Spray-Drying Microencapsulation of Bioactive Compounds from Lemon Verbena Green Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. L. citriodora Extract Preparation
2.3. Microencapsulation of MAE L. Citriodora Extract by Spray Drying
2.4. Evaluation of Variable Responses
2.4.1. Process Yield Assessment (Y%)
2.4.2. Encapsulation Efficiency Assessment (EE%)
2.4.3. Recovery of Compounds after Spray Drying Process (R%)
2.5. Evaluation of Polar Compounds in L. Citriodora Microparticles by HPLC-ESI-TOF/MS
3. Results
3.1. Quantitative Characterization of Phytochemical by HPLC-ESI-TOF-MS
3.2. RSM Analysis of Spray Drying Process
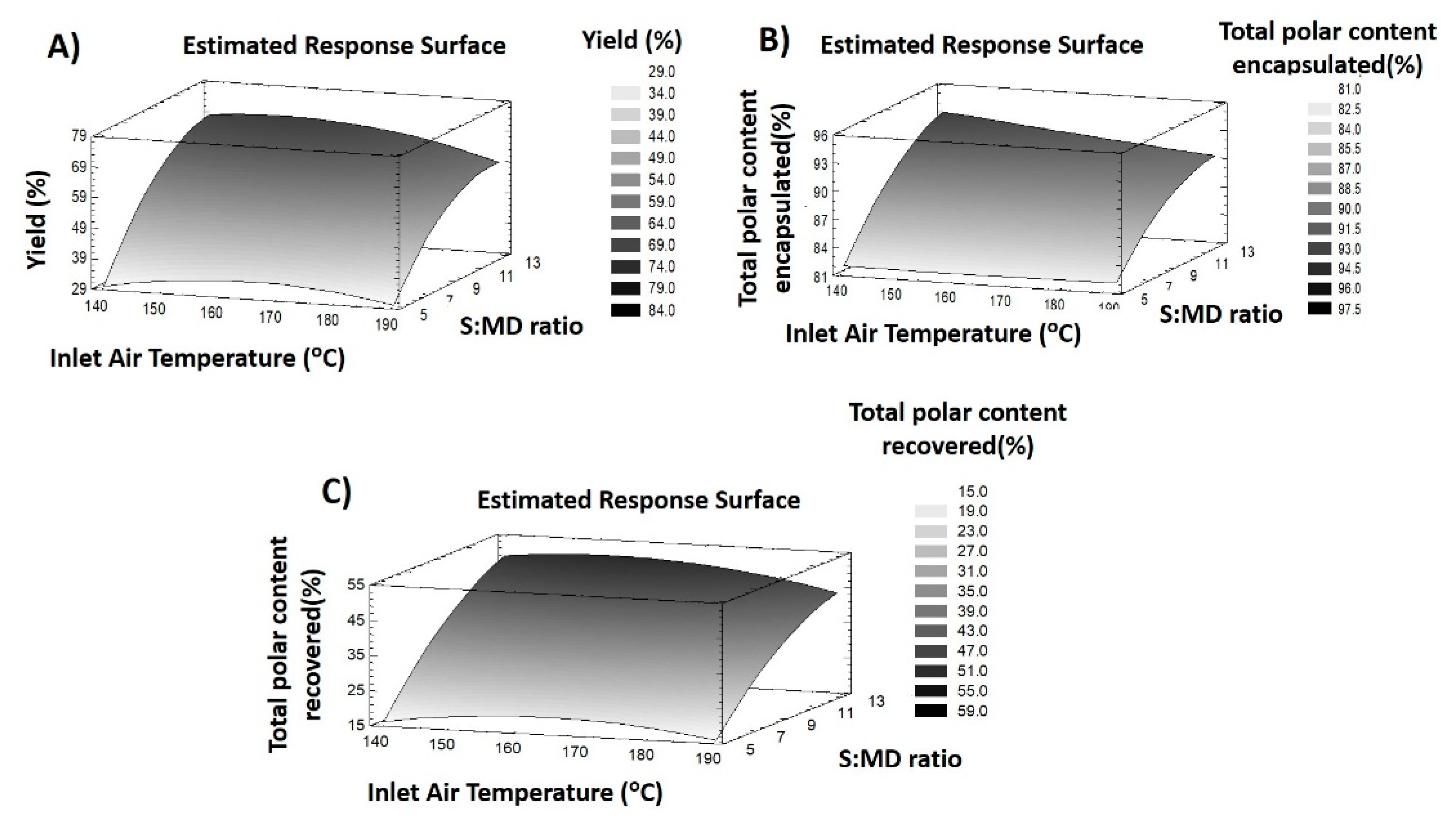
3.2.1. Response Variables of MD Particles
Process Yield
Encapsulation of Total Polar Compounds
Recovery of Total Polar Compounds
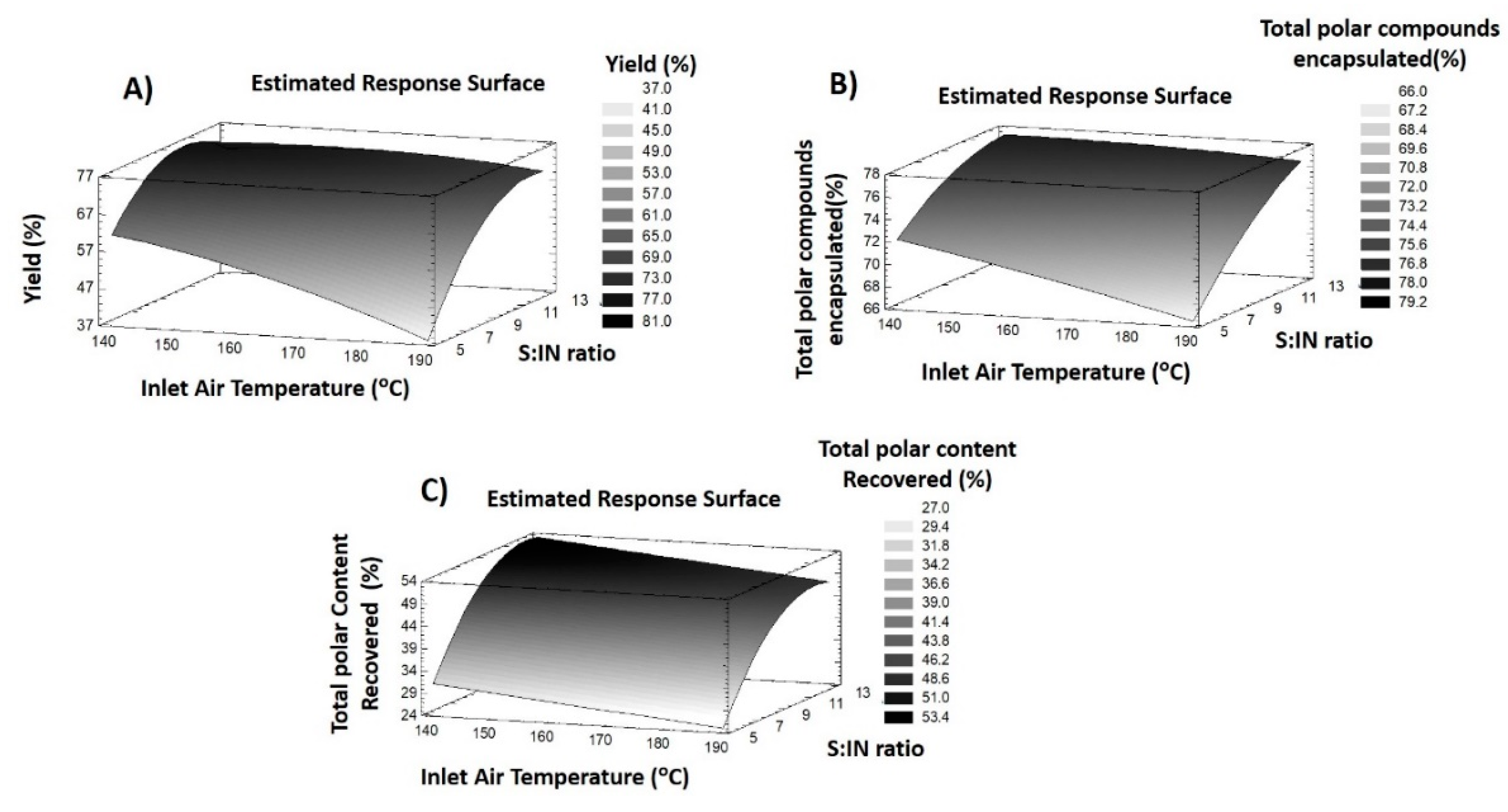
3.2.2. Response Variables of IN Particles
Process Yield
Encapsulation of Total Polar Compounds
Recovery of Total Polar Compounds
3.3. Comparative Evaluation of Effects Induced by Encapsulation Agents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolida, S.; Tuohy, K.; Gibson, G.R. Prebiotic effects of inulin and oligofructose. Br. J. Nutr. 2002, 87, S193–S197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413. [Google Scholar] [CrossRef] [PubMed]
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- González Cañete, N.; Durán Agüero, S. Soya isoflavones and evidences on cardiovascular protection. Nutr. Hosp. 2014, 29, 1271–1282. [Google Scholar]
- Melo, M.N.D.O.; Oliveira, A.P.; Wiecikowski, A.F.; Carvalho, R.S.; Castro, J.D.L.; De Oliveira, F.A.G.; Pereira, H.M.G.; Da Veiga, V.F.; Capella, M.M.A.; Rocha, L.; et al. Phenolic compounds from Viscum album tinctures enhanced antitumor activity in melanoma murine cancer cells. Saudi Pharm. J. 2018, 26, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.D.L.L.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships between chemical structure and antioxidant activity of isolated phytocompounds from lemon verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.L.L.; Micol, V.; Joven, J.; Segura-Carretero, A.; Fernández-Arroyo, S. Different behavior of polyphenols in energy metabolism of lipopolysaccharide-stimulated cells. Food Res. Int. 2018, 118, 96–100. