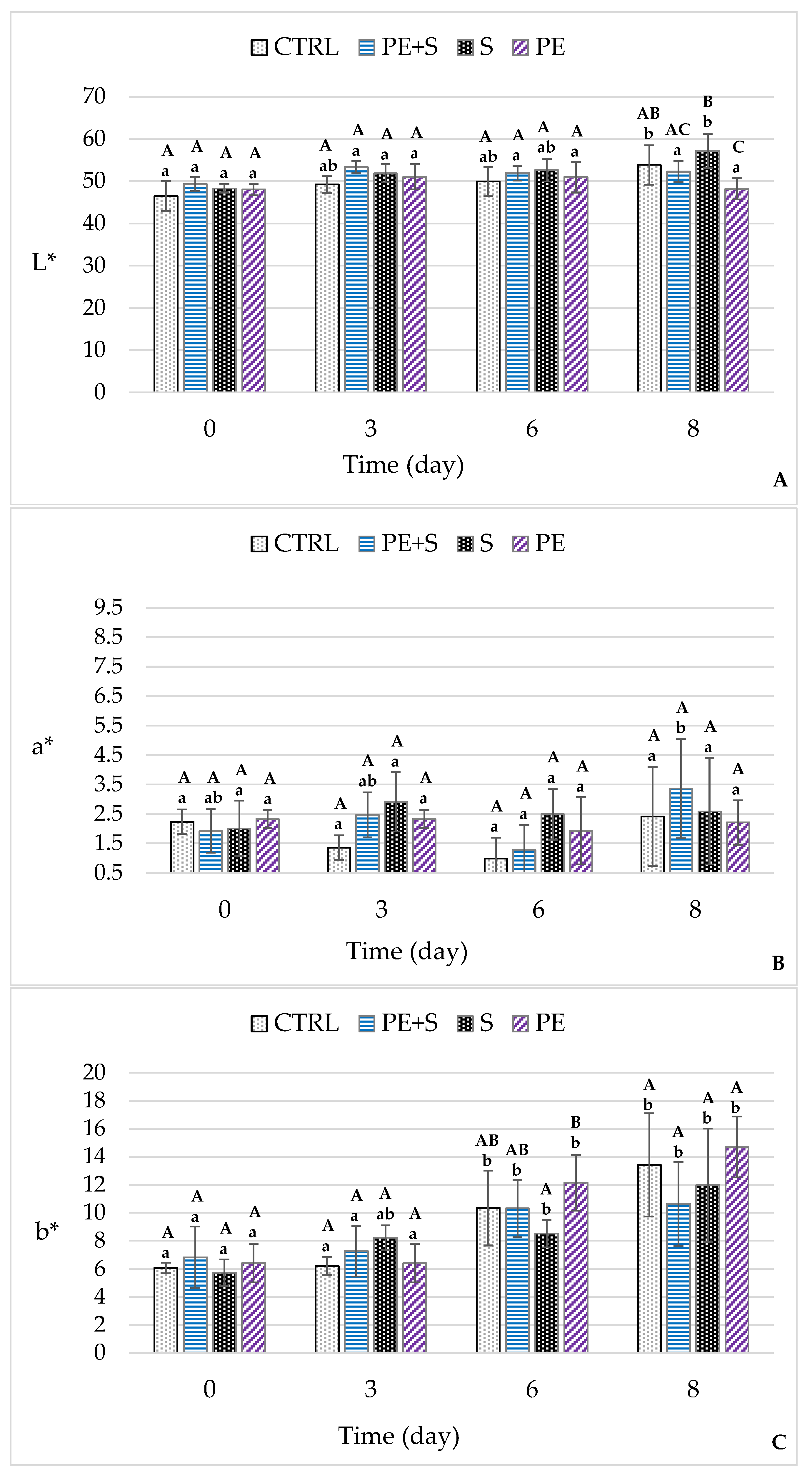
3.2. Physical and Chemical Quality Analysis
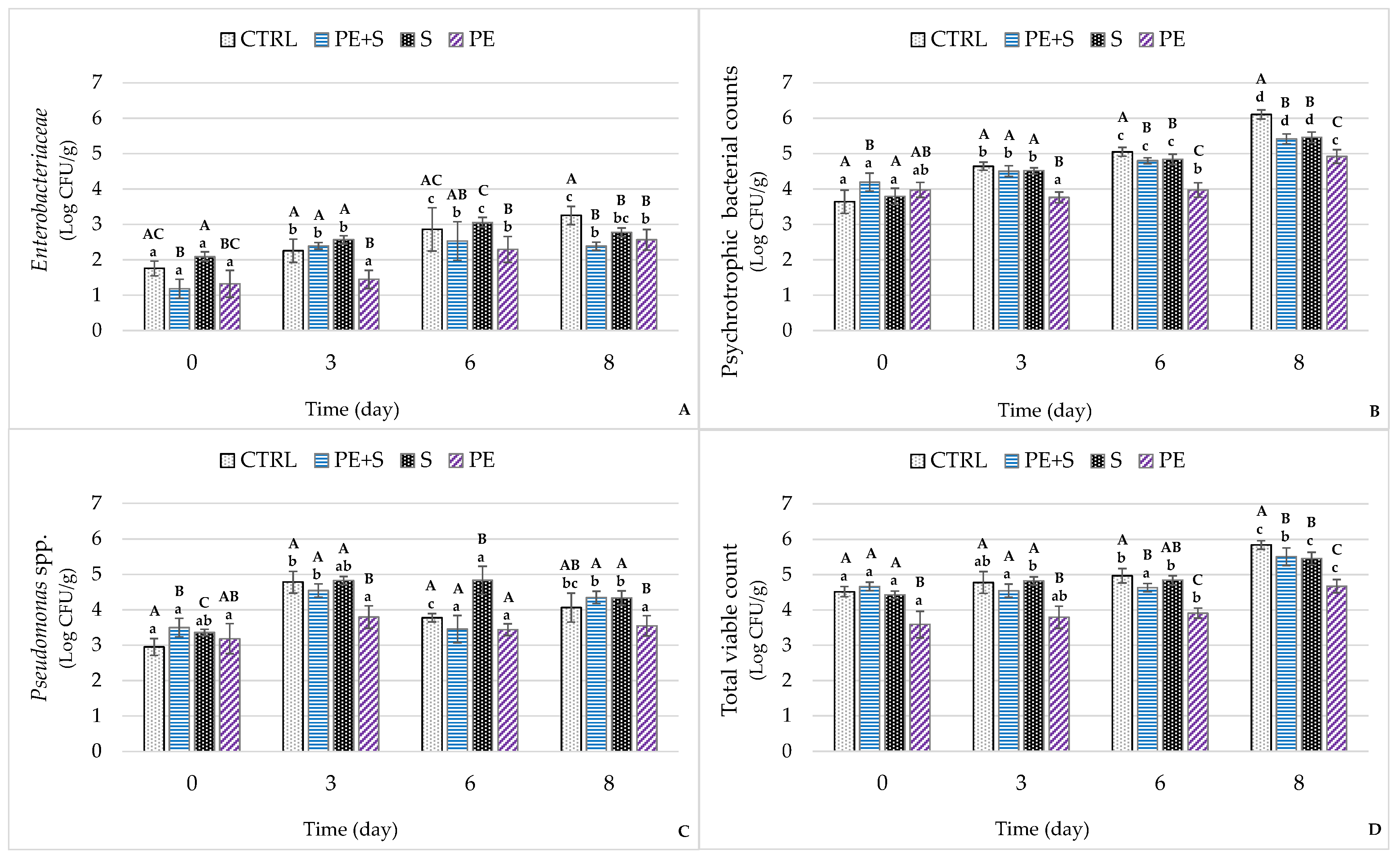
3.3. Microbiological Analysis
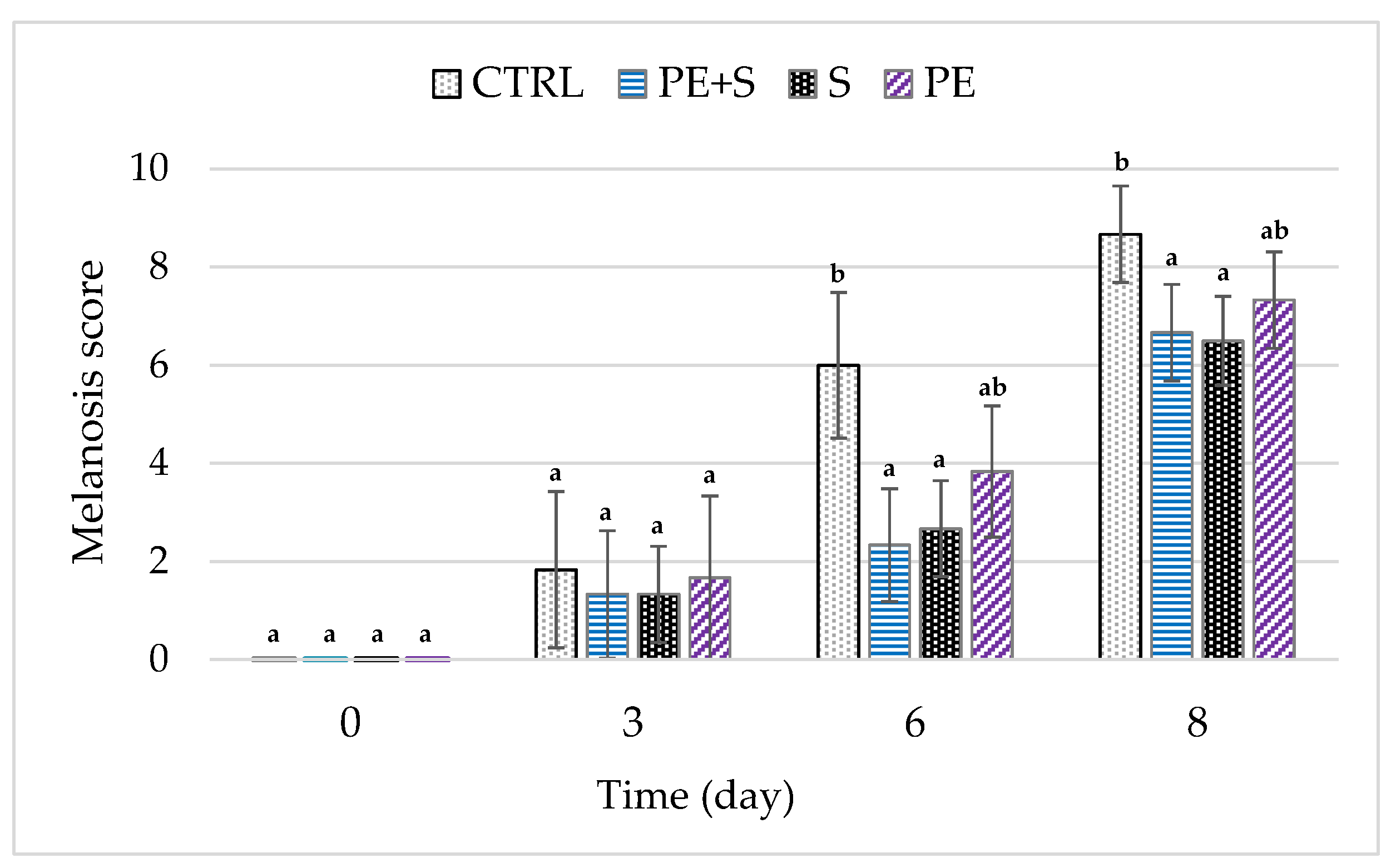
3.4. Melanosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Catalogue. Vol.1—Shrimps and Prawns of the World; FAO: Rome, Italy, 1980. [Google Scholar]
- Arculeo, M.; Cannizzaro, L.; Lo Brutto, S.; Vitale, S. Growth and reproduction of the deep-water rose shrimp, Parapenaeus longirostris (lucas, 1846) (decapoda, penaeidae), in the Southern Tyrrhenian sea. Crustaceana 2014, 87, 1168–1184. [Google Scholar] [CrossRef]
- Cankirilig, E.C.; Berik, N. Changes in fatty acid and mineral compositions of rose-shrimp croquettes during production process. Am. J. Food Technol. 2017, 12, 254–261. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; de Oliveira, A.R.M. Melanosis in crustaceans: A review. LWT Food Sci. Technol. 2016, 65, 791–799. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Martínez-Alvarez, Ó.; Llamas, A.; Montero, P. Melanosis inhibition and SO2residual levels in shrimps (Parapenaeus longirostris) after different sulfite-based treatments. J. Sci. Food Agric. 2005, 85, 1143–1148. [Google Scholar] [CrossRef]
- Hardisson, A.; Rubio, C.; Frías, I.; Rodríguez, I.; Reguera, J.I. Content of sulphite in frozen prawns and shrimps. Food Control 2002, 13, 275–279. [Google Scholar] [CrossRef]
- Iammarino, M.; Di Taranto, A.; Ientile, A.R. Monitoring of sulphites levels in shrimps samples collected in Puglia (Italy) by ion-exchange chromatography with conductivity detection. Food Addit. Contam. Part B Surveill. 2014, 7, 84–89. [Google Scholar] [CrossRef]
- Gokoglu, N.; Yerlikaya, P. Inhibition effects of grape seed extracts on melanosis formation in shrimp (Parapenaeus longirostris). Int. J. Food Sci. Technol. 2008, 43, 1004–1008. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Balzan, S.; Taticchi, A.; Cardazzo, B.; Urbani, S.; Servili, M.; Di Lecce, G.; Zabalza, I.B.; Rodriguez-Estrada, M.T.; Novelli, E.; Fasolato, L. Effect of phenols extracted from a by-product of the oil mill on the shelf-life of raw and cooked fresh pork sausages in the absence of chemical additives. LWT Food Sci. Technol. 2017, 85, 89–95. [Google Scholar] [CrossRef]
