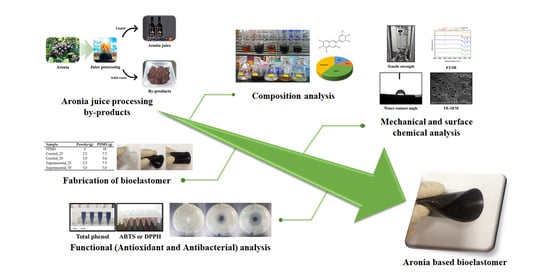
Fabrication of Functional Bioelastomer for Food Packaging from Aronia (Aronia melanocarpa) Juice Processing By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bioelastomers
2.3. Characterization of Bioelastomers
2.4. Antioxidant Activity of Bioelastomers
2.4.1. Total Polyphenol Content
2.4.2. Total Flavonoid Content
2.4.3. DPPH Radical Scavenging Activity
2.4.4. ABTS Radical Scavenging Activity
2.5. Antibacterial Activity of Bioelastomers
3. Results and Discussions
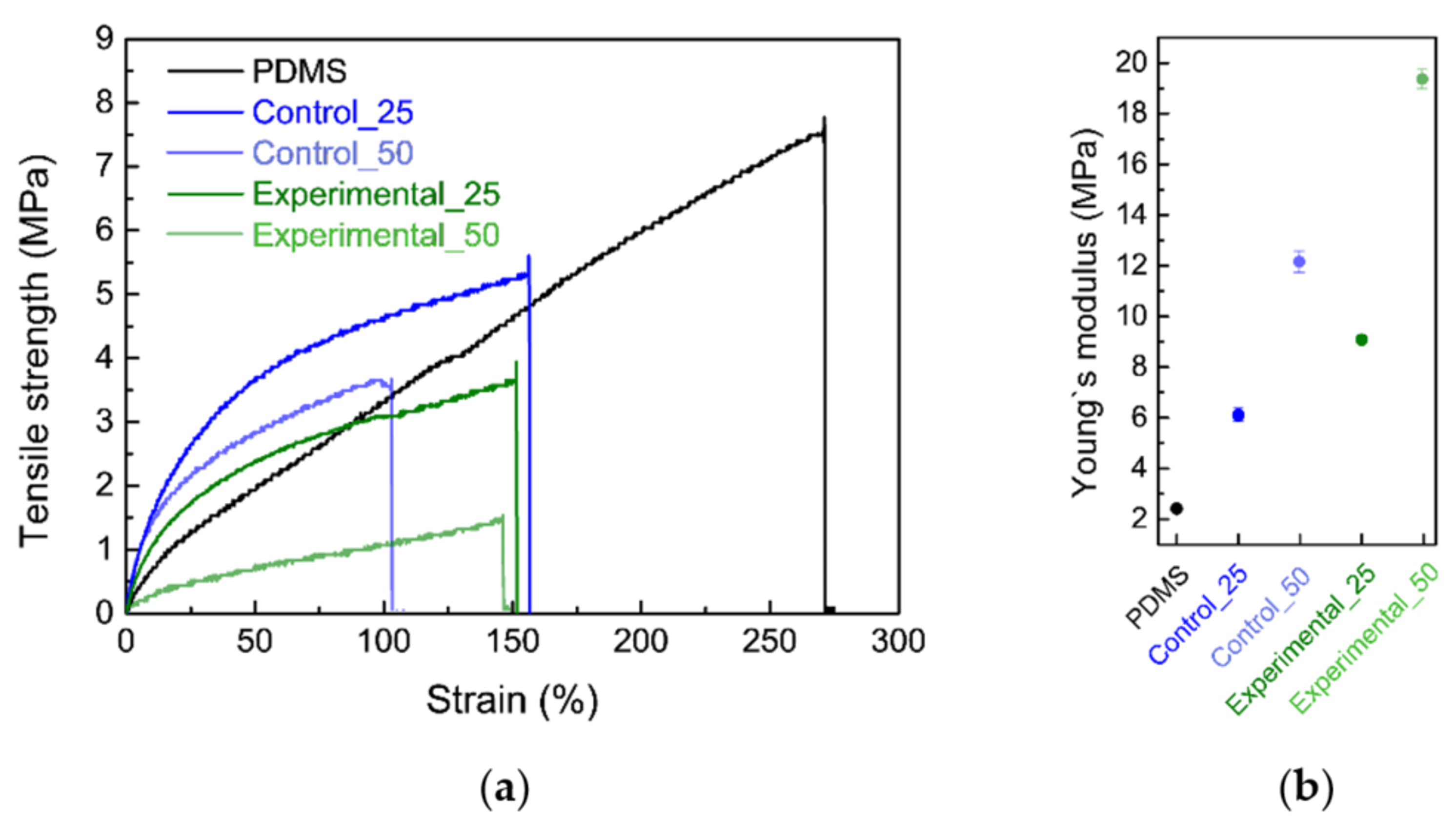
3.1. Mechanical Properties of Bioelastomers
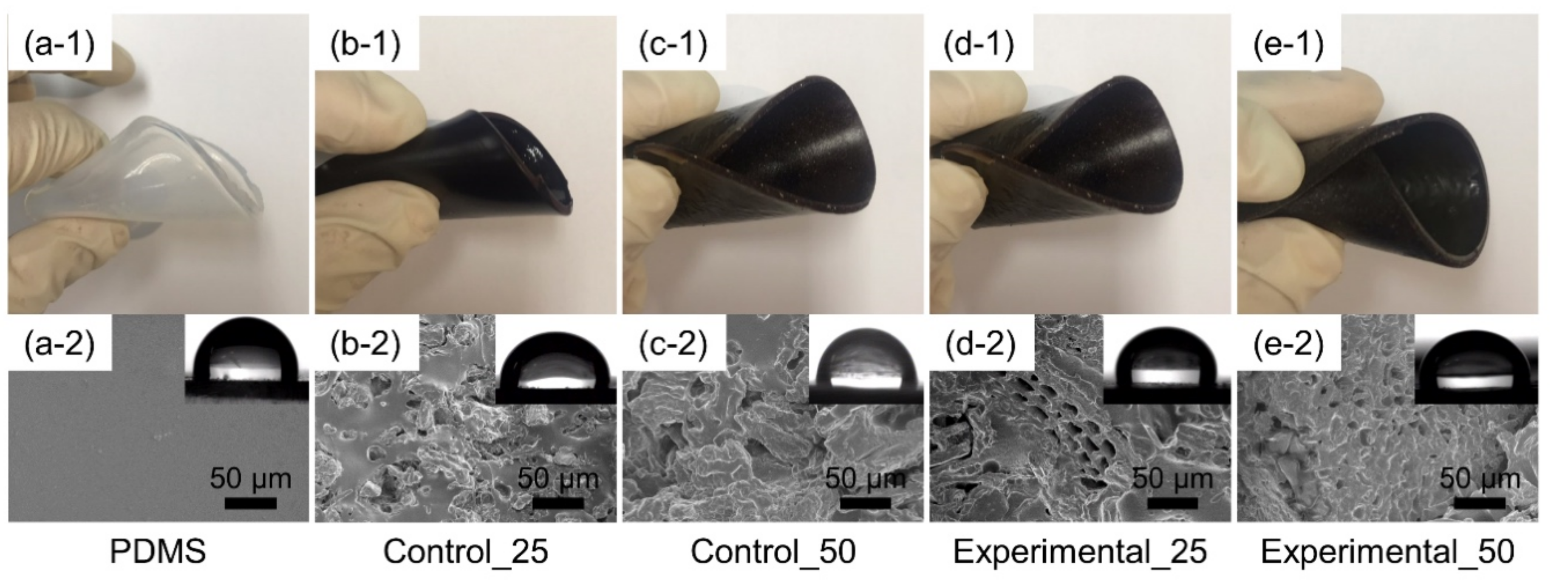
3.2. Surface Properties of Bioelastomers
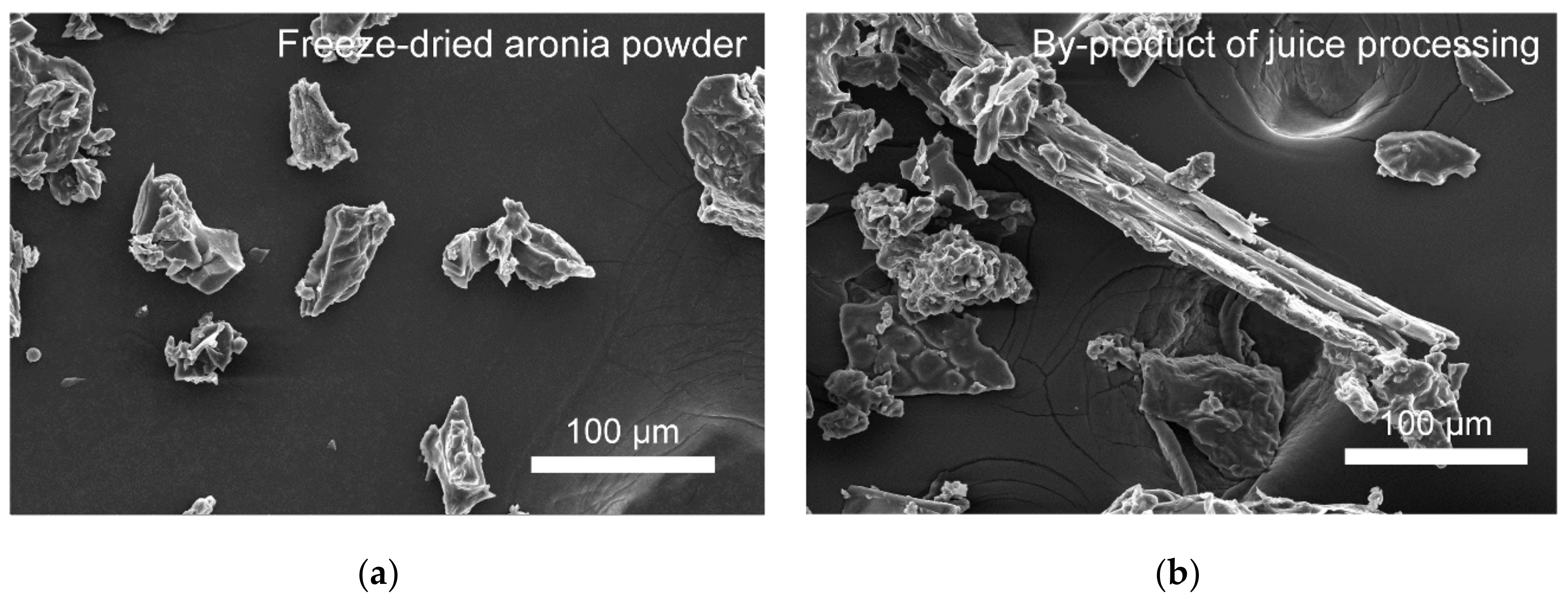
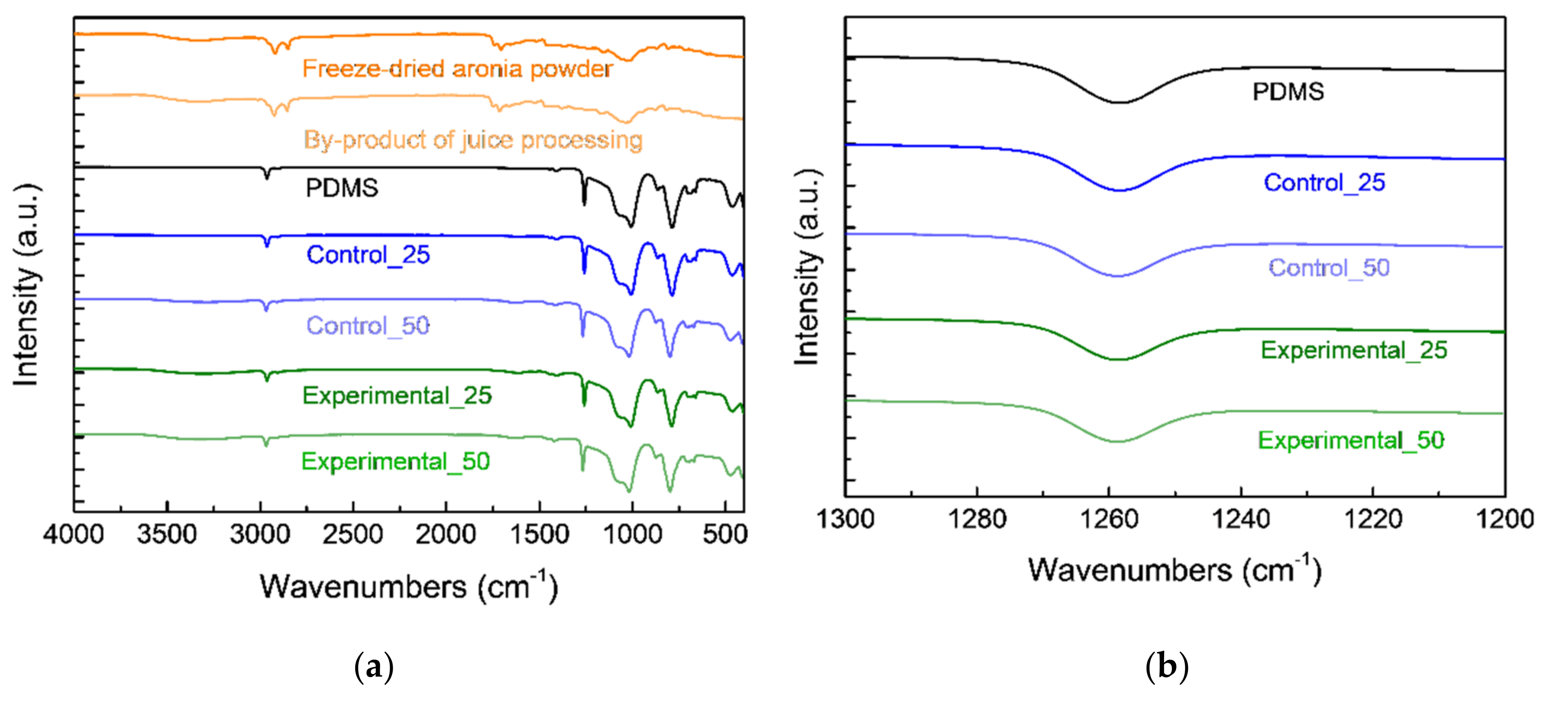
3.3. Chemical Properties of Aronia Powders and Bioelastomers
3.4. Antioxidant Activity of Aronia Powders and Bioelastomers
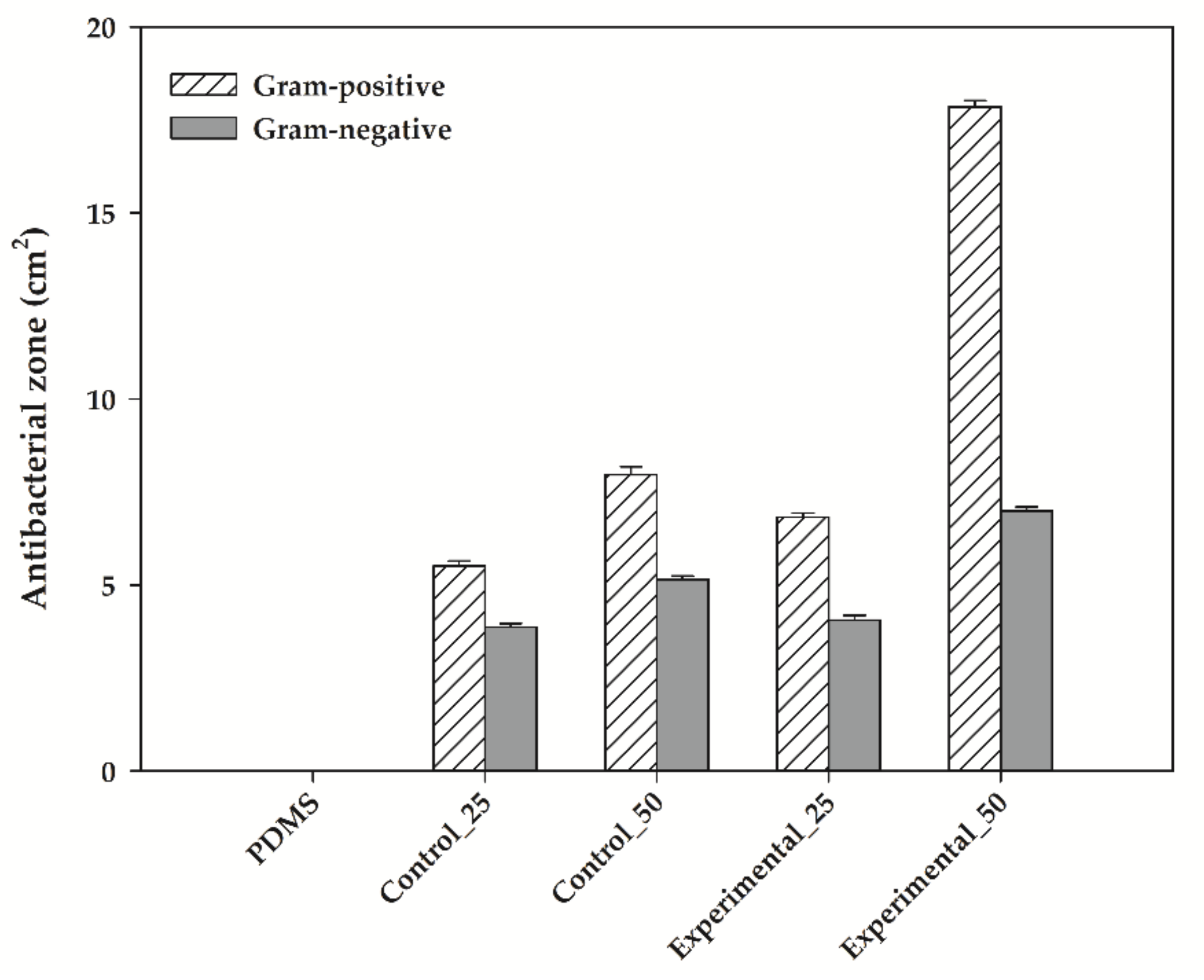
3.5. Antibacterial Activity of Bioelastomers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.H.; Yoo, H.Y.; Lee, S.K.; Chun, Y.; Kim, H.R.; Bankeeree, W.; Lotrakul, P.; Punnapayak, H.; Prasongsuk, S.; Kim, S.W. Significant Impact of Casein Hydrolysate to Overcome the Low Consumption of Glycerol by Klebsiella aerogenes ATCC 29007 and Its Application to Bioethanol Production. Energy Convers. Manag. 2020, 221, 113181. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H.Y.; Kim, Y.H.; Kim, I.K.; Byun, E.H.; Yang, Y.H.; Park, S.J.; Na, J.G.; Shon, H.; Lee, T.; et al. Improved Reutilization of Industrial Crude Lysine to 1,5-diaminopentane by Enzymatic Decarboxylation Using Various Detergents and Organic Solvents. Korean J. Chem. Eng. 2018, 35, 1854–1859. [Google Scholar] [CrossRef]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- BCC Research Staff. Sustainable Biopolymers: A BCC Research Outlook, BCC Research: Market Research Reports. 2019. Available online: https://www.bccresearch.com (accessed on 6 July 2020).
