Lactococcus lactis subsp. cremoris Produces Zinc Protoporphyrin IX Both Aerobically and Anaerobically and Improves the Bright Red Color of Fermented Meat Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Starter Culture
2.1.1. Starter Culture for Sausage
2.1.2. Bacterial Culture for Aseptic Meat Homogenate Model System
2.2. Preparation of Sausage
2.3. The Total and Lactic Acid Bacterial Counts
2.4. Water Activity, pH, and Titratable Acidity
2.5. Imaging and ZnPP Autofluorescence
2.6. Measurement of ZnPP in Dry-Cured Sausages
2.7. Color Analysis
2.8. Aseptic Meat Homogenate Model System and Measurement of ZnPP Fluorescence Intensity
2.9. Statistical Analysis
3. Results
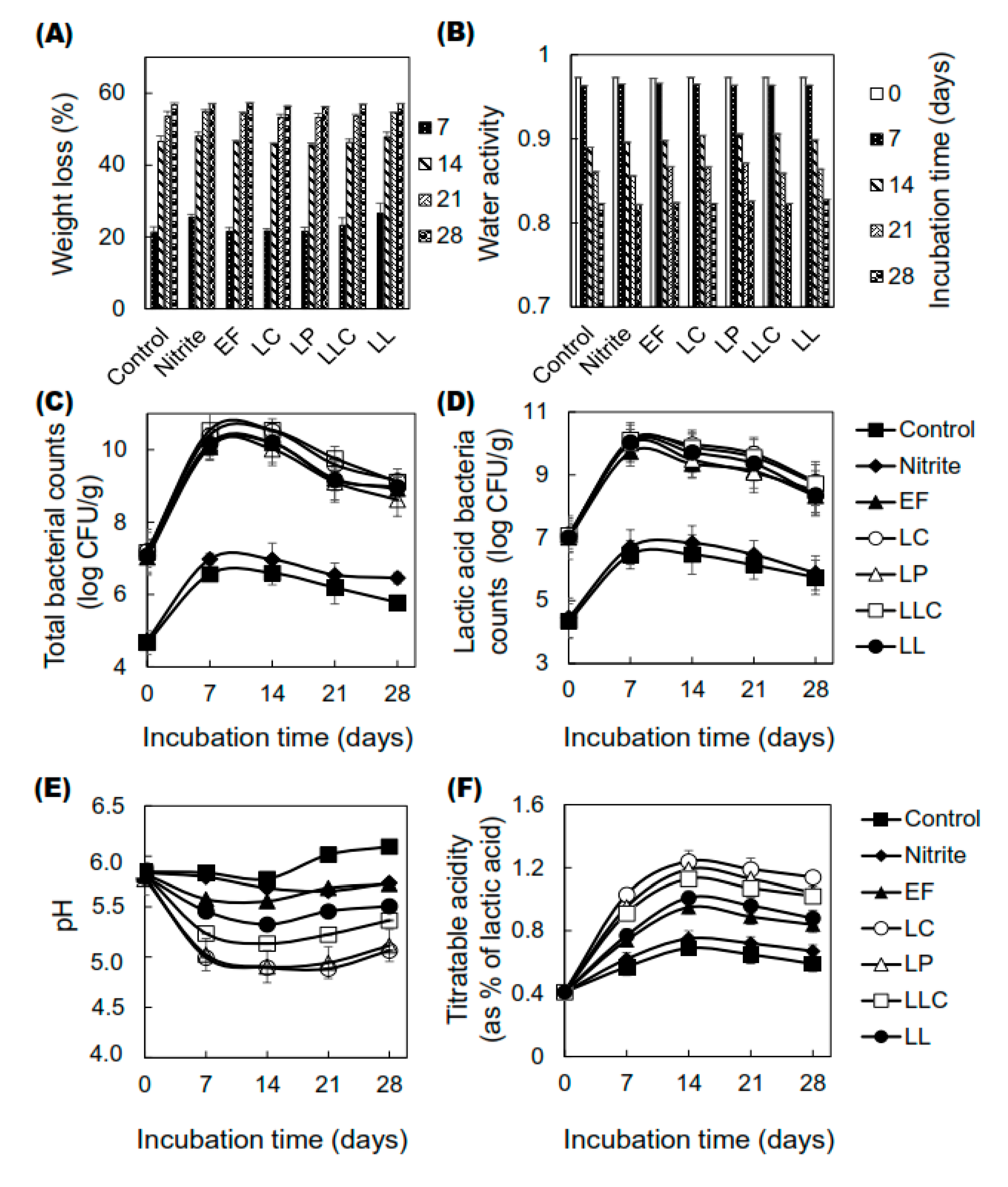
3.1. Changes in Technological Properties of Dry-Cured Sausages
3.2. Evaluation of the Color-Improving Ability of High ZnPP-Forming LAB in Dry-Cured Sausages
3.2.1. Changes in the External Color of Dry-Cured Sausages
3.2.2. Changes in the Internal Color of Dry-Cured Sausages and Observation of ZnPP Distribution
3.2.3. Changes in the ZnPP Content of Dry-Cured Sausages
3.2.4. Color Profiles of the Dry-Cured Sausages
3.3. Effect of Oxygen on ZnPP Formation by the ZnPP-Forming Inoculated-LAB
4. Discussion
4.1. Internal Color and Anaerobic ZnPP Formation
4.2. External Color and Aerobic ZnPP Formation
4.3. Technological Properties of the Dry-Cured Sausages and ZnPP Formation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pegg, R.B.; Boles, J.A. CURING | Production Procedures. Encyclopedia of Meat Sciences, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 442–452. [Google Scholar] [CrossRef]
- Masuda, M.; Mower, H.F.; Pignatelli, B.; Celan, I.; Friesen, M.D.; Nishino, H.; Ohshima, H. Formation of N-nitrosamines and N-nitramines by the reaction of secondary amines peroxynitrite and other reactive nitrogen species: Comparison with nitrotyrosine formation. Chem. Res. Toxicol. 2000, 13, 301–308. [Google Scholar] [CrossRef]
- Skibsted, L.H. Nitric oxide and quality and safety of muscle based foods. Nitric Oxide Biol. Chem. 2011, 24, 176–183. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Red Meat and Processed Meat; International Agency for Research on Cancer: Lyon, France, 2018; Volume 114, ISBN 9789283201809.
- Wakamatsu, J.; Nishimura, T.; Hattori, A. A Zn-porphyrin complex contributes to bright red color in Parma ham. Meat Sci. 2004, 67, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.K.S.; Adamsen, C.E.; Catharino, R.R.; Skibsted, L.H.; Eberlin, M.N. Mass spectrometric evidence for a zinc-porphyrin complex as the red pigment in dry-cured Iberian and Parma ham. Meat Sci. 2007, 75, 203–210. [Google Scholar] [CrossRef] [PubMed]