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Vezza, T.; Rodríguez-Nogales, A.; Hidalgo-Garcia, L.; Garrido-Mesa, J.; Ruiz-Malagon, A.; Molina-Tijeras, J.A.; Romero, M.; Robles-Vera, I.; Leyva-Jiménez, F.J.; et al. The Beneficial Effects of Lippia Citriodora Extract on Diet-Induced Obesity in Mice Are Associated with Modulation in the Gut Microbiota Composition. Mol. Nutr. Food Res. 2020, 64, 2000005. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.L.L.; Olivares-Vicente, M.; Herranz-López, M.; Arráez-Román, D.; Fernández-Arroyo, S.; Micol, V.; Segura-Carretero, A. Bioassay-guided purification of Lippia citriodora polyphenols with AMPK modulatory activity. J. Funct. Foods 2018, 46, 514–520. [Google Scholar] [CrossRef]
- Olivares-Vicente, M.; Sánchez-Marzo, N.; Encinar, J.A.; Cádiz-Gurrea, M.D.L.L.; Lozano-Sánchez, J.; Segura-Carretero, A.; Arraez-Roman, D.; Riva, C.; Barrajón-Catalán, E.; Herranz-López, M.; et al. The Potential Synergistic Modulation of AMPK by Lippia citriodora Compounds as a Target in Metabolic Disorders. Nutrients 2019, 11, 2961. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of Phenolic Compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Shao, P.; Xuan, S.; Wu, W.; Qu, L. Encapsulation efficiency and controlled release of Ganoderma lucidum polysaccharide microcapsules by spray drying using different combinations of wall materials. Int. J. Biol. Macromol. 2019, 125, 962–969. [Google Scholar] [CrossRef]
- Tan, S.; Ebrahimi, A.; Langrish, T. Controlled release of caffeine from tablets of spray-dried casein gels. Food Hydrocoll. 2019, 88, 13–20. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols-A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar]