- Cinelli, P.; Coltelli, M.B.; Signori, F.; Morganti, P.; Lazzeri, A. Cosmetic Packaging to Save the Environment: Future Perspectives. Cosmetics 2019, 6, 26. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of Bacterial Cellulose from Alternative Cheap and Waste Resources: A Step for Cost Reduction with Positive Environmental Aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef]
- Zerva, I.; Remmas, N.; Ntougias, S. Biocatalyst Potential of Cellulose-Degrading Microorganisms Isolated from Orange Juice Processing Waste. Beverages 2019, 5, 21. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Seo, H.S.; Park, B.H. Phenolic Compound Extraction from Spent Coffee Grounds for Antioxidant Recovery. Korean J. Chem. Eng. 2020, 36, 186–190. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Tran, T.N.; Athanassiou, A.; Basit, A.; Bayer, I.S. Starch-based Bio-elastomers Functionalized with Red Beetroot Natural Antioxidant. Food Chem. 2017, 216, 324–333. [Google Scholar] [CrossRef]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; de-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef]
- Cerruti, P.; Malinconico, M.; Rychly, J.; Matisova-Rychla, L.; Carfagna, C. Effect of Natural Antioxidants on the Stability of Polypropylene Films. Polym. Degrad. Stab. 2009, 94, 2095–2100. [Google Scholar] [CrossRef]
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct Transformation of Edible Vegetable Waste into Bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Hajji, S.; Chaker, A.; Jridi, M.; Maalej, H.; Jellouli, K.; Boufi, S.; Nasri, M. Structural Analysis, and Antioxidant and Antibacterial Properties of Chitosan-poly(vinyl alcohol) Biodegradable Films. Environ. Sci. Pollut. Res. Int. 2016, 23, 15310–15320. [Google Scholar] [CrossRef]
- Iyer, K.A.; Zhang, L.; Torkelson, J.M. Direct Use of Natural Antioxidant-rich Agro-wastes as Thermal Stabilizer for Polymer: Processing and Recycling. ACS Sustain. Chem. Eng. 2016, 4, 881–889. [Google Scholar] [CrossRef]
- Tran, T.N.; Heredia-Guerrero, J.A.; Mai, B.T.; Ceseracciu, L.; Marini, L.; Athanassiou, A.; Bayer, I.S. Bioelastomers Based on Cocoa Shell Waste with Antioxidant Ability. Adv. Sustain. Syst. 2017, 1, 1700002. [Google Scholar] [CrossRef]
- Perotto, G.; Ceseracciu, L.; Simonutti, R.; Paul, U.C.; Guzman-Puyol, S.; Tran, T.N.; Bayer, I.S.; Athanassiou, A. Bioplastics from Vegetable Waste: Via an Eco-friendly Water-based Process. Green Chem. 2018, 20, 894–902. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Lu, J.; Cheng, Y.; Qian, F.; Zhai, S.; An, Q.; Wang, H. The Fabrication of a Degradable Film with High Antimicrobial and Antioxidant Activities. Ind. Crops. Prod. 2019, 140, 111692. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; Velasco, J.I.; de Oliveira, R.A. Active Edible Films Based on Arrowroot Starch with Microparticles of Blackberry Pulp Obtained by Freeze-Drying for Food Packaging. Polymers 2019, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black Chokeberry (Aronia melanocarpa) and Its Products as Potential Health-promoting Factors—An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Hidayat, M.A.; Maharani, D.A.; Purwanto, D.A.; Kuswandi, B.; Yuwono, M. Simple and Sensitive Paper-based Colorimetric Biosensor for Determining Total Polyphenol Content of the Green Tea Beverages. Biotechnol. Bioprocess Eng. 2020, 25, 255–263. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Mayer-Miebach, E.; Adamiuk, M.; Behsnilian, D. Stability of Chokeberry Bioactive Polyphenols during Juice Processing and Stabilization of a Polyphenol-Rich Material from the By-Product. Agriculture 2012, 2, 244–258. [Google Scholar] [CrossRef]