- Tarnavölgyi, G. Analysis of consumers’ attitudes towards food additives using focus group survey. Agric. Conspec. Sci. 2003, 68, 193–196. [Google Scholar]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Retardation of quality changes of Pacific white shrimp by green tea extract treatment and modified atmosphere packaging during refrigerated storage. Int. J. Food Microbiol. 2011, 149, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lv, H.; Yuan, G.; Fang, X. Effect of grape seed extracts on the melanosis and quality of pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Sci. Technol. Res. 2014, 20, 671–677. [Google Scholar] [CrossRef]
- Fei, P.; Xu, Y.; Zhao, S.; Gong, S.; Guo, L. Olive oil polyphenol extract inhibits vegetative cells of Bacillus cereus isolated from raw milk. J. Dairy Sci. 2019, 102, 3894–3902. [Google Scholar] [CrossRef]
- Miraglia, D.; Esposto, S.; Branciari, R.; Urbani, S.; Servili, M.; Perucci, S.; Ranucci, D. Effect of a Phenolic extract from olive vegetation water on fresh salmon steak quality during storage. Ital. J. Food Saf. 2016, 5, 224–228. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Santacatalina, J.V.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Influence of drying on the retention of olive leaf polyphenols infused into dried apple. Food Bioprocess Technol. 2014, 8, 885–894. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2006, 100, 998–1004. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G.F. Improvement of bioactive phenol content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment. Food Chem. 2011, 124, 1308–1315. [Google Scholar] [CrossRef]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste: Recent Advances for Sustainable Management; Elsevier: New York, NY, USA, 2017; pp. 1–28. ISBN 9780128053140. [Google Scholar]
