Effects of Industrial Processing on Pesticide Multiresidues Transfer from Raw Tomatoes to Processed Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection and Processing
2.2. Chemicals and Reagents
2.3. Sample Preparation
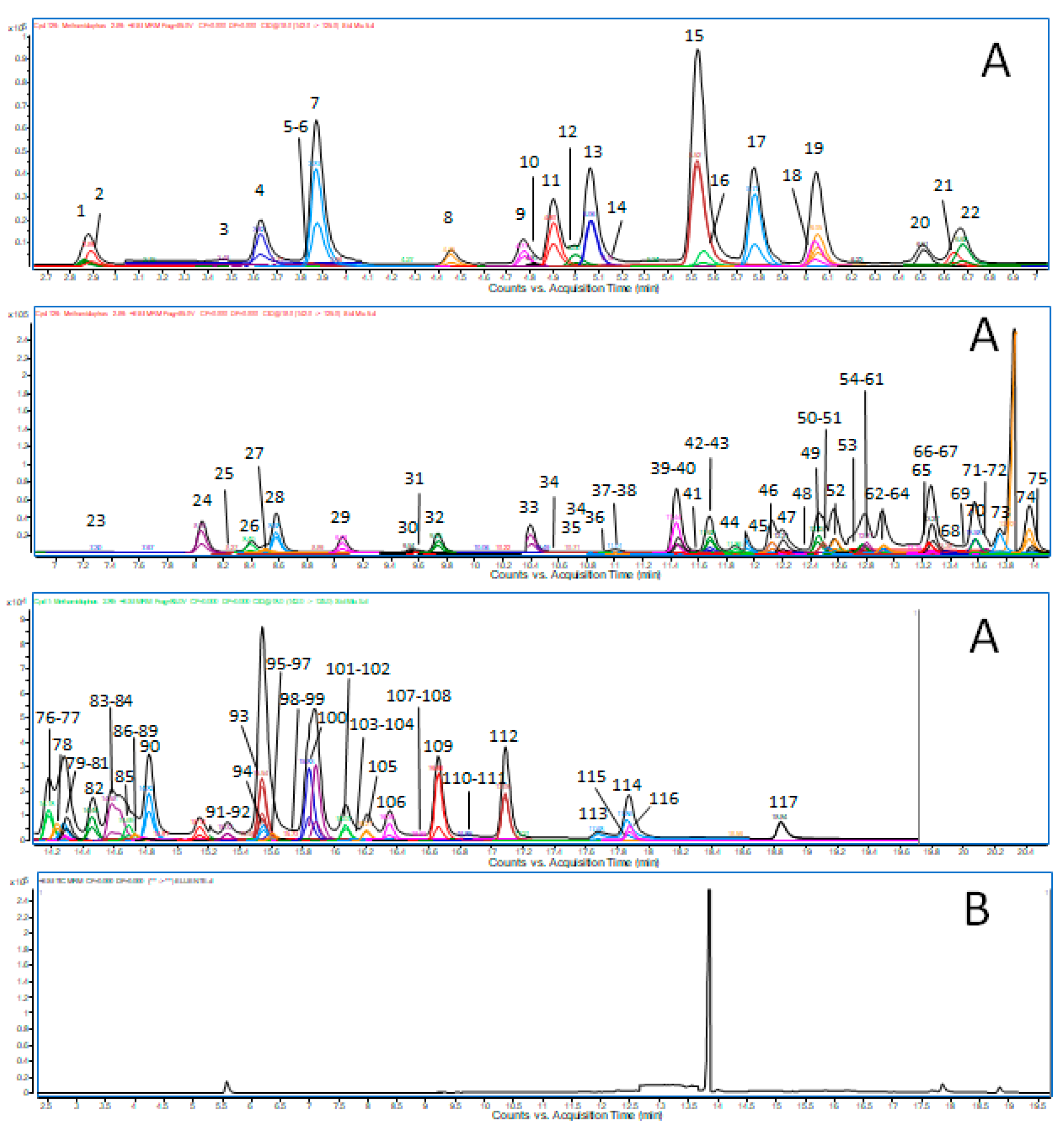
2.4. UHPLC-MS/MS Analysis
2.5. Method Validation
2.6. Industrial Processing
3. Results and Discussion
3.1. Validation Method
3.2. Analysis of Raw and Processed Tomatoes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Knapp, S.; Peralta, I.E. The Tomato (Solanum lycopersicum L., Solanaceae) and Its Botanical Relatives. In The Tomato Genome (Compendium of Plant Genomes); Causse, M., Giovannoni, J., Bouzayen, M., Zouine, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Chapter 2. [Google Scholar] [CrossRef]
- OECD. “Tomato (Solanum lycopersicum)”, in Safety Assessment of Transgenic Organisms in the Environment; OECD Consensus Documents; OECD Publishing: Paris, France, 2017; Volume 7. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization. 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 16 March 2020).
- Italian Processed Tomato Overview 2018. Gain Reports IT1838, USDA Foreign Agricultural Service, 29/03/2018. Available online: https://www.fas.usda.gov/data/italy-italian-processed-tomato-overview-2018 (accessed on 29 February 2020).
- ISTAT—Production Areas Jointed. Available online: http://dati.istat.it/ (accessed on 16 March 2020).
- DECRETO N. 501 DECA 11 DEL 18 MARZO. Rules of Integrated Tomato Production of the Sardinia Region. Defense of Tomato in Field and Industry. 2015. Available online: https://www.sardegnaambiente.it/documenti/1_19_20150320134850.pdf (accessed on 23 February 2020).
- REGULATION (EC) NO 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. Official Journal of the European Union. L 70/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R0396&from=EN (accessed on 16 March 2020).
- National Action Plan (PAN) for the Sustainable Use of Plant Protection Products in Italy. Available online: https://www.minambiente.it/sites/default/files/archivio/allegati/vari/pubbl_PAN.pdf (accessed on 22 January 2014).
- SANTE/E4/VW 10235/2016—Rev. 4, Commission Working Document, on the Evaluation of Data Submitted to Confirm MRLs. Following the Review of Existing MRLs. Brussels. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_sanco-10235-2016.pdf (accessed on 18 February 2020).