- Paseephol, T.; Small, D.M.; Sherkat, F. Rheology and texture of set yogurt as affected by inulin adition. J. Texture Stud. 2008, 39, 617–634. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. Manufacturing design to improve the attainment of functional ingredients from Aloysia citriodora leaves by advanced microwave technology. J. Ind. Eng. Chem. 2019, 79, 52–61. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. Comparative study of conventional and pressurized liquid extraction for recovering bioactive compounds from Lippia citriodora leaves. Food Res. Int. 2018, 109, 213–222. [Google Scholar] [CrossRef]
- Oehlert, G.W. A First Course in Design and Analysis of Experiments; W. H. Freeman and Company: New York, NY, USA, 2010; ISBN 0-7167-3510-5. [Google Scholar]
- Yusoff, N.I.; Leo, C.P. Microwave Assisted Extraction of Defatted Roselle (Hibiscus sabdariffa L.) Seed at Subcritical Conditions with Statistical Analysis. J. Food Qual. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D. Effects of spray drying conditions on the physicochemical and antioxidant properties of the Gac (Momordica cochinchinensis) fruit aril powder. J. Food Eng. 2010, 98, 385–392. [Google Scholar] [CrossRef]
- Reineccius, G.A. Spray-Drying of Food Flavors. ACS Symp. Ser. 1988, 370, 55–66. [Google Scholar]
- Bazaria, B.; Kumar, P. Compositional Changes in Functional Attributes of Vacuum Concentrated Beetroot Juice. J. Food Process. Preserv. 2016, 40, 1215–1222. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.; Lozano-Sánchez, J.; Fernández-Ochoa, Á.; Segura-Carretero, A. Enhancing the Yield of Bioactive Compounds from Sclerocarya birrea Bark by Green Extraction Approaches. Molecules 2019, 24, 966. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Phoungchandang, S.; Sertwasana, A. Spray-drying of ginger juice and physicochemical properties of ginger powders. Sci. Asia 2010, 36, 40–45. [Google Scholar] [CrossRef]
- Baur, E.; Ruhrberg, K.; Woishnis, W. Chemical Resistance of Engineering Thermoplastics; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780323473576. [Google Scholar]
- Avaltroni, F.; Bouquerand, P.; Normand, V. Maltodextrin molecular weight distribution influence on the glass transition temperature and viscosity in aqueous solutions. Carbohydr. Polym. 2004, 58, 323–334. [Google Scholar] [CrossRef]