- Esposto, S.; Taticchi, A.; Di Maio, I.; Urbani, S.; Veneziani, G.; Selvaggini, R.; Sordini, B.; Servili, M. Effect of an olive phenolic extract on the quality of vegetable oils during frying. Food Chem. 2015, 176, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Selvaggini, R.; Esposto, S.; Taticchi, A.; Urbani, S.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Optimization of the temperature and oxygen concentration conditions in the malaxation during the oil mechanical extraction process of four italian olive cultivars. J. Agric. Food Chem. 2014, 62, 3813–3822. [Google Scholar] [CrossRef]
- Castrica, M.; Menchetti, L.; Balzaretti, C.M.; Branciari, R.; Ranucci, D.; Cotozzolo, E.; Vigo, D.; Curone, G.; Brecchia, G.; Miraglia, D. Impact of dietary supplementation with goji berries (Lycium barbarum) on microbiological quality, physico-chemical, and sensory characteristics of rabbit meat. Foods 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–47. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S. Prevention of quality loss and melanosis of Pacific white shrimp by cashew leaf extracts. Food Control 2019, 95, 257–266. [Google Scholar] [CrossRef]
- Menchetti, L.; Canali, C.; Castellini, C.; Boiti, C.; Brecchia, G. The different effects of linseed and fish oil supplemented diets on insulin sensitivity of rabbit does during pregnancy. Res. Vet. Sci. 2018, 118, 126–133. [Google Scholar] [CrossRef]
- Powell, E.O. Growth rate and generation time of bacteria, with special reference to continuous culture. J. Gen. Microbiol. 1956, 15, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics using SPSS (and Sex and Drugs and Rock ‘n’ Roll), 3rd ed.; Sage Pubblications: London, UK, 2009. [Google Scholar]
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G.F. Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. JAOCS J. Am. Oil Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Mendes, R.; Huidobro, A.; Caballero, E.L. Indole levels in deepwater pink shrimp (Parapenaeus longirostris) from the Portuguese coast. Effects of temperature abuse. Eur. Food Res. Technol. 2002, 214, 125–130. [Google Scholar] [CrossRef]