- De Maere, H.; Fraeye, I.; De Mey, E.; Dewulf, L.; Michiels, C.; Paelinck, H.; Chollet, S. Formation of naturally occurring pigments during the production of nitrite-free dry fermented sausages. Meat Sci. 2016, 114, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Niu, J.; Sakata, R.; Nagata, Y. Red Pigment of Parma Ham and Bacterial Influence on its Formation. J. Food Sci. 1996, 61, 1021–1023. [Google Scholar] [CrossRef]
- Adamsen, C.E.; Møller, J.K.S.; Hismani, R.; Skibsted, L.H. Thermal and photochemical degradation of myoglobin pigments in relation to colour stability of sliced dry-cured Parma ham and sliced dry-cured ham produced with nitrite salt. Eur. Food Res. Technol. 2004, 218, 403–409. [Google Scholar] [CrossRef]
- Wakamatsu, J.; Kawazoe, H.; Ohya, M.; Hayakawa, T.; Kumura, H. Improving the color of meat products without adding nitrite/nitrate using high zinc protoporphyrin IX-forming microorganisms. Meat Sci. 2020, 161, 107989. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Ohya, M.; Kumura, H.; Hayakawa, T.; Wakamatsu, J. Searching for high ZnPP-forming edible bacteria to improve the color of fermented meat products without nitrite/nitrate. Meat Sci. 2020, 165, 108109. [Google Scholar] [CrossRef]
- da Costa, R.J.; Voloski, F.L.S.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of Meat Products with Bacteriocins Produced by Lactic Acid Bacteria Isolated from Meat. J. Food Qual. 2019, 2019, 4726510. [Google Scholar] [CrossRef]
- Kučerová, K.; Svobodová, H.; Tůma, Š.; Ondráčková, I.; Plocková, M. Production of biogenic amines by Enterococci. Czech J. Food Sci. 2010, 27, 50–55. [Google Scholar] [CrossRef]
- Alam, M.K.; Hayakawa, T.; Kumura, H.; Wakamatsu, J. A new technique to improve the color of meat products using food-grade lactic acid bacteria with high zinc protoporphyrin IX-forming ability as a potential substitute for nitrite/nitrate. Meat Sci. 2020. submitted. [Google Scholar]
- Wakamatsu, J.; Okui, J.; Ikeda, Y.; Nishimura, T.; Hattori, A. Establishment of a model experiment system to elucidate the mechanism by which Zn-protoporphyrin IX is formed in nitrite-free dry-cured ham. Meat Sci. 2004, 68, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, J.I.; Uemura, J.; Odagiri, H.; Okui, J.; Hayashi, N.; Hioki, S.; Nishimura, T.; Hattori, A. Formation of zinc protoporphyrin IX in Parma-like ham without nitrate or nitrite. Anim. Sci. J. 2009, 80, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Khozroughi, A.G.; Kroh, L.W.; Schlüter, O.; Rawel, H. Assessment of the bacterial impact on the post-mortem formation of zinc protoporphyrin IX in pork meat. Food Chem. 2018, 256, 25–30. [Google Scholar] [CrossRef]
