Powder and Reconstituted Properties of Commercial Infant and Follow-On Formulas †
Abstract
1. Introduction
2. Materials and Methods
2.1. Commercial Infant Formula
2.2. Powder Properties
2.3. Flowability
2.4. Rehydration Properties
2.5. Statistical Analysis
3. Results and Discussion
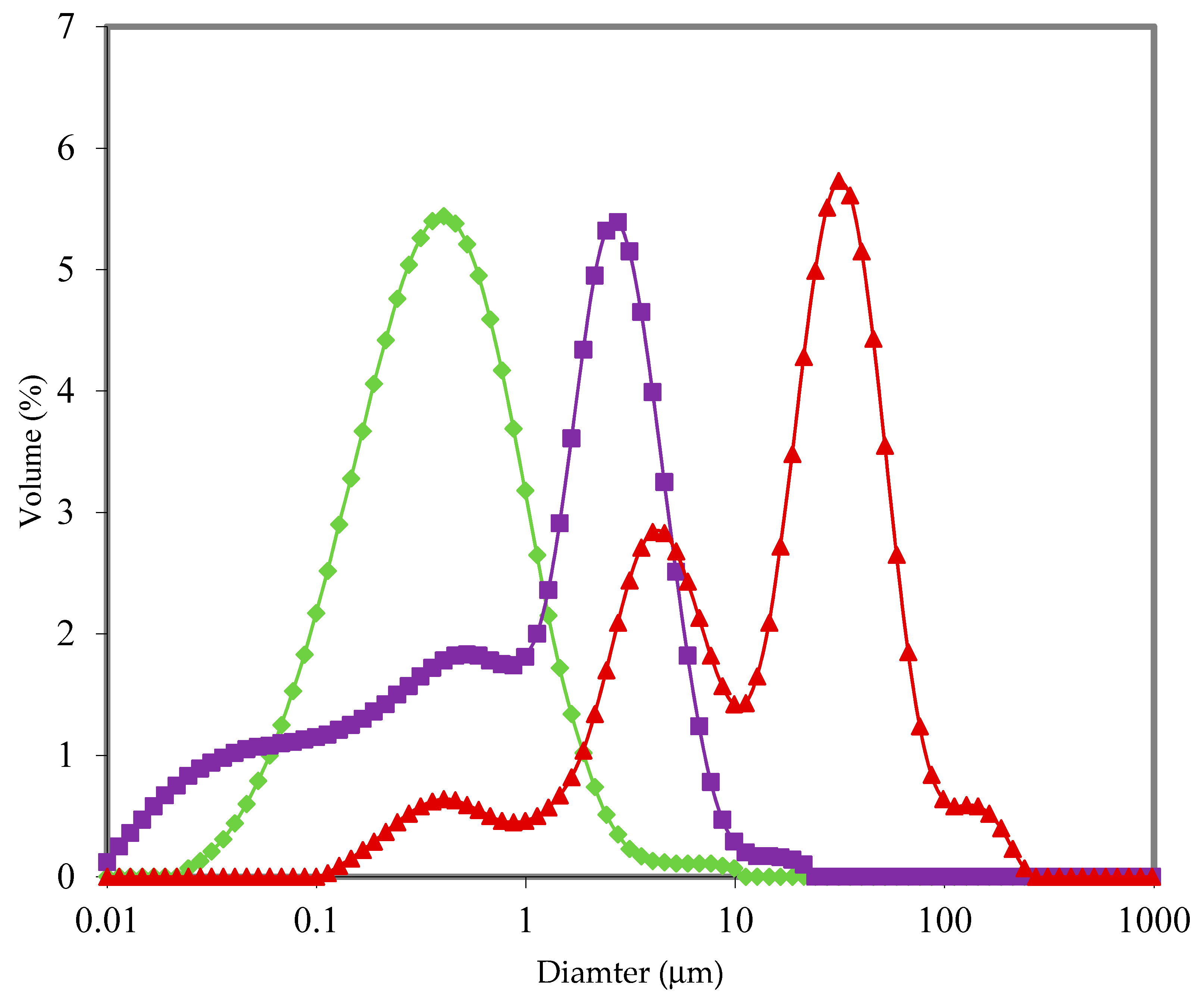
3.1. Powder Properties
3.2. Flowability
3.3. Compressibility
3.4. Rehydration to 12.5% w/w
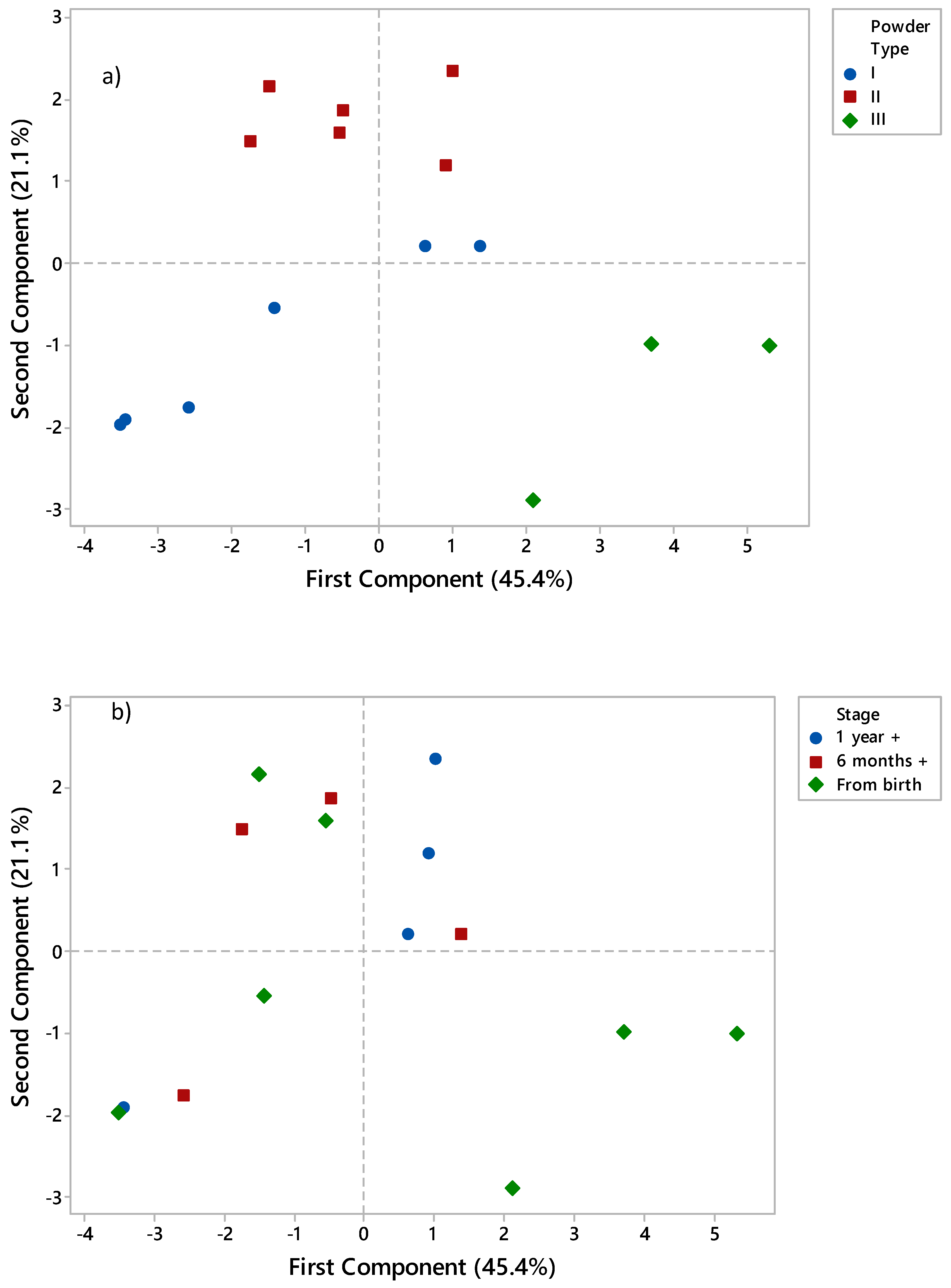
3.5. Principle Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maldonado, J.; Gil, A.; Narbona, E.; Molina, J.A. Special formulas in infant nutrition: A review. Early Hum. Dev. 1998, 53 (Suppl. S1), S23–S32. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kelly, A.L.; O’Mahony, J.A.; Hickey, D.K.; Chaurin, V.; Fenelon, M.A. Effect of protein content on emulsion stability of a model infant formula. Int. Dairy J. 2012, 25, 80–86. [Google Scholar] [CrossRef]
- Mullane, N.R.; Whyte, P.; Wall, P.G.; Quinn, T.; Fanning, S. Application of pulsed-field gel electrophoresis to characterise and trace the prevalence of Enterobacter sakazakii in an infant formula processing facility. Int. J. Food Microbiol. 2007, 116, 73–81. [Google Scholar] [CrossRef]
- De Vilder, J.; Martens, R.; Naudts, M. Influence of process variables on some whole milk powder characterisitcs. Milchwissenschaft 1976, 31, 396–400. [Google Scholar]