- Krishna, V.V.; Qaim, M. Consumer attitudes toward GM food and pesticide residues in India. Eur. Rev. Agric. Econ. 2008, 30, 233–251. [Google Scholar] [CrossRef]
- Suresh, A.; Kjha, G.; Raghav, S.; Supriya, P.; Lama, A.; Punera, B.; Kumar, R.; Handral, A.R.; Sethy, J.; Gidey, R.G.; et al. Food safety concerns of consumers: A case study of pesticide residues on vegetables in Delhi. Eur. Rev. Agric. Econ. 2015, 28, 229–236. [Google Scholar] [CrossRef]
- Relyea, R.A. A cocktail of contaminants: How mixtures of pesticides at low concentrations affect aquatic communities. Oecologia 2008, 2, 159. [Google Scholar] [CrossRef] [PubMed]
- Pesticide Action Network. Pesticide Cocktail in European Food, Brussels, Press Release. Available online: https://www.pan-europe.info/press-releases/2019/07/pesticide-cocktails-european-food (accessed on 16 March 2020).
- Keikotlhaile, B.M.; Spanoghe, P.; Steurbaut, W. Effect of food processing on pesticides residues in fruits and vegetables: A meta-analysis approach. Food Chem. Toxicol. 2010, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dordević, T.; Durović-Pejčev, R. Food processing as a means for pesticide residue dissipation. Pestic. Phytomed. 2016, 31, 89–105. [Google Scholar] [CrossRef]
- Holland, P.T.; Hamilton, D.; Ohlin, B.; Skidmore, M.W. Effects of storage and processing on pesticide residues in plant products. J. Macromol. 1994, 66, 335–356. [Google Scholar]
- Kaushik, G.; Satya, S.; Naik, S.N. Food processing a tool to pesticide residue dissipation—A review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Chavarri, M.J.; Herrera, A.; Arino, A. The decrease in pesticides in fruit and vegetables during commercial processing. Int. J. Food. Sci. Technol. 2005, 40, 205–211. [Google Scholar] [CrossRef]
- Krol, W.J.; Arsenault, T.L.; Pylypiw, H.M.; Mattina, M.J.I. Reduction of pesticide residues on produce by rinsing. J. Agric. Food Chem. 2000, 48, 4666–4670. [Google Scholar] [CrossRef]
- Sannino, A.; Bolzoni, L.; Bandini, M. Application of liquid chromatography with electrospray tandem mass spectrometry to the determination of a new generation of pesticides in processed fruits and vegetables. J. Chrom. A 2004, 1036, 161–169. [Google Scholar] [CrossRef]
- Silva-Rodríguez, A.; Acedo-Valenzuela, M.I.; Diez, N.M.M.; de la Peña, A.M.; Galeano-Díaz, T. Multiresidue method for the control of pesticide residues in tomatoes and derived products. Anal. Methods 2012, 4, 2543–2549. [Google Scholar] [CrossRef]
- Salamzadeh, J.; Shakoori, A.; Moradi, V. Occurrence of multiclass pesticide residues in tomato samples collected from different markets of Iran. J. Environ. Health Sci. Eng. 2018, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sartain, M.; Fandino, A.; Glauner, T. Routine Multiresidue Pesticide Analysis using the Agilent 6470 Triple Quadrupole Mass Spectrometer. In Agilent Application Note; AgilentTechnologies Inc.: Wood Dale, IL, USA, 2015. [Google Scholar]
- Jahanmard, E.; Ansari, F.; Feizi, M. Evaluation of QuEChERS sample preparation and GC mass spectrometry method for the determination of 15 pesticide residues in tomatoes used in salad production plants. Iran J. Public Health 2016, 45, 230–238. [Google Scholar]
- Golge, O.; Kabak, B. Evaluation of QuEChERS sample preparation and liquid chromatography–triple-quadrupole mass spectrometry method for the determination of 109 pesticide residues in tomatoes. Food Chem. 2015, 176, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Amvrazi, E.G.; Papadi-Psyllou, A.T.; Tsiropoulos, N.G. Pesticide enrichment factors and matrix effects on the determination of multiclass pesticides in tomato samples by single-drop microextraction (SDME) coupled with gas chromatography and comparison study between SDME and acetone-partition extraction procedure. Int. J. Environ. Anal. Chem. 2010, 90, 245–259. [Google Scholar]
- Cengiz, M.F.; Başlar, M.; Basançelebi, O.; Kılıçlh, M. Reduction of pesticide residues from tomatoes by low intensity electrical current and ultrasound applications. Food Chem. 2018, 267, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Graziela, C.R.; Andrade, M.; Monteiro, S.H.; Francisco, J.G.; Figueiredo, L.A.; Rocha, A.A.; Tornisielo, V.L. Effects of types of washing and peeling in relation to pesticide residues in tomatoes. J. Braz. Chem. Soc. 2015, 26, 10. [Google Scholar] [CrossRef]
- Bonnechère, A.; Hanot, V.; Bragard, C.; Bedoret, T.; van Loco, J. Effect of household and industrial processing on levels of pesticide residues and degradation products in melons. Food Addit. Contam. 2012, 25, 397–406. [Google Scholar] [CrossRef]
- Kontou, S.; Tsipi, D.; Oreopoulou, V.; Tzia, C. Determination of ETU in tomatoes and tomato products by HPLC-PDA. Evaluation of cleanup procedures. J. Agric. Food Chem. 2001, 49, 1090–1097. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Certel, M. Effects of chlorine, hydrogen peroxide, and ozone on the reduction of mancozeb residues on tomatoes. Turk. J. Agric. For. 2014, 38, 371–376. [Google Scholar] [CrossRef]
- Keikotlhaile, B.M.; Spanoghe, P. Pesticide residues in fruits and vegetables. In Pesticides—Formulations, Effects, Fate; Stoytcheva, M., Ed.; InTech: London, UK, 2011; Available online: http://www.intechopen.com/books/pesticides-formulations-effects-fate/pesticide-residues-in-fruits-and-vegetables (accessed on 17 March 2020).
- Rasolonjatovo, M.A.; Cemek, M.; Cengiz, M.F.; Ortaç, D.; Büşra Konuk, H.; Karaman, E.; Kocaman, A.T.; Göneş, S. Reduction of methomyl and acetamiprid residues from tomatoes after various household washing solutions. Int. J. Food Prop. 2017, 20, 2748–2759. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, T.K.; Hong, S.M.; Se, E.K.; Cho, N.J.; Kyung, K.S. Effect of household processing on pesticide residues in field-sprayed tomatoes. Food Sci. Biotechnol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Al-Taher, F.; Chen, Y.; Wylie, P.; Cappozzo, J. Reduction of pesticide residues in tomatoes and other produce. J. Food Prot. 2013, 76, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Anastasiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- SANTE/12682/2019. Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2019-12682.pdf (accessed on 1 January 2020).