- Chau, T.T.; Ishigaki, M.; Kataoka, T.; Taketani, S. Ferrochelatase catalyzes the formation of Zn-protoporphyrin of dry-cured ham via the conversion reaction from heme in meat. J. Agric. Food Chem. 2011, 59, 12238–12245. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Moraes, P.M.; Martins Perin, L.; Silva Júnior, A.; Nero, L.A. Comparison of phenotypic and molecular tests to identify lactic acid bacteria. Braz. J. Microbiol. 2013, 44, 109–112. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; p. 780. Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 15 October 2020).
- Wakamatsu, J.; Okui, J.; Hayashi, N.; Nishimura, T.; Hattori, A. Zn protoporphyrin IX is formed not from heme but from protoporphyrin IX. Meat Sci. 2007, 77, 580–586. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kawabuchi, T.; Kawakami, Y.; Sato, M.; Numata, M.; Matsumoto, K. Formation of Zinc Protoporphyrin IX and Protoporphyrin IX from Oxymyoglobin in Porcine Heart Mitochondria. Food Sci. Technol. Res. 2007, 13, 85–88. [Google Scholar] [CrossRef]
- Grossi, A.B.; do Nascimento, E.S.P.; Cardoso, D.R.; Skibsted, L.H. Proteolysis involvement in zinc-protoporphyrin IX formation during Parma ham maturation. Food Res. Int. 2014, 56, 252–259. [Google Scholar] [CrossRef]
- Fernández, M.; Martín, A.; Benito, M.J.; Casquete, R.; Recio, I.; Córdoba, M.D.G. Influence of starter cultures on the generation of antioxidant nitrogen compounds in Iberian dry-fermented sausages. Int. J. Food Sci. 2016, 51, 435–443. [Google Scholar] [CrossRef]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin Chemistry and Meat Color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, Y.; Ma, C.; Song, H.; Li, H.; Wang, Z.; Xiao, S. Physico-chemical characteristics and free fatty acid composition of dry fermented mutton sausages as affected by the use of various combinations of starter cultures and spices. Meat Sci. 2011, 88, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Nuryana, I.; Andriani, A.; Lisdiyanti, P. Yopi Analysis of organic acids produced by lactic acid bacteria. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 251. [Google Scholar] [CrossRef]
- Wakamatsu, J.; Akter, M.; Honma, F.; Hayakawa, T.; Kumura, H.; Nishimura, T. Optimal pH of zinc protoporphyrin IX formation in porcine muscles: Effects of muscle fiber type and myoglobin content. LWT 2019, 101, 599–606. [Google Scholar] [CrossRef]