- Rotllant, G.; Arnau, F.; García, J.A.; García, N.; Rodríguez, M.; Sardà, F. Note. Effect of Metabisulphite Treatments and Freezing on Melanosis Inhibition in Rose Shrimp Aristeus Antennatus (Risso, 1816). Food Sci. Technol. Int. 2002, 8, 243–247. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Martínez-Alvarez, O.; Gómez-Guillén, M.D.C.; Montero, P. Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis-inhibiting formulations during chilled storage. Int. J. Food Sci. Technol. 2007, 42, 1029–1038. [Google Scholar] [CrossRef]
- Castrica, M.; Chiesa, L.M.; Nobile, M.; De Battisti, F.; Siletti, E.; Pessina, D.; Panseri, S.; Balzaretti, C.M. Rapid safety and quality control during fish shelf-life by using a portable device. J. Sci. Food Agric. 2020, 101, 315–326. [Google Scholar] [CrossRef]
- Bahmani, Z.A.; Rezai, M.; Hosseini, S.V.; Regenstein, J.M.; Böhme, K.; Alishahi, A.; Yadollahi, F. Chilled storage of golden gray mullet (Liza aurata). LWT Food Sci. Technol. 2011, 44, 1894–1900. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Martínez-Álvarez, O.; Gómez-Guillén, M.C.; Montero, P. Several melanosis-inhibiting formulas to enhance the quality of deepwater pink shrimp (Parapenaeus longirostris). Innov. Food Sci. Emerg. Technol. 2019, 51, 91–99. [Google Scholar] [CrossRef]
- Yuan, G.; Zhang, X.; Tang, W.; Sun, H. Effect of chitosan coating combined with green tea extract on the melanosis and quality of Pacific white shrimp during storage in ice. CYTA J. Food 2016, 51, 114–121. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Q.; Qiu, M.; Li, S. Chitosan-based edible coatings for quality preservation of postharvest whiteleg shrimp (Litopenaeus vannamei). J. Food Sci. 2012, 77, C491–C496. [Google Scholar] [CrossRef]