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Angioni, A.; Garau, V.L.; Aguilera, A.; Del Real, M.; Melis, E.V.; Minelli, C.; Tuberoso, C.; Cabras, P. GC-ITMS determination and degradation of captan during winemaking. J. Agric. Food Chem. 2003, 51, 6761–6766. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.A.Z.; De Queiroz, M.E.L.R.; De Oliveira, A.F.; Neves, A.A.; Heleno, F.F.; Zambolim, L.; Freitas, J.F.; Morais, E.H.C. Pesticide residue removal in classic domestic processing of tomato and its effects on product quality. J. Environ. Sci. Health B 2017, 52, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Reiler, E.; Jørs, E.; Bælum, J.; Huici, O.; Alvarez Caero, M.M.; Cedergreen, N. The influence of tomato processing on residues of organochlorine and organophosphate insecticides and their dietary risk. Sci. Total Environ. 2015, 262–269. [Google Scholar] [CrossRef]
- Angioni, A.; Schirra, M.; Garau, V.L.; Melis, M.; Tuberoso, C.I.G.; Cabras, P. Residues of azoxystrobin, fenhexamid and pyrimethanil in strawberry following field treatments and the effect of domestic washing. Food Addit. Contam. 2004, 21, 1065–1070. [Google Scholar] [CrossRef]
- Tianxi Yang, T.; Doherty, J.; Zhao, B.; Kinchla, A.J.; Clark, J.M.; He, L. Effectiveness of Commercial and Homemade Washing Agents in Removing Pesticide Residues on and in Apples. J. Agric. Food Chem. 2017, 65, 9744–9752. [Google Scholar] [CrossRef]
- Commission Directive 2006/125/EC of 5 December 2006 on Processed Cereal-Based Foods and Baby Foods for Infants and Young Children. Available online: http://data.europa.eu/eli/dir/2006/125/oj (accessed on 17 March 2020).
- Commission Directive 2006/141/EC of 22 December 2006 on Infant Formulae and Follow-On Formulae and Amending Directive 1999/21/EC. Available online: http://data.europa.eu/eli/dir/2006/141/oj (accessed on 17 March 2020).
| Pesticide | Linearity | Linear Regression Equation | R2 ± RSD% | MRL | LOD | LOQ | Apparent Recovery (%, n = 15) | RSDr (5×LOQ) | RSDwR (5×LOQ) | U * | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | (g kg−1) | (g kg−1) | LOQ | 10xLOQ | n = 30 | n = 60 | ||||
| Cyromazine | LOQ–411 | y = 2741924x + 8425 | 0.9988 ± 0.07 | 0.60 | 1.37 | 4.11 | 115.3 ± 4.9 | 108.8 ± 1.7 | 11.2 | 12.4 | 29.4 |
| Methamidophos | LOQ–410 | y = 4887931x + 21107 | 0.9989 ± 0.06 | 0.01 * | 1.37 | 4.10 | 105.4 ± 14.3 | 93.1 ± 10.8 | 9.0 | 12.9 | 38.3 |
| Acephate | LOQ–435 | y = 375881x − 144 | 1.0000 ± 0.10 | 0.01 * | 1.45 | 4.35 | 96.5 ± 13.3 | 90.6 ± 19.4 | 12.0 | 17.1 | 39.8 |
| Formetanate | LOQ–413 | y = 14913520x − 5868 | 1.0000 ± 0.01 | 0.30 | 1.38 | 4.13 | 84.5 ± 1.9 | 84.4 ± 9.3 | 8.6 | 16.9 | 33.6 |
| Pymetrozine | LOQ–473 | y = 696973x + 6250 | 0.9959 ± 0.08 | 0.50 | 1.58 | 4.73 | 101.3 ± 6.7 | 98.2 ± 5.8 | 13.9 | 14.0 | 16.5 |
| Omethoate | LOQ–621 | y = 32351x + 372 | 0.9987 ± 0.07 | 0.01 * | 2.07 | 6.21 | 87.4 ± 7.5 | 85.1 ± 5.9 | 7.5 | 15.1 | 31.8 |
| Propamocarb | LOQ–417 | y = 43247201x − 8829 | 0.9998 ± 0.18 | 4.00 | 1.39 | 4.17 | 99.6 ± 6.7 | 98.5 ± 2.7 | 13.5 | 12.9 | 14.3 |
| Oxamyl | LOQ–401 | y = 5159083x − 5579 | 1.0000 ± 0.01 | 0.01 | 1.34 | 4.01 | 94.8 ± 11.1 | 80.9 ± 0.3 | 5.3 | 8.1 | 38.6 |
| Methomyl | LOQ–410 | y = 5917725x + 7746 | 0.9997 ± 0.14 | 0.01 | 1.37 | 4.10 | 111.8 ± 10.4 | 107.2 ± 2.3 | 9.6 | 10.3 | 31.0 |
| Flonicamid | LOQ–394 | y = 784761x + 1544 | 0.9998 ± 0.13 | 0.50 | 1.31 | 3.94 | 95.5 ± 9.6 | 91.0 ± 12.9 | 15.4 | 11.4 | 29.4 |
| Thiamethoxam | LOQ–524 | y = 11189678x + 71281 | 0.9986 ± 0.08 | 0.20 | 1.75 | 5.24 | 107.8 ± 8.3 | 104.5 ± 2.5 | 7.4 | 9.8 | 22.6 |
| Carbendazim | LOQ–400 | y = 5329930x + 3082 | 1.0000 ± 0.01 | 0.30 * | 1.33 | 4.00 | 76.6 ± 1.7 | 83.7 ± 4.1 | 8.1 | 12.8 | 43.2 |
| Monocrotophos | LOQ–414 | y = 17644763x + 44731 | 0.9993 ± 0.11 | 0.01 * | 1.38 | 4.14 | 96.4 ± 11.1 | 79.8 ± 7.4 | 6.5 | 10.7 | 41.8 |
| Chlordimeform | LOQ–396 | y = 853728x − 1979 | 0.9998 ± 0.01 | - | 1.32 | 3.96 | 85.6 ± 7.4 | 75.5 ± 0.7 | 14.2 | 11.4 | 43.1 |