- Dienaitė, L.; Pukalskas, A.; Pukalskienė, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Phytochemical Composition, Antioxidant and Antiproliferative Activities of Defatted Sea Buckthorn (Hippophaë rhamnoides L.) Berry Pomace Fractions Consecutively Recovered by Pressurized Ethanol and Water. Antioxidants 2020, 9, 274. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef]
- Choi, M.; Kang, Y.R.; Zu, H.D.; Lim, I.S.; Jung, S.K.; Chang, Y.H. Effects of Time on Phenolics and in vitro Bioactivity in Autoclave Extraction of Graviola (Annona muricata) Leaf. Biotechnol. Bioprocess Eng. 2020, 25, 9–15. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yang, C.Y. Ultrasound-Assisted Extraction of Bioactive Compounds and Antioxidant Capacity for the Valorization of Elaeocarpus serratus L. Leaves. Processes 2020, 8, 1218. [Google Scholar] [CrossRef]
- Jayakumar, A.; Vedhaiyan, R.K. Rapid Synthesis of Phytogenic Silver Nanoparticles Using Clerodendrum splendens: Its Antibacterial and Antioxidant Activities. Korean J. Chem. Eng. 2020, 36, 1869–1881. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S.; Kim, Y.; Jeong, Y.; Kim, J.E.; Paek, N.S.; Kang, C.H. Antioxidant and Probiotic Properties of Lactobacilli and Bifidobacteria of Human Origins. Biotechnol. Bioprocess Eng. 2020, 25, 421–430. [Google Scholar] [CrossRef]
- Shim, S.E.; Yashin, V.V.; Isayev, A.I. Environmentally-friendly Physico-chemical Rapid Ultrasonic Recycling of Fumed Silica-filled Poly(dimethyl siloxane) Vulcanizate. Green Chem. 2004, 6, 291–294. [Google Scholar] [CrossRef]
- Anatoly, C.; Pavel, Z.; Tatiana, C.; Alexei, R.; Svetlana, Z. Water Vapor Permeability through Porous Polymeric Membranes with Various Hydrophilicity as Synthetic and Natural Barriers. Polymers 2020, 12, 282. [Google Scholar] [CrossRef]
- Gu, Y.; Qiu, Y.; Wei, X.; Li, Z.; Hu, Z.; Gu, Y.; Zhao, Y.; Wang, Y.; Yue, T.; Yuan, Y. Characterization of Selenium-containing Polysaccharides Isolated from Selenium-enriched Tea and Its Bioactivities. Food Chem. 2020, 316, 126371. [Google Scholar] [CrossRef]
- Ceseracciu, L.; Heredia-Guerrero, J.A.; Dante, S.; Athanassiou, A.; Bayer, I.S. Robust and Biodegradable Elastomers Based on Corn Starch and Polydimethylsiloxane (PDMS). ACS Appl. Mater. Interfaces 2015, 7, 3742–3753. [Google Scholar] [CrossRef]
- Esteves, A.C.C.; Brokken-Zijp, J.; Laven, J.; Huinink, H.P.; Reuvers, N.J.W.; van, M.P.; de With, G. Influence of Cross-linker Concentration on the Cross-linking of PDMS and the Network Structures Formed. Polymer 2009, 50, 3955–3966. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and Evaluation of a Novel Antioxidant and pH Indicator Film Based on Chitosan and Food Waste Sources of Antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of Extraction Conditions on Ultrasound-Assisted Recovery of Bioactive Phenolics from Blueberry Pomace and Their Antioxidant Activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Wang, Y.; Shi, T.; Zhang, L.; Chen, Y.; Xie, M. Comparison of (Poly)phenolic Compounds and Antioxidant Properties of Pomace Extracts from Kiwi and Grape Juice. Food Chem. 2019, 15, 425–432. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound Increases the Aqueous Extraction of Phenolic Compounds with High Antioxidant Activity from Olive Pomace. LWT 2018, 89, 284–290. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Chen, X. Bioactive Flavonoids in Moringa oleifera and their Health-promoting Properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Hervalejo, A.; Pereira-Caro, G.; Muñoz-Redondo, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J.; Moreno-Rojas, J.M. Effect of Rootstock and Harvesting Period on the Bioactive Compounds and Antioxidant Activity of Two Orange Cultivars (‘Salustiana’ and ‘Sanguinelli’) Widely Used in Juice Industry. Processes 2020, 8, 1212. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Hwang, S.J.; Yoon, W.B.; Lee, O.H.; Cha, S.J.; Kim, J.D. Radical-scavenging-linked Antioxidant Activities of Extracts from Black Chokeberry and Blueberry Cultivated in Korea. Food Chem. 2014, 146, 71–77. [Google Scholar] [CrossRef]
- Línzembold, I.; Czett, D.; Böddi, K.; Kurtán, T.; Király, S.B.; Gulyás-Fekete, G.; Takátsy, A.; Lóránd, T.; Deli, J.; Agócs, A.; et al. Study on the Synthesis, Antioxidant Properties, and Self-Assembly of Carotenoid–Flavonoid Conjugates. Molecules 2020, 25, 636. [Google Scholar] [CrossRef]
- Ushasree, M.V.; Lee, E.Y. Flavonoids, Terpenoids, and Polyketide Antibiotics: Role of Glycosylation and Biocatalytic Tactics in Engineering Glycosylation. Biotechnol. Adv. 2020, 41, 107550. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.C. Monoolein Cubosomes for Enhancement of in vitro Anti-oxidative Efficacy of Bambusae Caulis in Taeniam Extract Toward Carcinogenic Fine Dust-stimulated RAW 264.7 cells. Korean J. Chem. Eng. 2019, 36, 1466–1473. [Google Scholar] [CrossRef]
- Kusumah, J.; Real Hernandez, L.M.; Gonzalez de Mejia, E. Antioxidant Potential of Mung Bean (Vigna radiata) Albumin Peptides Produced by Enzymatic Hydrolysis Analyzed by Biochemical and In Silico Methods. Foods 2020, 9, 1241. [Google Scholar] [CrossRef] [PubMed]
- Wan Yahaya, W.A.; Abu Yazid, N.; Mohd Azman, N.A.; Almajano, M.P. Antioxidant Activities and Total Phenolic Content of Malaysian Herbs as Components of Active Packaging Film in Beef Patties. Antioxidants 2019, 8, 204. [Google Scholar] [CrossRef]
- Xiao, X.N.; Wang, F.; Yuan, Y.T.; Liu, J.; Liu, Y.Z.; Yi, X. Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules 2019, 24, 2831. [Google Scholar] [CrossRef]
- Liang, S.; Wang, L. A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract. Polymers 2018, 10, 71. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Yang, Y.N.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Drug Release and Antioxidant/antibacterial Activities of Silymarin-zein Nanoparticle/bacterial Cellulose Nanofiber Composite Films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Kurahachi, M.; Nonaka, A.; Miyamoto, T. A Study of the Antibacterial Mechanism of Catechins: Isolation and Identification of Escherichia coli Cell Surface Proteins that Interact with Epigallocatechin Gallate. Food Control 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Demirbas, A.; Yilmaz, V.; Ildiz, N.; Baldemir, A.; Ocsoy, I. Anthocyanins-rich Berry Extracts Directed Formation of Ag NPs with the Investigation of their Antioxidant and Antimicrobial Activities. J. Mol. Liq. 2017, 248, 1044–1049. [Google Scholar] [CrossRef]
| By-Products | Polymers | Methods | Applications | Ref. |
|---|---|---|---|---|
| Red beetroot (RB) | Polydimethylsiloxane (PDMS) | RB and corn starch were added to 14 mL of heptane | Food packaging | [13] |
| White and red grape seeds and, tomato skins and seeds | Polypropylene (PP) | Blending under a nitrogen stream at 180 °C, increasing the screw rate from 20 to 32 rpm for 10 min | Stabilization against thermal-oxidative degradation | [15] |
