Comparison of Physicochemical Properties and Metabolite Profiling Using 1H NMR Spectroscopy of Korean Wheat Malt
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Physicochemical Characteristics of Wheat Kernel
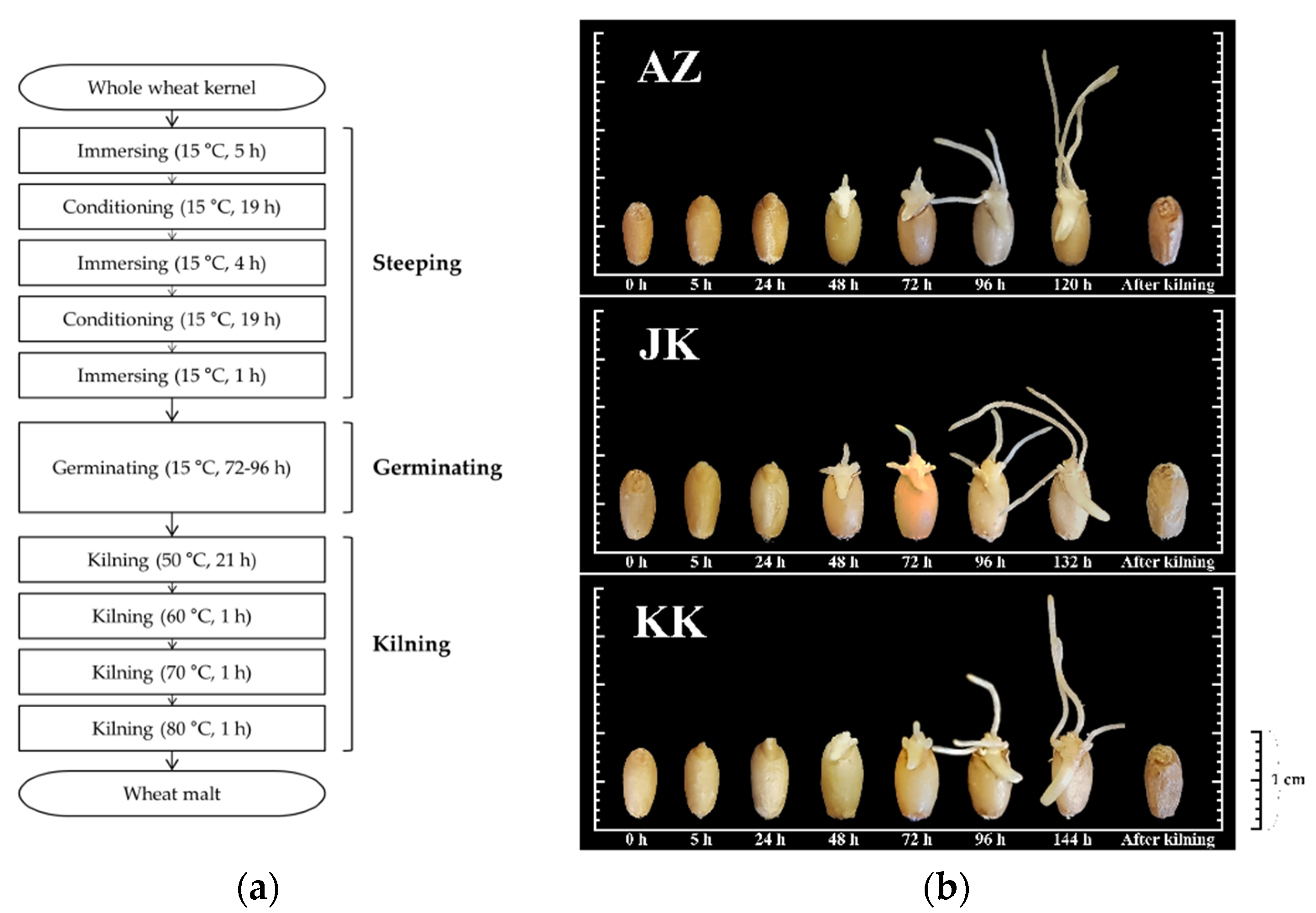
2.2. Preparation of Samples and the Malting Process
2.3. Physicochemical Characteristics of Wheat Malt
2.4. Enzyme Activities of Wheat Malts
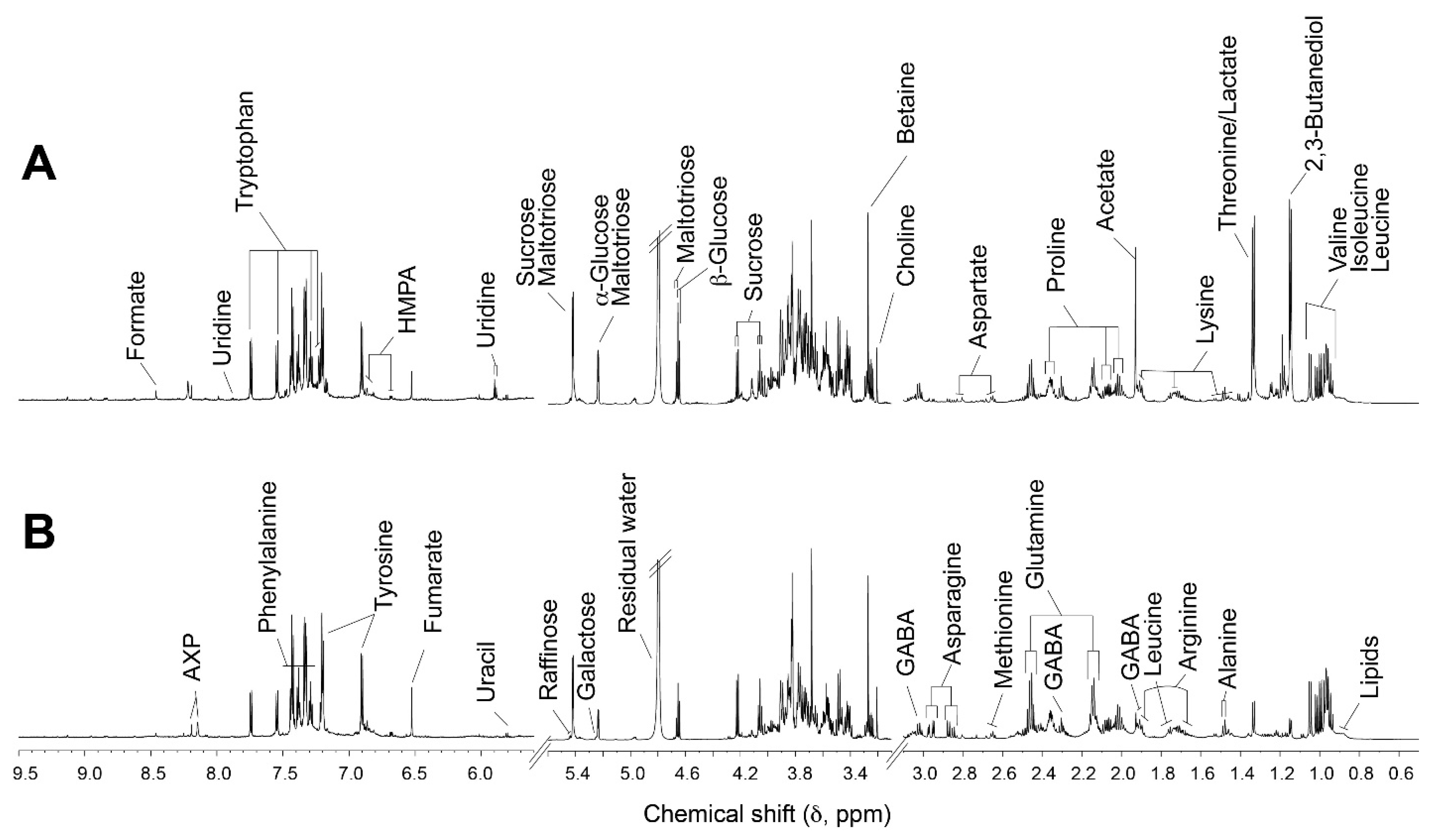
2.5. Metabolite Analysis of Wheat Malts