| Cypermethrin | LOQ–518 | y = 5132597x + 20901 | 0.9979 ± 0.12 | 0.50 | 1.73 | 5.18 | 86.6 ± 4.1 | 86.3 ± 7.4 | 12.6 | 11.8 | 29.7 |
| Imidacloprid | LOQ–321 | y = 5358564x + 20834 | 0.9990 ± 0.20 | 0.50 | 1.07 | 3.21 | 107.4 ± 13.7 | 107.0 ± 1.2 | 4.2 | 6.7 | 32.1 |
| Methiocarb | LOQ–387 | y = 31899495x + 73553 | 0.9994 ± 0.16 | 0.20 | 1.29 | 3.87 | 76.9 ± 1.9 | 80.0 ± 5.2 | 5.6 | 9.0 | 44.7 |
| Dimethoate | LOQ–407 | y = 9144917x + 45650 | 0.9984 ± 0.08 | 0.02 | 1.36 | 4.07 | 105.3 ± 5.0 | 96.7 ± 2.7 | 12.3 | 8.4 | 17.7 |
| Acetamiprid | LOQ–398 | y = 13742437x + 39503 | 0.9992 ± 0.79 | 0.50 | 1.33 | 3.98 | 98.1 ± 8.7 | 88.1 ± 1.8 | 6.1 | 10.7 | 26.7 |
| Cymoxanil | LOQ–435 | y = 7081921x + 5097 | 0.9998 ± 0.00 | 0.40 | 1.45 | 4.35 | 99.1 ± 5.8 | 88.6 ± 5.8 | 11.4 | 14.9 | 24.1 |
| Thiacloprid | LOQ–439 | y = 4714164x + 21318 | 0.9988 ± 0.55 | 0.50 | 1.46 | 4.39 | 111.8 ± 5.7 | 106.6 ± 1.2 | 11.2 | 12.8 | 24.4 |
| Atrazine-desethyl | LOQ–430 | y = 8730484x + 17192 | 0.9996 ± 0.03 | - | 1.43 | 4.30 | 102.0 ± 5.2 | 93.5 ± 3.8 | 8.4 | 11.5 | 18.0 |
| Aldicarb | LOQ–458 | y = 22774x − 3 | 0.9989 ± 0.04 | 0.02 * | 1.53 | 4.58 | 99.8 ± 0.2 | 99.9 ± 0.2 | 10.0 | 8.1 | 10.6 |
| Pirimicarb | LOQ–410 | y = 30848679x + 25212 | 0.9994 ± 0.02 | 0.50 | 1.37 | 4.10 | 109.8 ± 9.6 | 108.2 ± 1.8 | 9.5 | 7.8 | 27.7 |
| Dichlorvos | LOQ–410 | y = 450147x + 175 | 1.0000 ± 0.01 | 0.01 * | 1.37 | 4.10 | 87.2 ± 4.3 | 81.7 ± 2.0 | 8.7 | 17.1 | 32.6 |
| Thiophanate-methyl | LOQ–406 | y = 17268518x − 53036 | 0.9997 ± 0.32 | 1.00 | 1.35 | 4.06 | 93.1 ± 10.0 | 80.2 ± 4.6 | 18.1 | 7.9 | 38.4 |
| Metribuzin | LOQ–427 | y = 3761325x + 17279 | 0.9983 ± 0.74 | 0.10 | 1.42 | 4.27 | 98.9 ± 10.7 | 108.4 ± 2.4 | 5.6 | 12.4 | 26.3 |
| Carbofuran | LOQ–416 | y = 27252840x + 33458 | 0.9996 ± 0.01 | 0.002 * | 1.39 | 4.16 | 96.3 ± 2.9 | 94.9 ± 1.5 | 8.5 | 7.7 | 10.7 |
| Carbaryl | LOQ–410 | y = 14046644x + 7098 | 0.9998 ± 0.22 | 0.01 * | 1.37 | 4.10 | 110.4 ± 5.0 | 100.2 ± 2.1 | 8.4 | 11.5 | 22.9 |
| Imazalil | LOQ–431 | y = 972258x + 947 | 0.9999 ± 0.21 | 0.50 | 1.44 | 4.31 | 78.8 ± 8.2 | 77.5 ± 2.2 | 13.0 | 16.7 | 46.0 |
| Fosthiazate | LOQ–397 | y = 720338x + 1656 | 0.9997 ± 0.12 | 0.02 | 1.32 | 3.97 | 97.2 ± 2.1 | 98.7 ± 0.1 | 7.5 | 10.6 | 6.4 |
| Disulfoton-Sulfoxide | LOQ–471 | y = 12702349x + 19964 | 0.9998 ± 0.32 | - | 1.57 | 4.71 | 110.8 ± 7.0 | 107.6 ± 1.2 | 4.6 | 16.4 | 24.7 |
| Flutriafol | LOQ–470 | y = 3625x + 183 | 0.9986 ± 0.04 | 0.80 | 1.57 | 4.70 | 106.9 ± 8.1 | 104.9 ± 1.0 | 15.2 | 12.4 | 21.0 |
| Metalaxyl | LOQ–390 | y = 4371x + 183 | 0.9986 ± 0.04 | 0.20 | 1.30 | 3.90 | 85.6 ± 6.9 | 84.3 ± 13.1 | 9.5 | 10.2 | 36.7 |
| Methidathion | LOQ–424 | y = 396329x − 101 | 0.9994 ± 0.14 | 0.02 * | 1.41 | 4.24 | 93.6 ± 11.4 | 92.9 ± 2.9 | 10.2 | 11.1 | 26.2 |
| Azinphos-methyl | LOQ–464 | y = 398216x + 390 | 0.9998 ± 0.58 | 0.05 * | 1.55 | 4.64 | 98.2 ± 9.9 | 93.6 ± 2.3 | 8.5 | 19.4 | 22.5 |
| Chlorantraniliprole | LOQ–399 | y = 585240x + 2 | 0.9997 ± 0.01 | 0.60 | 1.33 | 3.99 | 92.1 ± 5.8 | 89.6 ± 4.5 | 7.4 | 15.7 | 22.2 |
| Pyrimethanil | LOQ–395 | y = 1208059x − 4250 | 0.9991 ± 0.05 | 1.00 | 1.32 | 3.95 | 109.7 ± 5.5 | 105.4 ± 0.3 | 7.4 | 15.7 | 20.9 |
| Azoxystrobin | LOQ–431 | y = 37027632 + 20546 | 0.9999 ± 0.31 | 3.00 | 1.44 | 4.31 | 103.4 ± 5.5 | 99.7 ± 4.2 | 6.7 | 19.8 | 14.5 |
| Diethofencarb | LOQ–385 | y = 17914008x +75801 | 0.9990 ± 1.21 | 0.70 * | 1.28 | 3.85 | 97.0 ± 14.2 | 83.9 ± 14.6 | 6.4 | 15.5 | 43.4 |
| Propanil | LOQ–392 | y = 1172102x − 4083 | 0.9972 ± 0.09 | 0.01 * | 1.31 | 3.92 | 90.9 ± 5.1 | 91.0 ± 5.8 | 12.5 | 13.0 | 22.0 |
| Fenamidone | LOQ–390 | y = 19532597x + 43718 | 0.9995 ± 1.06 | 1.00 | 1.30 | 3.90 | 96.5 ± 9.5 | 103.2 ± 1.3 | 9.4 | 14.5 | 21.5 |
| Diclobutrazol | LOQ–391 | y = 145538x + 476 | 0.9993 ± 0.22 | - | 1.30 | 3.91 | 101.5 ± 0.7 | 102.0 ± 0.2 | 8.7 | 10.3 | 3.8 |
| Boscalid | LOQ–408 | y = 4252887x + 877 | 1.0000 ± 0.30 | 3.00 | 1.36 | 4.08 | 104.5 ± 8.6 | 105.9 ± 0.3 | 14.1 | 15.5 | 20.6 |