- Wu, S. Effect of chitosan-based edible coating on preservation of white shrimp during partially frozen storage. Int. J. Biol. Macromol. 2014, 65, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Chen, H.; Fang, X.; Mao, J.; Gao, H. Effect of cinnamaldehyde on melanosis and spoilage of Pacific white shrimp (Litopenaeus vannamei) during storage. J. Sci. Food Agric. Food Agric. 2012, 92, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Bono, G.; Okpala, C.O.R.; Alberio, G.R.A.; Messina, C.M.; Santulli, A.; Iacalone, G.; Spagna, G. Toward shrimp consumption without chemicals: Combined effects of freezing and modified atmosphere packaging (MAP) on some quality characteristics of Giant Red Shrimp (Aristaeomorpha foliacea) during storage. Food Chem. 2015, 197, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Sallam, K. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Jones, Y.M.; Kitts, D. Seafood Safety, Processing, and Biotechnology; CRC Press: Lancaster, PA, USA, 1997; ISBN 9781566765732. [Google Scholar]
- Schormüller, J. Handbuch der Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 1969; Volume 9. [Google Scholar]
- Bonnell, D. Quality Assurance in Seafood Processing: A Practical Guide; Springer: Cham, Switzerland, 1994. [Google Scholar]
- Koka, R.; Weimer, B.C. Influence of growth conditions on heat-stable phospholipase activity in Pseudomonas. J. Dairy Res. 2001, 68, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Cadun, A.; Kişla, D.; Çakli, Ş. Marination of deep-water pink shrimp with rosemary extract and the determination of its shelf-life. Food Chem. 2008, 109, 81–87. [Google Scholar] [CrossRef]
- Senapati, S.R.; Kumar, G.P.; Singh, C.B.; Xavier, K.A.M.; Chouksey, M.K.; Nayak, B.B.; Balange, A.K. Melanosis and quality attributes of chill stored farm raised whiteleg shrimp (Litopenaeus vannamei). J. Appl. Nat. Sci. 2017, 9, 626–631. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Balzan, S.; Carraro, L.; Taticchi, A.; Montemurro, F.; Novelli, E. Effect of minimum bactericidal concentration of phenols extracted from oil vegetation water on spoilers, starters and food-borne bacteria. Ital. J. Food Saf. 2015, 4, 75–77. [Google Scholar] [CrossRef][Green Version]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Menchetti, L.; Taticchi, A.; Esposto, S.; Servili, M.; Ranucci, D.; Branciari, R.; Miraglia, D. The influence of phenolic extract from olive vegetation water and storage temperature on the survival of Salmonella Enteritidis inoculated on mayonnaise. LWT Food Sci. Technol. 