2.6. Statistical Analysis
2.6.1. Multivariate Data Analysis for Physicochemical Experiments
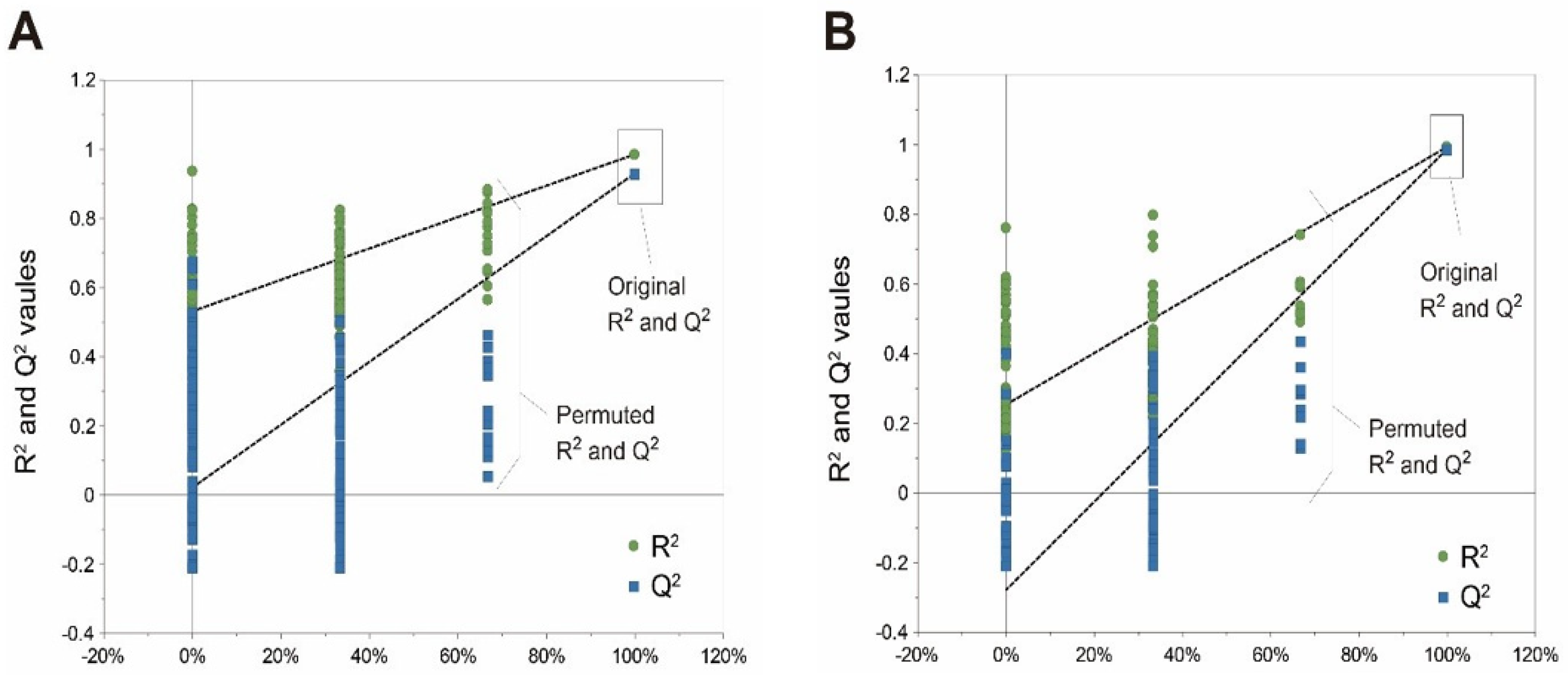
2.6.2. Spectral Processing and Multivariate Data Analysis for Metabolites Analysis