| Dimethomorph | LOQ–394 | y = 14639124x + 39105 | 0.9980 ± 0.16 | 1.00 | 1.31 | 3.94 | 93.8 ± 17.4 | 97.9 ± 2.5 | 8.9 | 8.7 | 35.5 |
| Mandipropamid | LOQ–455 | y = 5148550x − 20963 | 0.9983 ± 5.08 | 3.00 | 1.52 | 4.55 | 85.6 ± 11.9 | 82.1 ± 8.4 | 5.6 | 14.8 | 40.6 |
| Benthiavalicarb | LOQ–235 | y = 4160577x + 5366 | 0.9994 ± 0.97 | 0.30 | 0.78 | 2.35 | 111.4 ± 5.8 | 106.8 ± 0.9 | 9.7 | 11.5 | 24.0 |
| Molinate | LOQ–621 | y = 47567x − 140 | 0.9999 ± 0.93 | 0.01 * | 2.07 | 6.21 | 92.2 ± 3.1 | 85.9 ± 4.6 | 8.1 | 15.4 | 24.5 |
| Chloroxuron | LOQ–425 | y = 12252052x − 45885 | 0.9984 ± 0.19 | 0.01 * | 1.42 | 4.25 | 105.7 ± 5.7 | 102.3 ± 0.4 | 4.5 | 10.5 | 15.7 |
| Myclobutanil | LOQ–411 | y = 123049x − 122 | 0.9997 ± 0.07 | 0.30 | 1.37 | 4.11 | 83.1 ± 6.1 | 91.8 ± 0.7 | 13.4 | 13.7 | 32.5 |
| Bifenazate | LOQ–399 | y = 1008299x + 76836 | 0.9961 ± 1.07 | 0.50 | 1.33 | 3.99 | 87.0 ± 14.1 | 90.8 ± 19.6 | 5.6 | 15.5 | 44.4 |
| Cyproconazole 1 | LOQ–410 | y = 14106016x + 110236 | 0.9985 ± 4.29 | 0.05 | 1.37 | 4.10 | 109.2 ± 7.2 | 105.7 ± 1.1 | 10.8 | 18.6 | 22.6 |
| Triadimenol | LOQ–495 | y = 3828694x + 27482 | 0.9970 ± 5.79 | 1.00 | 1.65 | 4.95 | 84.9 ± 13.3 | 96.4 ± 8.1 | 8.4 | 15.7 | 38.8 |
| Iprovalicarb | LOQ–408 | y = 4408560x + 40826 | 0.9967 ± 0.22 | 0.70 | 1.36 | 4.08 | 90.6 ± 14.0 | 102.9 ± 0.4 | 12.4 | 13.5 | 33.7 |
| Fenhexamid | LOQ–436 | y = 3699942x + 18108 | 0.9991 ±1.57 | 2.00 | 1.45 | 4.36 | 102.8 ± 5.9 | 82.3 ± 3.6 | 3.4 | 18.5 | 36.8 |
| Azinphos-ethyl | LOQ–391 | y = 367433x + 492 | 0.9997 ± 0.39 | 0.02 * | 1.30 | 3.91 | 79.1 ± 9.5 | 95.7 ± 0.5 | 7.6 | 15.4 | 43.2 |
| Tetraconazole | LOQ–516 | y = 9182219x − 30061 | 0.9993 ± 0.09 | 0.10 | 1.72 | 5.16 | 106.8 ± 7.4 | 109.5 ± 0.9 | 12.1 | 15.3 | 22.4 |
| Cyproconazole 2 | LOQ–410 | y = 5446075x − 17111 | 0.9994 ± 5.95 | 0.05 | 1.37 | 4.10 | 105.3 ± 7.5 | 102.7 ± 1.1 | 7.7 | 13.5 | 18.3 |
| Mepanipyrim | LOQ–407 | y = 328904x − 348 | 0.9991 ± 2.87 | 1.50 | 1.36 | 4.07 | 96.6 ± 2.1 | 100.8 ± 0.6 | 13.3 | 19.1 | 8.5 |
| Spirotetramat | LOQ–503 | y = 2820842x + 73839 | 0.9997 ± 0.67 | 2.00 | 1.68 | 5.03 | 85.6 ± 5.6 | 98.5 ± 1.7 | 6.5 | 18.3 | 29.9 |
| Flufenacet | LOQ–414 | y = 10522988x + 50386 | 0.9983 ± 8.65 | 0.05 | 1.38 | 4.14 | 89.9 ± 8.6 | 89.9 ± 0.4 | 10.5 | 14.8 | 28.4 |
| Ethoprop | LOQ–377 | y = 5056608x + 20031 | 0.9990 ± 2.42 | 0.02 | 1.26 | 3.77 | 89.5 ± 10.1 | 88.4 ± 1.2 | 8.9 | 10.4 | 29.2 |
| Bupirimate | LOQ–396 | y = 7147770x + 5118 | 0.9998 ± 0.37 | 2.00 | 1.32 | 3.96 | 91.8 ± 4.5 | 81.9 ± 10.2 | 5.4 | 18.8 | 33.1 |
| Cyazofamid | LOQ–405 | y = 54070x + 229 | 0.9992 ± 0.72 | 0.60 | 1.35 | 4.05 | 101.7 ± 1.0 | 102.7 ± 0.2 | 5.6 | 12.8 | 5.0 |
| Flusilazole | LOQ–457 | y = 9842141x + 54831 | 0.9982 ± 2.81 | 0.01 * | 1.52 | 4.57 | 106.4 ± 8.2 | 104.1 ± 0.2 | 5.8 | 14.9 | 20.7 |
| Cyprodinil | LOQ–408 | y = 2997671x + 13504 | 0.9992 ± 0.05 | 1.50 | 1.36 | 4.08 | 93.9 ± 15.1 | 79.8 ± 1.9 | 4.6 | 18.7 | 45.0 |
| Fenamiphos | LOQ–547 | y = 2610314x + 116029 | 0.9991 ± 0.92 | 0.04 | 1.82 | 5.47 | 103.9 ± 7.1 | 100.7 ± 1.2 | 11.2 | 15.6 | 16.5 |
| Iprodione | LOQ–455 | y = 82937x + 89 | 0.9997 ± 0.22 | 5.00 | 1.52 | 4.55 | 94.4 ± 4.0 | 96.5 ± 4.2 | 9.9 | 15.8 | 14.0 |
| Aclonifen | LOQ–393 | y = 96057x + 89 | 0.9997 ± 0.22 | 0.01 | 1.31 | 3.93 | 97.5 ± 1.2 | 96.2 ± 0.7 | 12.4 | 12.5 | 7.0 |
| Penconazole | LOQ–398 | y = 6090424x + 15097 | 0.9992 ± 0.43 | 0.10 | 1.33 | 3.98 | 104.6 ± 6.2 | 102.4 ± 0.1 | 9.1 | 11.4 | 15.2 |
| Tebuconazole | LOQ–401 | y = 19544180x + 77610 | 0.9989 ± 0.56 | 0.90 | 1.34 | 4.01 | 102.1 ± 2.1 | 104.4 ± 1.1 | 7.1 | 10.4 | 8.4 |
| Napropamide | LOQ–413 | y = 6724980x + 62380 | 0.9964 ± 0.81 | 0.10 | 1.38 | 4.13 | 108.4 ± 6.2 | 104.8 ± 11.1 | 8.5 | 13.8 | 25.6 |
| Benalaxyl | LOQ–481 | y = 23788991x + 92142 | 0.9982 ± 0.19 | 0.50 | 1.60 | 4.81 | 100.6 ± 4.9 | 108.6 ± 1.1 | 8.9 | 9.4 | 17.4 |
| Spinosyn A | LOQ–432 | y = 1125007x − 3076 | 0.9997 ± 0.01 | 0.70 | 1.44 | 4.32 | 77.0 ± 4.1 | 76.7 ± 0.6 | 15.5 | 14.5 | 46.8 |