| Parsley and spinach stems, cocoa pod husks, and rice hulls | Microcrystalline cellulose (MCC) | Solution of 3% by weight of solids in Tetrahydrofuran for 29 days | Packaging and biomedicine | [16] |
| Chitin and chitosan from crab (Carious mediterraneus) | Polyvinyl alcohol (PVA) | Two grams of chitosan was dissolved in 100 mL of 2% (v/v) acetic acid at 25 °C for 24 h and 2 g of PVA was dissolved in 100 mL of distilled water at 80 °C for 6 h | Food packaging | [17] |
| Chardonnay grape pomace and turmeric waste (Curcumina longa) | Low-density polyethylene (LDPE) | Sample of 96/4 wt% LDPE/grape pomace waste, melt state at 140 °C with 160 rpm | [18] | |
| Cocoa shell waste (CSW) | Polydimethylsiloxane (PDMS) | Micronized CSW was added to 10 mL of heptane | Food packaging and biomedical device | [19] |
| Carrot, radicchio, parsley, and cauliflower | Polyvinyl alcohol (PVA) | Powders were dispersed in 5% (w/w) water solution of HCl | Cosmetics and biodegradable polymer | [20] |
| Apricot (Prunus armeniaca L.) kernel skin (AKS) | Soy protein isolate (SPI) | SPI and glycerol were dispersed in 50 mL deionized water containing AKS at 80 °C for 30 min with 150 rpm | Food and drug packaging | [21] |
| Frozen blackberries (Rubus fruticosus) | Arrowroot starch | Arrowroot starch was dispersed in distilled water (4%, w/w) and mixed with glycerol and blackberry powder | Food packaging | [22] |
| Sample | Powder (g) | PDMS (g) | Powder (wt%) | PDMS (wt%) |
|---|---|---|---|---|
| PDMS | 0 | 10 | 0 | 100 |
| Control_25 1 | 2.5 | 7.5 | 25 | 75 |
| Control_50 1 | 5.0 | 5.0 | 50 | 50 |
| Experimental_25 2 | 2.5 | 7.5 | 25 | 75 |
| Experimental_50 2 | 50 | 5.0 | 50 | 50 |
| Polyphenol Contents (mg GAE1/g Dry Powder) | Flavonoid Contents (mg RE2/g Dry Powder) | |
|---|---|---|
| Aronia freeze-dried powder | 42.0 ± 1.4 | 20.0 ± 1.1 |
| Control_25 | 16.0 ± 0.5 | 8.0 ± 0.3 |
| Control_50 | 18.0 ± 0.8 | 12.0 ± 0.4 |
| Aronia juice processing by-products | 37.2 ± 1.3 | 18.5 ± 0.8 |
| Experimental_25 | 13.1 ± 0.3 | 4.4 ± 0.2 |
| Experimental_50 | 15.3 ± 0.4 | 8.8 ± 0.3 |
| Radical Scavenging Activity (%) | ||
|---|---|---|
| DPPH | ABTS | |
| Aronia freeze-dried powder | 89.4 ± 0.8 | 62.9 ± 2.6 |
| Control_25 | 35.4 ± 0.1 | 16.7 ± 0.1 |
| Control_50 | 49.6 ± 0.6 | 26.8 ± 1.1 |
| Aronia juice processing by-products | 63.8 ± 0.1 | 29.4 ± 0.3 |
| Experimental_25 | 26.2 ± 0.2 | 12.4 ± 0.2 |
| Experimental_50 | 35.7 ± 0.4 | 16.8 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.H.; Chun, Y.; Jang, Y.W.; Lee, S.K.; Kim, H.R.; Lee, J.H.; Kim, S.W.; Park, C.; Yoo, H.Y. Fabrication of Functional Bioelastomer for Food Packaging from Aronia (Aronia melanocarpa) Juice Processing By-Products. Foods 2020, 9, 1565. https://doi.org/10.3390/foods9111565
Lee KH, Chun Y, Jang YW, Lee SK, Kim HR, Lee JH, Kim SW, Park C, Yoo HY. Fabrication of Functional Bioelastomer for Food Packaging from Aronia (Aronia melanocarpa) Juice Processing By-Products. Foods. 2020; 9(11):1565. https://doi.org/10.3390/foods9111565
Chicago/Turabian StyleLee, Kang Hyun, Youngsang Chun, Ye Won Jang, Soo Kweon Lee, Hyeong Ryeol Kim, Ju Hun Lee, Seung Wook Kim, Chulhwan Park, and Hah Young Yoo. 2020. "Fabrication of Functional Bioelastomer for Food Packaging from Aronia (Aronia melanocarpa) Juice Processing By-Products" Foods 9, no. 11: 1565. https://doi.org/10.3390/foods9111565
APA StyleLee, K. H., Chun, Y., Jang, Y. W., Lee, S. K., Kim, H. R., Lee, J. H., Kim, S. W., Park, C., & Yoo, H. Y. (2020). Fabrication of Functional Bioelastomer for Food Packaging from Aronia (Aronia melanocarpa) Juice Processing By-Products. Foods, 9(11), 1565. https://doi.org/10.3390/foods9111565