3. Results and Discussion
3.1. Grain Properties of Korean Wheat Varieties
3.2. Wheat Malt Properties
3.3. Enzyme Activities of Wheat Malts
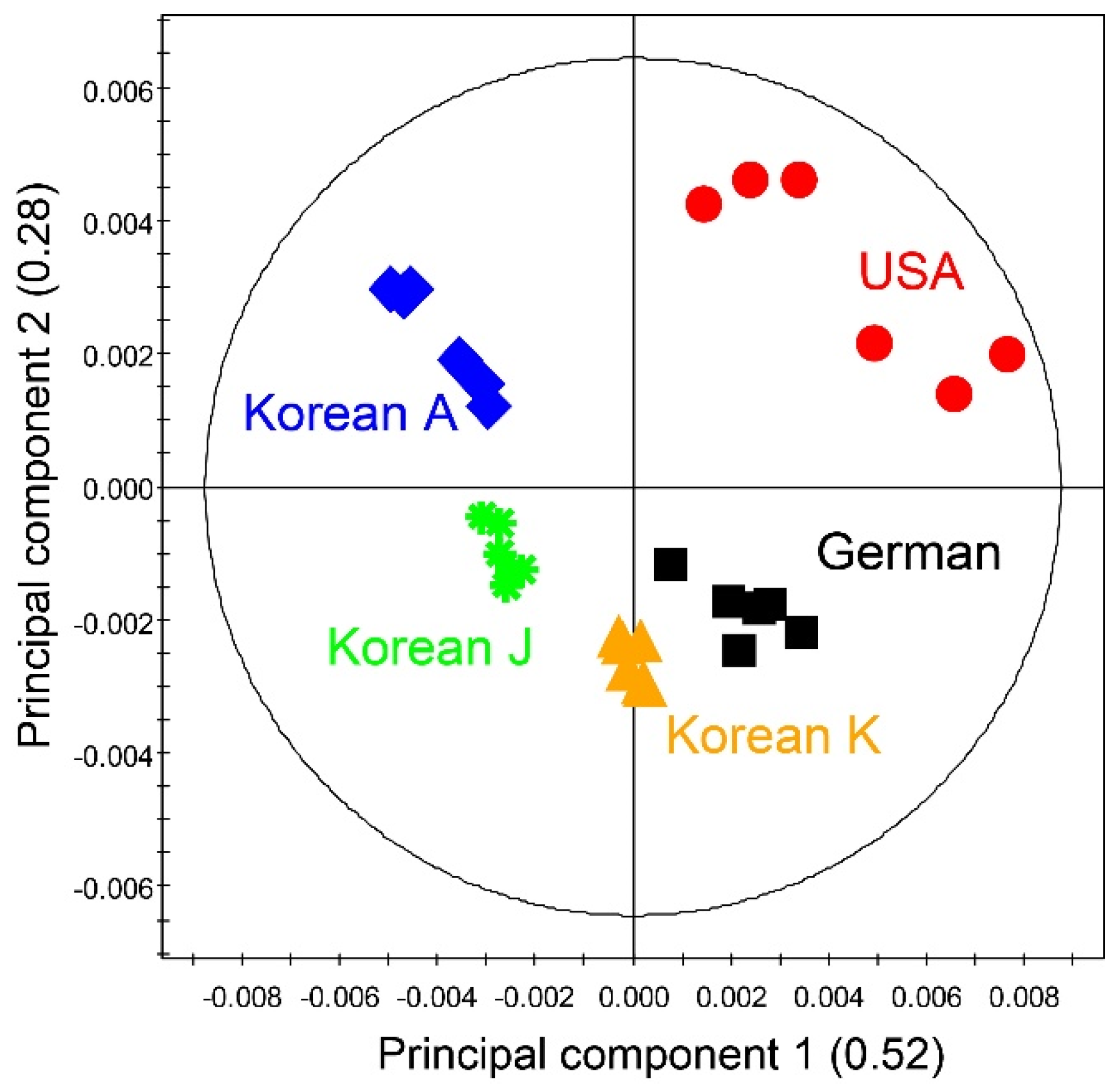
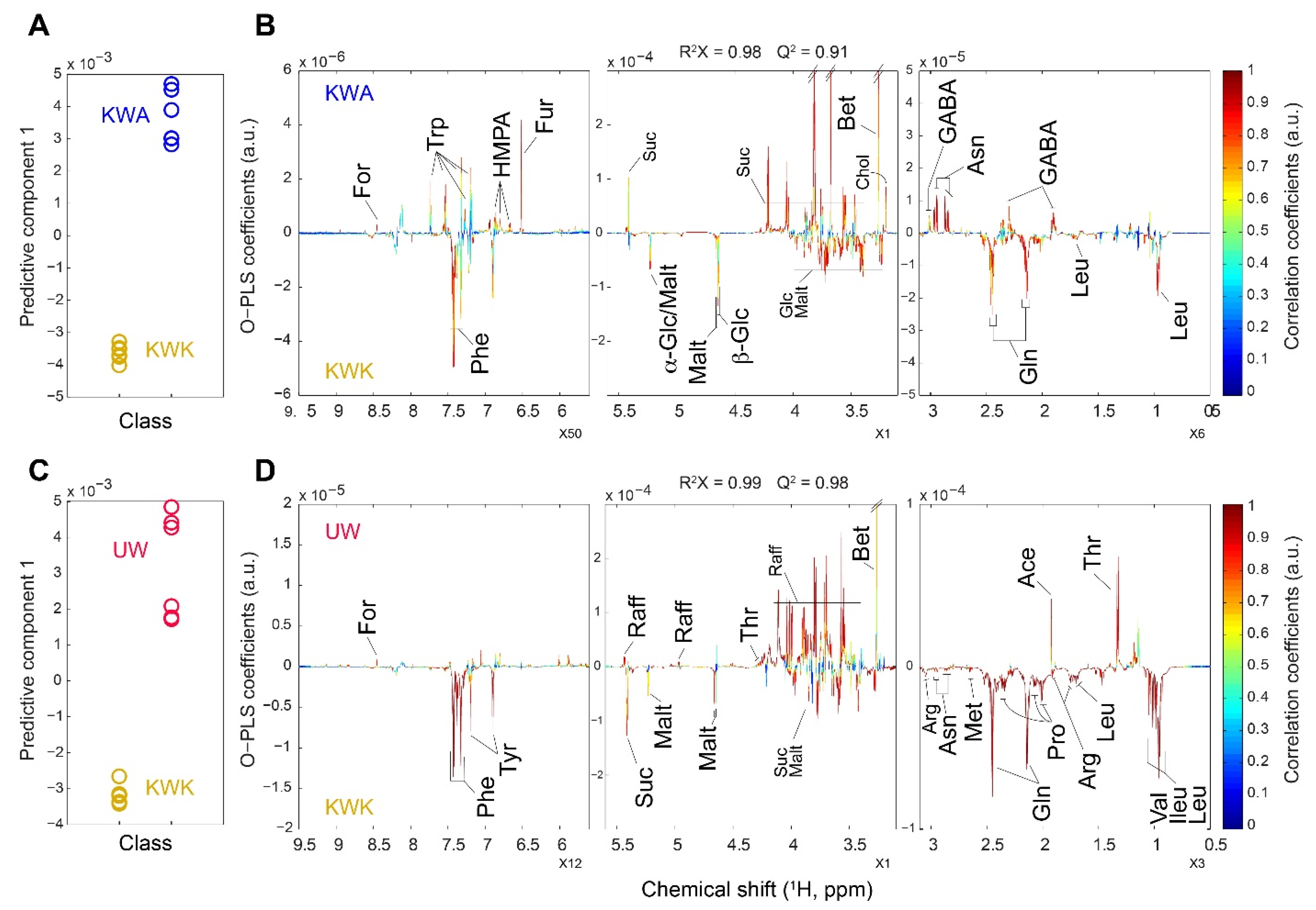