| Zoxamide | LOQ–410 | y = 5772043x + 12727 | 0.9994 ± 0.04 | 0.5 | 1.37 | 4.10 | 92.1 ± 9.4 | 81.5 ± 4.8 | 16.5 | 13.2 | 35.7 |
| Pyraclostrobin | LOQ–418 | y = 13354734x − 5243 | 1.0000 ± 3.84 | 0.30 | 1.39 | 4.18 | 94.5 ± 8.2 | 92.8 ± 3.9 | 5.8 | 15.7 | 21.0 |
| Cyflufenamid | LOQ–408 | y = 5945864x + 38431 | 0.9977 ± 0.65 | 0.04 | 1.36 | 4.08 | 87.5 ± 17.6 | 91.1 ± 1.1 | 17.4 | 18.5 | 40.0 |
| Bitertanol | LOQ–406 | y = 6031192x + 40609 | 0.9969 ± 0.80 | 0.01 * | 1.35 | 4.06 | 105.1 ± 1.7 | 102.6 ± 0.9 | 11.2 | 14.6 | 9.8 |
| Clofentezin | LOQ–408 | y = 1910622x − 533 | 0.9999 ± 0.95 | 0.30 | 1.36 | 4.08 | 83.2 ± 11.4 | 103.7 ± 3.8 | 8.9 | 8.1 | 42.8 |
| Phosalone | LOQ–578 | y = 6702437x + 16186 | 0.9995 ± 0.02 | 0.01 * | 1.93 | 5.78 | 99.6 ± 3.4 | 94.2 ± 3.1 | 3.4 | 12.8 | 12.8 |
| Metrafenone | LOQ–468 | y = 9239760x + 20728 | 0.9999 ± 0.02 | 0.40 | 1.56 | 4.68 | 96.4 ± 1.9 | 101.0 ± 4.9 | 5.6 | 8.4 | 11.4 |
| Difenconazole | LOQ–485 | y = 20779327x + 20116 | 0.9999 ± 0.02 | 2.00 | 1.62 | 4.85 | 97.4 ± 7.3 | 95.8 ± 1.8 | 10.0 | 9.4 | 16.3 |
| Chlorpyrifos-methyl | LOQ–414 | y = 65820x − 225 | 0.9999 ± 0.71 | 0.50 | 1.38 | 4.14 | 91.2 ± 7.9 | 79.1 ± 2.3 | 9.1 | 11.4 | 37.5 |
| Ametoctradin | LOQ–317 | y = 8626247x + 1875 | 0.9999 ± 0.01 | 2.00 | 1.06 | 3.17 | 96.1 ± 7.1 | 92.8 ± 0.4 | 8.4 | 18.7 | 18.2 |
| Spinosyn D | LOQ–432 | y = 209477x − 615 | 0.9997 ± 0.41 | 0.70 | 1.44 | 4.32 | 92.9 ± 7.6 | 89.4 ± 4.1 | 12.7 | 5.6 | 23.8 |
| Indoxacarb | LOQ–450 | y = 1790071x − 2915 | 0.9985 ± 0.01 | 0.50 | 1.50 | 4.50 | 94.1 ± 1.5 | 82.2 ± 4.1 | 8.4 | 18.9 | 29.5 |
| Cycloate | LOQ–502 | y = 598248x − 1864 | 0.9974 ± 0.15 | - | 1.67 | 5.02 | 101.6 ± 6.7 | 101.3 ± 0.1 | 10.1 | 15.4 | 13.8 |
| Hexaflumuron | LOQ–419 | y = 633012x − 654 | 0.9977 ± 1.28 | - | 1.40 | 4.19 | 81.8 ± 10.2 | 81.7 ± 1.3 | 5.6 | 14.7 | 41.0 |
| Trifloxystrobin | LOQ–432 | y = 17415017x + 17609 | 0.9999 ± 0.27 | 0.70 | 1.44 | 4.32 | 96.7 ± 7.3 | 94.7 ± 2.0 | 7.6 | 8.4 | 17.1 |
| Quizalofop-ethyl | LOQ–398 | y = 3145820x − 5888 | 0.9998 ± 0.18 | 0.40 | 1.33 | 3.98 | 100.8 ± 5.7 | 100.1 ± 1.5 | 11.3 | 5.4 | 11.8 |
| Cycloxydim | LOQ–407 | y = 137254x + 708 | 0.9986 ± 0.01 | 1.50 | 1.36 | 4.07 | 85.2 ± 7.8 | 96.3 ± 0.8 | 14.2 | 12.1 | 30.9 |
| Buprofezin | LOQ–465 | y = 20507228x + 51039 | 0.9998 ± 0.89 | 1.00 | 1.55 | 4.65 | 95.0 ± 8.0 | 93.9 ± 0.7 | 8.4 | 14.0 | 19.2 |
| Tebufenpyrad | LOQ–401 | y = 4341528x + 15573 | 0.9994 ± 1.27 | 0.80 | 1.34 | 4.01 | 83.6 ± 6.8 | 81.7 ± 1.8 | 9.7 | 9.4 | 36.7 |
| Emamectin Benzoate | LOQ–522 | y = 3632831x − 14712 | 0.9998 ± 0.25 | 0.02 | 1.74 | 5.22 | 98.8 ± 5.2 | 95.2 ± 3.1 | 12.4 | 7.4 | 13.7 |
| Propaquizafop | LOQ–431 | y = 2547342x − 6777 | 0.9971 ± 0.02 | 0.05 | 1.44 | 4.31 | 99.6 ± 8.8 | 100.0 ± 2.5 | 15.4 | 16.1 | 18.1 |
| Metaflumizone | LOQ–410 | y = 949238x − 6681 | 0.9983 ± 0.03 | 0.60 | 1.37 | 4.10 | 90.1 ± 3.8 | 75.6 ± 8.6 | 10.6 | 13.0 | 41.8 |
| Oxadiazon | LOQ–403 | y = 726169x − 4189 | 0.9983 ± 0.05 | 0.05 | 1.34 | 4.03 | 94.3 ± 4.8 | 88.1 ± 0.4 | 8.4 | 8.4 | 21.4 |
| Allethrin | LOQ–649 | y = 439397x − 5650 | 0.9963 ± 0.22 | - | 2.16 | 6.49 | 87.7 ± 4.3 | 81.2 ± 0.1 | 10.1 | 10.4 | 32.7 |
| Piperonyl butoxide | LOQ–404 | y = 31536094x + 11745 | 0.9999 ± 0.01 | - | 1.35 | 4.04 | 104.8 ± 5.5 | 100.9 ± 1.8 | 5.6 | 9.7 | 14.6 |
| Chlorpyriphos | LOQ–395 | y = 638457x − 2533 | 0.9992 ± 0.01 | 0.01 | 1.32 | 3.95 | 104.5 ± 5.1 | 109.5 ± 6.0 | 11.4 | 7.4 | 16.1 |
| Hexythiazox | LOQ–358 | y = 10457257x − 49960 | 0.9987 ± 0.01 | 0.50 | 1.19 | 3.58 | 97.9 ± 5.8 | 97.1 ± 0.5 | 14.3 | 14.1 | 12.5 |
| Pyriproxyfen | LOQ–418 | y = 6633898x − 13776 | 0.9994 ± 0.02 | 1.00 | 1.39 | 4.18 | 83.4 ± 4.8 | 77.7 ± 3.5 | 15.8 | 12.8 | 40.2 |
| Pendimethalin | LOQ–333 | y = 1172935x − 2381 | 0.9999 ± 0.01 | 0.05 | 1.11 | 3.33 | 84.2 ± 6.3 | 81.0 ± 4.6 | 14.2 | 8.4 | 36.9 |
| Flufenoxuron | LOQ–391 | y = 3953910x + 11436 | 0.9990 ± 0.48 | 0.50 * | 1.30 | 3.91 | 81.9 ± 7.4 | 79.5 ± 1.5 | 9.7 | 9.5 | 40.7 |