2020, 109648. [Google Scholar] [CrossRef]
- Alparslan, Y.; Yapici, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT Food Sci. Technol. 2016, 72, 457–466. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miraglia, D.; Castrica, M.; Menchetti, L.; Esposto, S.; Branciari, R.; Ranucci, D.; Urbani, S.; Sordini, B.; Veneziani, G.; Servili, M. Effect of an Olive Vegetation Water Phenolic Extract on the Physico-Chemical, Microbiological and Sensory Traits of Shrimp (Parapenaeus longirostris) during the Shelf-Life. Foods 2020, 9, 1647. https://doi.org/10.3390/foods9111647
Miraglia D, Castrica M, Menchetti L, Esposto S, Branciari R, Ranucci D, Urbani S, Sordini B, Veneziani G, Servili M. Effect of an Olive Vegetation Water Phenolic Extract on the Physico-Chemical, Microbiological and Sensory Traits of Shrimp (Parapenaeus longirostris) during the Shelf-Life. Foods. 2020; 9(11):1647. https://doi.org/10.3390/foods9111647
Chicago/Turabian StyleMiraglia, Dino, Marta Castrica, Laura Menchetti, Sonia Esposto, Raffaella Branciari, David Ranucci, Stefania Urbani, Beatrice Sordini, Gianluca Veneziani, and Maurizio Servili. 2020. "Effect of an Olive Vegetation Water Phenolic Extract on the Physico-Chemical, Microbiological and Sensory Traits of Shrimp (Parapenaeus longirostris) during the Shelf-Life" Foods 9, no. 11: 1647. https://doi.org/10.3390/foods9111647
APA StyleMiraglia, D., Castrica, M., Menchetti, L., Esposto, S., Branciari, R., Ranucci, D., Urbani, S., Sordini, B., Veneziani, G., & Servili, M. (2020). Effect of an Olive Vegetation Water Phenolic Extract on the Physico-Chemical, Microbiological and Sensory Traits of Shrimp (Parapenaeus longirostris) during the Shelf-Life. Foods, 9(11), 1647. https://doi.org/10.3390/foods9111647