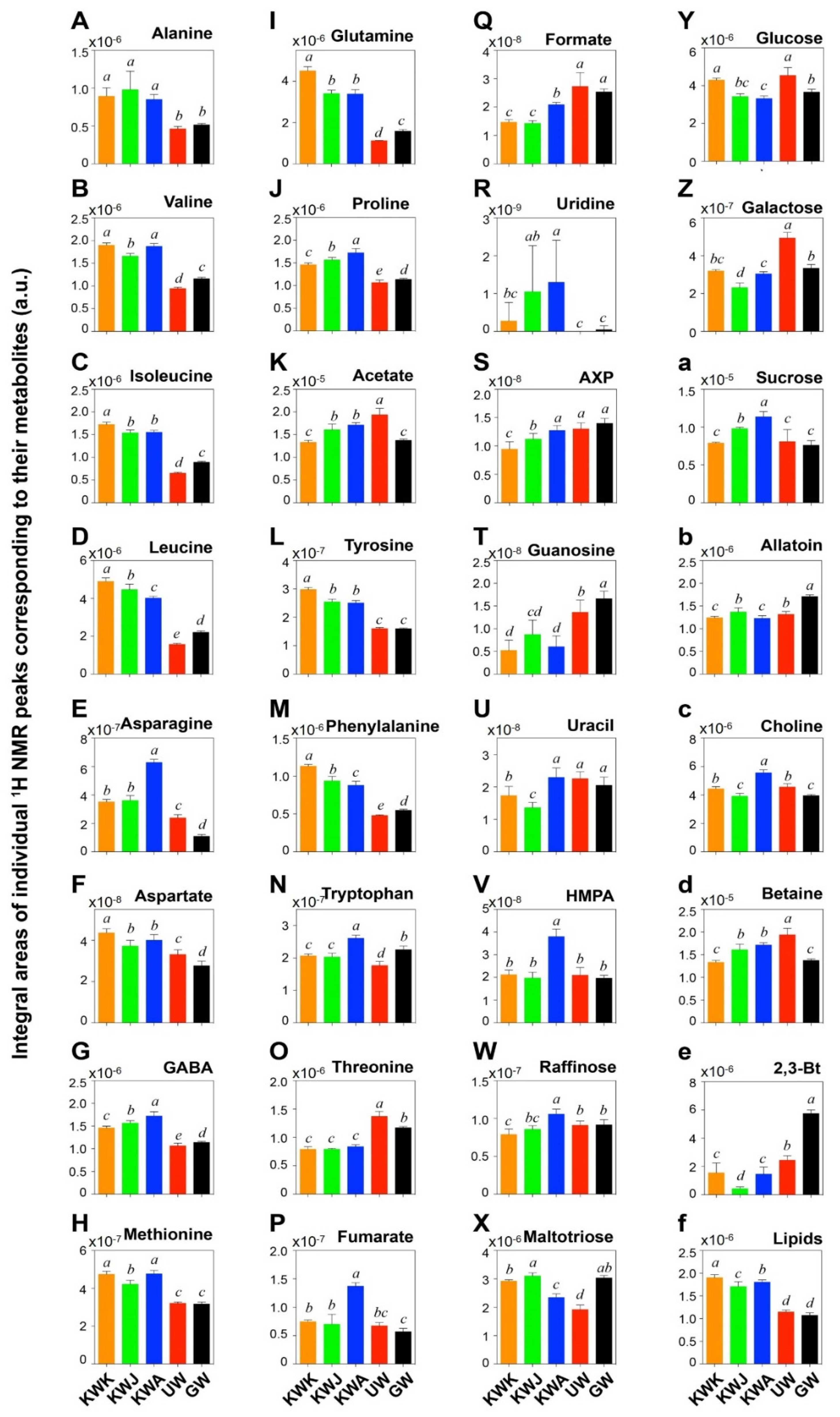
3.4. Metabolites Profile from Wheat Malts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). FAO Cereal Supply and Demand Brief. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 9 January 2019).