- Adamsen, C.E.; Møller, J.K.S.; Laursen, K.; Olsen, K.; Skibsted, L.H. Zn-porphyrin formation in cured meat products: Effect of added salt and nitrite. Meat Sci. 2006, 72, 672–679. [Google Scholar] [CrossRef]
- Chau, T.T.; Ishigaki, M.; Kataoka, T.; Taketani, S. Porcine ferrochelatase: The relationship between iron-removal reaction and the conversion of heme to Zn-protoporphyrin. Biosci. Biotechnol. Biochem. 2010, 74, 1415–1420. [Google Scholar] [CrossRef]





| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
|---|---|---|---|---|---|
| Control | 0.19 ± 0.00 a | 1.07 ± 0.01 b | 1.41 ± 0.01 b | 1.72 ± 0.04 b | 2.03 ± 0.03 b |
| Nitrite | 0.15 ± 0.00 a | 0.37 ± 0.01 a | 0.48 ± 0.02 a | 0.54 ± 0.01 a | 0.57 ± 0.01 a |
| EF | 0.19 ± 0.00 a | 2.04 ± 0.30 c | 2.92 ± 0.05 c | 3.58 ± 0.05 c | 3.80 ± 0.08 c |
| LC | 0.17 ± 0.01 a | 3.75 ± 0.04 e,f | 4.66 ± 0.05 e,f | 5.42 ± 0.12 e | 6.10 ± 0.16 d |
| LP | 0.17 ± 0.01 a | 3.58 ± 0.03 e | 4.37 ± 0.12 e | 4.94 ± 0.11 e | 5.66 ± 0.08 d |
| LLC | 0.16 ± 0.00 a | 3.89 ± 0.02 f | 4.87 ± 0.09 f | 5.40 ± 0.13 e | 6.08 ± 0.12 d |
| LL | 0.19 ± 0.01 a | 2.71 ± 0.05 d | 3.55 ± 0.06 d | 4.17 ± 0.12 d | 4.47 ± 0.18 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauser-Ul-Alam, M.; Toba, Y.; Hioki, S.; Hayakawa, T.; Kumura, H.; Wakamatsu, J.-i. Lactococcus lactis subsp. cremoris Produces Zinc Protoporphyrin IX Both Aerobically and Anaerobically and Improves the Bright Red Color of Fermented Meat Products. Foods 2020, 9, 1583. https://doi.org/10.3390/foods9111583
Kauser-Ul-Alam M, Toba Y, Hioki S, Hayakawa T, Kumura H, Wakamatsu J-i. Lactococcus lactis subsp. cremoris Produces Zinc Protoporphyrin IX Both Aerobically and Anaerobically and Improves the Bright Red Color of Fermented Meat Products. Foods. 2020; 9(11):1583. https://doi.org/10.3390/foods9111583
Chicago/Turabian StyleKauser-Ul-Alam, Md., Yu Toba, Shoji Hioki, Toru Hayakawa, Haruto Kumura, and Jun-ichi Wakamatsu. 2020. "Lactococcus lactis subsp. cremoris Produces Zinc Protoporphyrin IX Both Aerobically and Anaerobically and Improves the Bright Red Color of Fermented Meat Products" Foods 9, no. 11: 1583. https://doi.org/10.3390/foods9111583
APA StyleKauser-Ul-Alam, M., Toba, Y., Hioki, S., Hayakawa, T., Kumura, H., & Wakamatsu, J.-i. (2020). Lactococcus lactis subsp. cremoris Produces Zinc Protoporphyrin IX Both Aerobically and Anaerobically and Improves the Bright Red Color of Fermented Meat Products. Foods, 9(11), 1583. https://doi.org/10.3390/foods9111583