- Araujo-Díaz, S.B.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kasapis, S. Glass Transition Phenomena in Dehydrated Model Systems and Foods: A Review. Dry. Technol. 2005, 23, 731–757. [Google Scholar] [CrossRef]
- Krishnan, S.; Bhosale, R.; Singhal, R. Microencapsulation of cardamom oleoresin: Evaluation of blends of gum arabic, maltodextrin and a modified starch as wall materials. Carbohydr. Polym. 2005, 61, 95–102. [Google Scholar] [CrossRef]
- Saenz, C.; Tapia, S.; Chavez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
| Encapsulation Conditions | Total Polar Content | Iridoids | Flavonoids | Phenylprop | Yield | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | EE | R | TC | EE | R | TC | EE | R | TC | EE | R | ||
| 1 | 133,745 ± 11,561 | 91.96 | 50.31 | 3626 ± 38 | 94.82 | 58.71 | 6719 ± 225 | 86.77 | 52.30 | 1,234,000 ± 1298 | 92.20 | 49.41 | 65.36 |
| 2 | 88.47 | 37.90 | 93.86 | 44.59 | 86.83 | 39.69 | 88.29 | 37.18 | 58.10 | ||||
| 3 | 92.51 | 47.98 | 95.68 | 61.95 | 88.80 | 51.60 | 92.83 | 46.48 | 66.86 | ||||
| 4 | 89.04 | 40.70 | 94.25 | 48.85 | 88.44 | 41.74 | 88.69 | 39.99 | 61.27 | ||||
| 5 | 89.00 | 41.55 | 94.41 | 50.66 | 89.07 | 43.07 | 88.52 | 40.69 | 61.32 | ||||
| 6 | 82.56 | 18.54 | 91.65 | 21.99 | 86.98 | 17.87 | 81.18 | 18.40 | 33.74 | ||||
| 7 | 88.17 | 39.22 | 94.27 | 48.36 | 88.41 | 40.65 | 87.61 | 38.38 | 60.86 | ||||
| 8 | 91.19 | 38.45 | 94.44 | 49.43 | 87.93 | 42.92 | 91.45 | 37.04 | 60.31 | ||||
| 9 | 80.47 | 13,42 | 90.49 | 16.19 | 87.10 | 12.69 | 78.70 | 13.34 | 26.10 | ||||
| 10 | 91.41 | 46.93 | 94.77 | 59.84 | 88.95 | 50.95 | 91.51 | 45.44 | 64.94 | ||||
| 11 | 80.75 | 14.26 | 90.55 | 16.96 | 86.21 | 13.97 | 79.13 | 14.11 | 26.41 | ||||
| 12 | 86.67 | 27.23 | 93.31 | 36.79 | 87.79 | 28.45 | 85.85 | 26.39 | 42.31 | ||||
| Source | Encapsulation | Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yield | Total Polar Content | Iridoids | Flavonoids | Phenylprop | Total Polar Content | Iridoids | Flavonoids | Phenylprop | |
| p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | |
| Model | 0.001 b | 0.000 b | 0.000 b | 0.278 | 0.000 b | 0.000 b | 0.000 b | 0.000 b | 0.000 b |
| X1:Temperature | 0.027 b | 0.022 b | 0.160 | 0.785 | 0.017 b | 0.093 | 0.162 | 0.034 b | 0.104 |
| X2: S:EA ratio | 0.000 b | 0.000 b | 0.000 b | 0.196 | 0.000 b | 0.000 b | 0.000 b | 0.000 b | 0.000 b |
| X1 X2 | 0.057 | 0.039 b | 0.025 b | 0.766 | 0.038 b | 0.197 | 0.256 | 0.216 | 0.191 |
| 0.008 b | 0.751 | 0.429 | 0.962 | 0.745 | 0.016 b | 0.155 | 0.024 b | 0.013 b | |
| 0.001 b | 0.003 b | 0.004 b | 0.278 | 0.003 b | 0.010 b | 0.015 b | 0.005 b | 0.011 b | |
| Lack-of-fit | 0.015 b | 0.040 b | 0.193 | 0.429 | 0.023 b | 0.069 | 0.126 | 0.029 b | 0.071 |
| R2 | 0.94 | 0.96 | 0.98 | 0.41 | 0.96 | 0.97 | 0.96 | 0.96 | 0.97 |
| CV | <15 | <1 | <0.3 | <1 | <2 | <12 | <10 | <12 | <13 |
| Run | Total Polar Content | Iridoids | Flavonoids | Phenylprop | Yield | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | EE | R | TC | EE | R | TC | EE | R | TC | EE | R | ||
| 1 | 133,745 ± 1561 | 77.27 | 49.97 | 3626 ± 38 | 80.07 | 76.05 | 6719 ± 225 | 83.74 | 53.62 | 123,400 ± 1298 | 75.88 | 47.63 | 70.07 |
| 2 | 73.78 | 43.51 | 76.14 | 54.67 | 84.54 | 39.62 | 72.12 | 43.31 | 69.83 | ||||
| 3 | 77.44 | 51.99 | 78.55 | 59.98 | 84.18 | 48.24 | 76.43 | 51.99 | 73.53 | ||||
| 4 | 74.40 | 44.52 | 76.86 | 50.48 | 86.81 | 37.48 | 72.69 | 45.15 | 68.56 | ||||
| 5 | 74.90 | 46.77 | 74.86 | 57.90 | 82.88 | 40.62 | 73.93 | 46.91 | 71.18 | ||||
| 6 | 67.01 | 16.82 | 67.53 | 18.96 | 84.17 | 12.03 | 65.22 | 17.38 | 28.51 | ||||
| 7 | 75.22 | 51.05 | 75.81 | 56.70 | 86.38 | 39.59 | 73.93 | 52.36 | 74.47 | ||||
| 8 | 75.85 | 49.17 | 74.07 | 51.26 | 84.33 | 38.46 | 75.02 | 50.61 | 74.70 | ||||
| 9 | 67.29 | 27.12 | 66.61 | 27.88 | 83.67 | 18.24 | 65.78 | 28.38 | 53.79 | ||||
| 10 | 76.83 | 47.03 | 78.71 | 43.13 | 87.77 | 34.30 | 75.59 | 49.18 | 65.43 | ||||
| 11 | 72.56 | 31.67 | 73.15 | 33.20 | 85.19 | 22.88 | 71.22 | 32.86 | 58.88 | ||||
| 12 | 71.40 | 47.75 | 73.59 | 46.45 | 85.78 | 32.99 | 69.85 | 50.02 | 71.27 | ||||