- Kwak, H.S.; Kim, T.J.; Joo, E.Y.; Cha, J.H.; Kim, A.J.; Kim, M.J.; Kim, S.S. Quality variation of domestic wheat compared to imported wheat depending on harvest year. J. Korean Soc. Food Sci. Nutr. 2017, 46, 146–151. [Google Scholar] [CrossRef]
- Kwak, H.S.; Kim, M.J.; Kim, S.S. Sensory profile, consumer acceptance, and physicochemical properties of pan bread made with imported or domestic commercial wheat flour. J. Sens. Stud. 2019, 34, 10. [Google Scholar] [CrossRef]
- Kim, M.; Kwak, H.; Kim, S. Effects of germination on protein, γ-aminobutyric acid, phenolic acids, and antioxidant capacity in wheat. Molecules 2018, 23, 2244. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kwak, H.S.; Lee, M.J.; Kim, S.S. Quality predictive models for whole flour of immature wheat during storage and consumer acceptance on its baked product. LWT Food Sci. Technol. 2017, 83, 42–49. [Google Scholar] [CrossRef]
- Mastanjević, K.; Šarkanj, B.; Krska, R.; Sulyok, M.; Warth, B.; Mastanjević, K.; Šantek, B.; Krstanović, V. From malt to wheat beer: A comprehensive multi-toxin screening, transfer assessment and its influence on basic fermentation parameters. Food Chem. 2018, 254, 115–121. [Google Scholar] [CrossRef]
- Briggs, D.E. Malts and Malting; Springer Science & Business Media: London, UK, 1998; pp. 54–70. [Google Scholar]
- Krahl, M.; Zarnkow, M.; Back, W.; Becker, T. Determination of the influence of malting parameters on the water-extractable arabinoxylan content of wheat (Triticum aestivum), rye (Secale cereale), and spelt wheat (Triticum aestivum spp. spelta). J. Am. Soc. Brew. Chem. 2010, 68, 34–40. [Google Scholar] [CrossRef]
- Faltermaier, A.; Zarnkow, M.; Becker, T.; Gastl, M.; Arendt, E.K. Common wheat (Triticum aestivum L.): Evaluating microstructural changes during the malting process by using confocal laser scanning microscopy and scanning electron microscopy. Eur. Food Res. Technol. 2015, 241, 239–252. [Google Scholar] [CrossRef]
- Jin, Y.; Du, J.; Zhang, K.; Guo, M. Relationships between the index of protein modification (Kolbach index) and hydrolytic enzyme production in a wheat malt. J. Inst. Brew. 2014, 120, 201–206. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Wilson, I.D. Metabolic profiling: Status, challenges, and perspective. In Metabolic Profiling; Humana Press: New York, NY, USA, 2018; pp. 3–13. [Google Scholar]
- Bettenhausen, H.M.; Barr, L.; Broeckling, C.D.; Chaparro, J.M.; Holbrook, C.; Sedin, D.; Heuberger, A.L. Influence of malt source on beer chemistry, flavor, and flavor stability. Food Res. Int. 2018, 113, 487–504. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Song, E.H.; Jeong, J.; Park, C.Y.; Kim, H.Y.; Kim, E.H.; Bang, E.; Hong, Y.S. Metabotyping of rice (Oryza sativa L.) for understanding its intrinsic physiology and potential eating quality. Food Res. Int. 2018, 111, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, B.J.; Chung, J.O.; Kim, H.N.; Kim, E.H.; Jung, S.; Lee, H.; Lee, S.J.; Hong, Y.S. Metabolomic unveiling of a diverse range of green tea (Camellia sinensis) metabolites dependent on geography. Food Chem. 2015, 174, 452–459. [Google Scholar] [CrossRef] [PubMed]
- American Association of Cereal Chemists (AACC). AACC Method 55-31.01. Available online: http://methods.aaccnet.org/summaries/55-31-01.aspx (accessed on 9 January 2019).
- European Brewing Convention (EBC). Analytica EBC Methods. Available online: https://brewup.eu/ebc-analytica (accessed on 17 May 2019).