| Propargite | LOQ–382 | y = 6439838x + 13171 | 0.9997 ± 1.64 | 0.01 * | 1.27 | 3.82 | 86.8 ± 6.0 | 81.8 ± 1.2 | 14.1 | 14.5 | 33.5 |
| Lufenuron | LOQ–437 | y = 568999x + 552 | 0.9993 ± 0.09 | 0.50 | 1.46 | 4.37 | 93.5 ± 5.4 | 96.3 ± 0.6 | 7.5 | 12.3 | 15.7 |
| Etoxazole | LOQ–516 | y = 393488x + 312 | 0.9998 ± 0.02 | 0.07 | 1.72 | 5.16 | 88.7 ± 6.6 | 83.2 ± 2.7 | 18.3 | 17.2 | 31.5 |
| Fenpyroximate(E) | LOQ–460 | y = 24740316x + 67928 | 0.9987 ± 0.41 | 0.20 | 1.53 | 4.60 | 96.2 ± 8.0 | 95.2 ± 1.2 | 10.3 | 9.3 | 18.0 |
| Deltamethrin | LOQ–345 | y = 212931x + 1529 | 0.9999 ± 0.01 | 0.07 | 1.15 | 3.45 | 96.8 ± 5.7 | 79.9 ± 1.1 | 4.8 | 10.3 | 36.0 |
| Acrinathrin | LOQ–475 | y = 48200x − 207 | 0.9998 ± 0.27 | 0.10 | 1.58 | 4.75 | 89.9 ± 12.2 | 88.8 ± 10.7 | 5.4 | 8.3 | 34.6 |
| Pyridaben | LOQ–418 | y = 18219756x + 7898 | 0.9999 ± 0.07 | 0.30 | 1.39 | 4.18 | 98.6 ± 2.6 | 88.7 ± 9.2 | 8.6 | 13.4 | 23.2 |
| Tau–Fluvalinate | LOQ–431 | y = 17740157x − 22927 | 0.9999 ± 0.13 | 0.10 | 1.44 | 4.31 | 85.0 ± 11.4 | 94.2 ± 3.8 | 4.5 | 14.1 | 34.7 |
| Fenarimol | LOQ–444 | y = 167645x + 140 | 0.9999 ± 0.57 | 0.02 * | 1.48 | 4.44 | 84.2 ± 19.4 | 86.3 ± 10.2 | 11.1 | 5.6 | 48.7 |
| Etofenprox | LOQ–387 | y = 5530818x + 37481 | 0.9981 ± 0.24 | 1.00 | 1.29 | 3.87 | 92.4 ± 5.6 | 93.3 ± 0.5 | 7.2 | 13.1 | 18.0 |
| Bifenthrin | LOQ–417 | y = 39808x + 128 | 0.9989 ± 0.58 | 0.30 * | 1.39 | 4.17 | 80.4 ± 12.6 | 76.2 ± 1.4 | 11.1 | 8.7 | 49.2 |
| Famoxadone | LOQ–376 | y = 168731x − 107 | 1.0000 ± 0.07 | 2.00 | 1.25 | 3.76 | 91.7 ± 10.9 | 94.4 ± 7.1 | 8.7 | 9.8 | 27.6 |
| Pesticide | Samples * | Min–Max (Average) (µg kg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Raw Tomatoes | Puree | Triple Concentrate | Pulp | Diced Tomatoes | |||||||
| Creso (31) | Dask (34) | Datterino (2) | Docet (31) | Rapidus (3) | Taylor (46) | Mixed (12) | ||||||
| Formetanate | 68 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ -20.58 (5.69) | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Propamocarb | 15 | <LOQ | <LOQ–26.87 (4.71) | <LOD | <LOQ | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD |
| Flonicamid | 9 | <LOQ | <LOQ–10.49 (5.73) | <LOD | <LOD | <LOD | 9.55 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Carbendazim * | 2 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD |
| Imidacloprid | 25 | <LOQ | <LOQ | <LOD | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD |
| Methiocarb | 8 | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD |
| Dimethoate | 1 | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Acetamiprid | 15 | <LOQ | <LOQ | <LOD | <LOQ | <LOQ | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Cymoxanil | 1 | <LOD | <LOD | <LOD | <LOD | <LOD | 6.51 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Thiacloprid | 4 | <LOQ | <LOD | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Atrazine-desethyl * | 1 | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Metribuzin | 1 | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Carbofuran * | 5 | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Chlorantraniliprole | 102 | <LOQ–111.76 (29.37) | <LOQ–205.19 (44.45) | 29.74–40.81 (35.28) | <LOQ–50.95 (16.04) | 51.78 | <LOQ–139.75 (23.11) | <LOQ–37.10 (22.03) | <LOD | <LOD | <LOD | <LOD |
| Pyrimethanil | 13 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Azoxystrobin | 141 | 17.99–201.98 (80.80) | <LOQ–32.75 (8.27) | <LOQ | <LOQ | 26.96 | <LOQ–129.65 (22.00) | 7.67–72.16 (31.15) | <LOD | <LOD | <LOD | <LOD |
| Fenamidone | 4 | <LOD | <LOQ | <LOQ | <LOD | <LOD | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Boscalid | 15 | <LOQ -45.13 | 7.59–69.62 (38.60) | <LOD | <LOQ | <LOQ | <LOQ–442.23 (112.86) | <LOD | <LOD | <LOD | <LOD | <LOD |
| Dimethomorph | 106 | <LOQ | <LOQ–170.19 (31.45) | <LOQ | <LOQ | 7.70 | 27.42–71.13 (47.23) | <LOQ–655.78 (264.91) | <LOD | <LOD | <LOD | <LOD |