| Source | Encapsulation | Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yield | Total Polar Content | Iridoids | Flavonoids | Phenylprop | Total Polar Content | Iridoids | Flavonoids | Phenylprop | |
| p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | |
| Model | 0.035 b | 0.000 b | 0.000 b | 0.457 | 0.000 b | 0.004 b | 0.003 b | 0.000 b | 0.010 b |
| X1:Temperature | 0.008 b | 0.006 b | 0.117 | 0.430 | 0.012 b | 0.092 | 0.023 b | 0.003 b | 0.162 |
| X2: S:EA ratio | 0.002 b | 0.000 b | 0.001 b | 0.613 | 0.001 b | 0.003 b | 0.001 b | 0.000 b | 0.005 b |
| X1 X2 | 0.022 b | 0.029 b | 0.070 | 0.292 | 0.066 | 0.236 | 0.715 | 0.327 | 0.203 |
| 0.143 | 0.530 | 0.280 | 0.664 | 0.853 | 0.647 | 0.014 b | 0.006 b | 0.913 | |
| 0.004 b | 0.034 b | 0.143 | 0.604 | 0.074 | 0.018 b | 0.032 b | 0.006 b | 0.023 b | |
| Lack-of-fit | 0.032 b | 0.324 | 0.203 | 0.843 | 0.586 | 0.219 | 0.055 | 0.028 b | 0.250 |
| R2 | 0.83 | 0.98 | 0.93 | 0.47 | 0.97 | 0.91 | 0.88 | 0.95 | 0.88 |
| CV | <20 | <1 | <1 | <1 | <1 | <15 | <13 | <12 | <15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Arráez-Román, D.; Segura-Carretero, A. Spray-Drying Microencapsulation of Bioactive Compounds from Lemon Verbena Green Extract. Foods 2020, 9, 1547. https://doi.org/10.3390/foods9111547
Leyva-Jiménez FJ, Lozano-Sánchez J, Cádiz-Gurrea MdlL, Fernández-Ochoa Á, Arráez-Román D, Segura-Carretero A. Spray-Drying Microencapsulation of Bioactive Compounds from Lemon Verbena Green Extract. Foods. 2020; 9(11):1547. https://doi.org/10.3390/foods9111547
Chicago/Turabian StyleLeyva-Jiménez, Francisco Javier, Jesús Lozano-Sánchez, María de la Luz Cádiz-Gurrea, Álvaro Fernández-Ochoa, David Arráez-Román, and Antonio Segura-Carretero. 2020. "Spray-Drying Microencapsulation of Bioactive Compounds from Lemon Verbena Green Extract" Foods 9, no. 11: 1547. https://doi.org/10.3390/foods9111547
APA StyleLeyva-Jiménez, F. J., Lozano-Sánchez, J., Cádiz-Gurrea, M. d. l. L., Fernández-Ochoa, Á., Arráez-Román, D., & Segura-Carretero, A. (2020). Spray-Drying Microencapsulation of Bioactive Compounds from Lemon Verbena Green Extract. Foods, 9(11), 1547. https://doi.org/10.3390/foods9111547