- Ji, H.G.; Lee, Y.R.; Lee, M.S.; Hwang, K.H.; Kim, E.H.; Park, J.S.; Hong, Y.S. Metabolic phenotyping of various tea (Camellia sinensis L.) cultivars and understanding of their intrinsic metabolism. Food Chem. 2017, 233, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ding, F.; Hao, F.; Yu, M.; Lei, H.; Wu, X.; Zhao, Z.; Guo, H.; Yin, J.; Wang, Y. Reprogramming of seed metabolism facilitates pre-harvest sprouting resistance of wheat. Sci. Rep. 2016, 6, 20593. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef]
- Cloarec, O.; Dumas, M.E.; Trygg, J.; Craig, A.; Barton, R.H.; Lindon, J.C.; Nicholson, J.K.; Holmes, E. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal. Chem. 2005, 77, 517–526. [Google Scholar] [CrossRef]
- Choi, I.; Kang, C.-S.; Lee, C.-K.; Kim, S.-L. Classification of 31 Korean wheat (Triticum aestivum L.) cultivars based on the chemical compositions. Prev. Nutr. Food Sci. 2016, 21, 393. [Google Scholar] [CrossRef][Green Version]
- Iqbal, Z.; Pasha, I.; Abrar, M.; Arif, A.M.; Masih, S. Single kernel characterization of wheat varieties in relation to milling quality. J. Glob. Innov. Agric. Soc. Sci. 2015, 3, 136–141. [Google Scholar] [CrossRef]
- Frančáková, H.; Líšková, M.; Bojňanská, T.; Mareček, J. Germination index as an indicator of malting potential. Czech. J. Food Sci. 2012, 30, 377–384. [Google Scholar] [CrossRef]
- Muñoz-Insa, A.; Selciano, H.; Zarnkow, M.; Becker, T.; Gastl, M. Malting process optimization of spelt (Triticum spelta L.) for the brewing process. LWT Food Sci. Technol. 2013, 50, 99–109. [Google Scholar] [CrossRef]
- Faltermaier, A.; Waters, D.; Becker, T.; Arendt, E.; Gastl, M. Common wheat (Triticum aestivum L.) and its use as a brewing cereal—A review. J. Inst. Brew. 2014, 120, 1–15. [Google Scholar] [CrossRef]
- Bamforth, C. Brewing: New Technologies; Woodhead Publishing: Cambridge, UK, 2006; pp. 52–88. [Google Scholar]
- Zhuang, S.; Shetty, R.; Hansen, M.; Fromberg, A.; Hansen, P.B.; Hobley, T.J. Brewing with 100% unmalted grains: Barley, wheat, oat and rye. Eur. Food Res. Technol. 2017, 243, 447–454. [Google Scholar] [CrossRef]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of wort amino acids on beer flavour; a review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Pires, E.; Brányik, T. Biochemistry of Beer Fermentation; Springer: New York, NY, USA, 2015; pp. 51–53. [Google Scholar]
- Lekkas, C.; Stewart, G.G.; Hill, A.; Taidi, B.; Hodgson, J. The importance of free amino nitrogen in wort and beer. Tech. Q Master Brew. Assoc. Am. 2005, 42, 113–116. [Google Scholar] [CrossRef]
- Szlavko, C.M. Tryptophol, tyrosol and phenylethanol- the aromatic higher alcohols in beer. J. Inst. Brew. 1973, 79, 283–288. [Google Scholar] [CrossRef]
- Ziegler, L.; Piendl, A. Nucleobases and nucleosides in malt. J. Am. Soc. Brew. Chem. 1976, 34, 174–181. [Google Scholar] [CrossRef]
- Mallik, M.; Williams, R.D. Allelopathic growth stimulation of plants and microorganisms. Allelopath. J. 2005, 16, 175–198. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=141087 (accessed on 11 March 2020).
- Browne, R.A.; Brindle, K.M. 1H NMR-based metabolite profiling as a potential selection tool for breeding passive resistance against Fusarium head blight (FHB) in wheat. Mol. Plant Pathol. 2007, 8, 401–410. [Google Scholar] [CrossRef]
- Gordon, R.; Power, A.; Chapman, J.; Chandra, S.; Cozzolino, D. A review on the source of lipids and their interactions during beer fermentation that affect beer quality. Fermentation 2018, 4, 89. [Google Scholar] [CrossRef]