| Iprovalicarb | 11 | 5.80–10.77 (15.60) | <LOD | <LOD | 15.59 | <LOQ | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Tetraconazole | 49 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ–34.21 (18.72) | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Spirotetramat | 67 | <LOQ | <LOQ | <LOQ | <LOQ | <LOD | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Penconazole | 4 | <LOQ | 4.22 | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Tebuconazole | 21 | <LOQ | <LOQ | <LOD | <LOQ | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Benalaxyl | 62 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Spinosyn A | 83 | <LOQ–70.61 (11.60) | <LOQ–216.78 (24.54) | <LOQ | <LOQ–39.65 (27.03) | <LOQ | 36.79 | <LOQ–9.83 (4.51) | <LOD | <LOD | <LOD | <LOD |
| Zoxamide | 5 | <LOQ | <LOQ | <LOD | <LOD | <LOQ | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Pyraclostrobin | 42 | <LOQ -128.01 (22.25) | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ–22.78 (11.50) | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Clofentezine | 1 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD |
| Phosalone * | 9 | <LOQ | <LOD | <LOQ | <LOQ | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD |
| Difenconazole | 14 | <LOQ–54.00 (24.87) | <LOQ | <LOD | <LOD | <LOD | 20.79 | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Ametoctradin | 60 | <LOQ–134.60 (27.30) | <LOQ–606.10 (86.72) | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Spinosyn D | 49 | <LOQ–97.36 (38.09) | <LOQ–352.24 (92.88) | <LOD | <LOQ–117.68 (86.79) | <LOD | <LOQ–349.00 (70.46) | 10.32–12.62 (11.46) | <LOD | <LOD | <LOD | <LOD |
| Indoxacarb | 4 | 11.20–17.60 (14.40) | 6.25 | <LOD | <LOD | <LOD | 7.31 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Trifloxystrobin | 2 | <LOD | <LOQ | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Quizalofop-ethyl | 2 | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Emamectin Benzoate | 72 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ–7.97 (5.58) | <LOQ–17.95 (6.92) | <LOD | <LOD | <LOD | <LOD |
| Piperonyl butoxide | 59 | <LOQ–4.62 (4.62) | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Chlorpyriphos | 14 | <LOQ | <LOQ | <LOD | <LOQ | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD |
| Hexythiazox | 38 | 4.87–18.57 (13.84) | 6.18–43.72 (16.65) | 9.56 | 4.87–11.52 (7.12) | <LOD | 7.76–23.47 (12.58) | 17.89 | <LOD | <LOD | <LOD | <LOD |
| Pyriproxyfen | 2 | <LOQ | <LOD | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Pendimethalin | 1 | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Fenpyroximate(E) | 2 | <LOD | 12.30 | <LOD | <LOD | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD |
| Deltamethrin | 26 | <LOQ–12.48 (8.02) | <LOQ–7.47 (4.05) | <LOQ–7.11 (5.57) | <LOQ–15.41 (7.19) | 4.78 | <LOD | <LOD | <LOD | <LOD | ||
| Fenarimol * | 97 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrias, F.; Atzei, A.; Lai, C.; Dedola, F.; Ibba, E.; Zedda, G.; Canu, F.; Angioni, A. Effects of Industrial Processing on Pesticide Multiresidues Transfer from Raw Tomatoes to Processed Products. Foods 2020, 9, 1497. https://doi.org/10.3390/foods9101497
Corrias F, Atzei A, Lai C, Dedola F, Ibba E, Zedda G, Canu F, Angioni A. Effects of Industrial Processing on Pesticide Multiresidues Transfer from Raw Tomatoes to Processed Products. Foods. 2020; 9(10):1497. https://doi.org/10.3390/foods9101497
Chicago/Turabian StyleCorrias, Francesco, Alessandro Atzei, Carla Lai, Fabrizio Dedola, Enrico Ibba, Gianluca Zedda, Francesca Canu, and Alberto Angioni. 2020. "Effects of Industrial Processing on Pesticide Multiresidues Transfer from Raw Tomatoes to Processed Products" Foods 9, no. 10: 1497. https://doi.org/10.3390/foods9101497
APA StyleCorrias, F., Atzei, A., Lai, C., Dedola, F., Ibba, E., Zedda, G., Canu, F., & Angioni, A. (2020). Effects of Industrial Processing on Pesticide Multiresidues Transfer from Raw Tomatoes to Processed Products. Foods, 9(10), 1497. https://doi.org/10.3390/foods9101497