- Dickie, K.H.; Cann, C.; Norman, E.C.; Bamforth, C.W.; Muller, R.E. Foam-negative materials. J. Am. Soc. Brew. Chem. 2001, 59, 17–23. [Google Scholar] [CrossRef]
| AZ 1 | JK | KK | |
|---|---|---|---|
| Wheat kernel characteristics | |||
| Moisture content (%) | 11.50 ± 0.03 c | 12.11 ± 0.01 b | 12.46 ± 0.02 a |
| Diameter (mm) | 2.74 ± 0.01 c | 3.02 ± 0.04 a | 2.93 ± 0.01 b |
| Length (mm) | 6.22 ± 0.03 | 7.01 ± 0.24 a | 6.48 ± 0.03 b |
| Width (mm) | 3.03 ± 0.06 c | 3.41 ± 0.05 a | 3.19 ± 0.03 b |
| Perimeter (mm) | 15.14 ± 0.16 c | 17.14 ± 0.48 a | 15.95 ± 0.09 b |
| Thousand-kernel weight (g) | 35.39 ± 0.31 c | 46.51 ± 1.35 a | 41.65 ± 0.52 b |
| Hardness index | 27.39 ± 0.37 c | 64.29 ± 1.77 a | 57.51 ± 0.64 b |
| Roundness | 1.27 ± 0.01 ns | 1.27 ± 0.01 | 1.27 ± 0.01 |
| Germinative properties | |||
| Germinative energy (%) | 96.83 ± 0.76 ns | 95.75 ± 0.25 | 96.08 ± 0.76 |
| Mean germination time (day) | 1.09 ± 0.02 b | 1.15 ± 0.03 a | 1.08 ± 0.02 b |
| Germination index | 9.20 ± 0.12 a | 8.72 ± 0.19 b | 9.29 ± 0.13 a |
| Control | Korean Wheat Malt | |||||
|---|---|---|---|---|---|---|
| GW 1 | UW | KWA | KWJ | KWK | ||
| Moisture content (%) | 4.1 ± 0.3 b | 5.1 ± 0.1 a | 5.2 ± 0.1 a | 4.1 ± 0.5 b | 4.3 ± 0.2 b | |
| pH | 5.96 ± 0.01 d | 6.18 ± 0.02 b | 6.24 ± 0.01 a | 6.09 ± 0.02 c | 6.20 ± 0.02 b | |
| Color (EBC units) | 3.7 ± 0.1 d | 4.8 ± 0.2 b | 3.5 ± 0.2 d | 5.6 ± 0.1 a | 4.1 ± 0.1 c | |
| Extract (%) | 81.9 ± 1.3 a | 82.0 ± 0.7 a | 80.1 ± 0.7 ab | 79.5 ± 1.2 b | 80.1 ± 1.5 ab | |
| Free amino nitrogen (mg/100g) | 114.8 ± 5.1 cd | 106.1 ± 4.9 d | 124.5 ± 6.6 bc | 151.6 ± 6.6 a | 136.0 ± 8.3 b | |
| Diastatic power (°WK) | 475.8 ± 3.5 a | 469.3 ± 8.7 a | 415.3 ± 10.6 b | 400.5 ± 11.5 bc | 383.0 ± 15.7 c | |
| Viscosity (mPa·s) | 2.20 ± 0.09 bc | 1.91 ± 0.08 d | 2.84 ± 0.21 a | 2.00 ± 0.14 cd | 2.38 ± 0.08 b | |
| Soluble nitrogen (mg/L) | 827.2 ± 26.2 bc | 775.4 ± 7.9 d | 795.0 ± 8.5 cd | 1140.0 ± 54.7 a | 848.8 ± 26.2 b | |
| Total nitrogen (%) | 1.79 ± 0.01 c | 1.74 ± 0.02 d | 2.42 ± 0.01 a | 2.41 ± 0.01 a | 2.24 ± 0.01 b | |
| Kolbach index (%) | 46.2 ± 0.4 ab | 44.7 ± 0.7 b | 32.9 ± 0.4 d | 47.3 ± 2.3 a | 38.0 ± 1.2 c | |
| Control | Korean Wheat Malt | |||||
|---|---|---|---|---|---|---|
| GW 1 | UW | KWA | KWJ | KWK | ||
| α-Amylase (U/g) | 73.67 ± 4.01 a | 56.39 ± 2.40 b | 46.01 ± 0.78 c | 43.63 ± 1.19 c | 42.04 ± 0.72 c | |
| β-Amylase (U/g) | 12.92 ± 0.07 c | 14.11 ± 0.02 a | 11.92 ± 0.12 d | 10.84 ± 0.06 e | 13.30 ± 0.12 b | |
| β-Endoxylanase (U/kg) | 50.89 ± 3.93 d | 93.12 ± 6.83 a | 60.02 ± 2.13 c | 70.93 ± 1.33 b | 46.38 ± 0.95 d | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, Y.S.; Lee, D.; Hong, Y.-S.; Lim, S.-T.; Kim, S.S.; Kwak, H.S. Comparison of Physicochemical Properties and Metabolite Profiling Using 1H NMR Spectroscopy of Korean Wheat Malt. Foods 2020, 9, 1436. https://doi.org/10.3390/foods9101436
Byeon YS, Lee D, Hong Y-S, Lim S-T, Kim SS, Kwak HS. Comparison of Physicochemical Properties and Metabolite Profiling Using 1H NMR Spectroscopy of Korean Wheat Malt. Foods. 2020; 9(10):1436. https://doi.org/10.3390/foods9101436
Chicago/Turabian StyleByeon, Yang Soo, Dabeen Lee, Young-Shick Hong, Seung-Taik Lim, Sang Sook Kim, and Han Sub Kwak. 2020. "Comparison of Physicochemical Properties and Metabolite Profiling Using 1H NMR Spectroscopy of Korean Wheat Malt" Foods 9, no. 10: 1436. https://doi.org/10.3390/foods9101436
APA StyleByeon, Y. S., Lee, D., Hong, Y.-S., Lim, S.-T., Kim, S. S., & Kwak, H. S. (2020). Comparison of Physicochemical Properties and Metabolite Profiling Using 1H NMR Spectroscopy of Korean Wheat Malt. Foods, 9(10), 1436. https://doi.org/10.3390/foods9101436