- De Vilder, J.; Martens, R.; Naudts, M. The influence of dry matter content, the homogenisation and the heating of concentrate on the physical characteristics of whole milk powder. Milchwissenschaft 1979, 34, 78–84. [Google Scholar]
- McCarthy, N.A.; Gee, V.L.; Hickey, D.K.; Kelly, A.L.; O’Mahony, J.A.; Fenelon, M.A. Effect of protein content on the physical stability and microstructure of a model infant formula. Int. Dairy J. 2013, 29, 53–59. [Google Scholar] [CrossRef]
- Murphy, E.G.; Tobin, J.T.; Roos, Y.H.; Fenelon, M.A. A high-solids steam injection process for the manufacture of powdered infant milk formula. Dairy Sci. Technol. 2013, 93, 463–475. [Google Scholar] [CrossRef]
- Jeurink, T.J.M.; De Kruif, C.G. Changes in milk on heating: Viscosity measurements. J. Dairy Res. 1993, 60, 139–150. [Google Scholar] [CrossRef]
- O’Loughlin, I.B.; Murray, B.A.; Kelly, P.M.; Fitzgerald, R.J.; Brodkorb, A. Enzymatic hydrolysis of heat-induced aggregates of whey protein isolate. J. Agric. Food Chem. 2012, 60, 4895–4904. [Google Scholar] [CrossRef]
- Murphy, E.G.; Fenelon, M.A.; Roos, Y.H.; Hogan, S.A. Decoupling macronutrient interactions during heating of model infant milk formulas. J. Agric. Food Chem. 2014, 62, 10585–10593. [Google Scholar] [CrossRef]
- Murphy, E.G.; Roos, Y.H.; Hogan, S.A.; Maher, P.G.; Flynn, C.G.; Fenelon, M.A. Physical stability of infant milk formula made with selectively hydrolysed whey proteins. Int. Dairy J. 2015, 40, 39–46. [Google Scholar] [CrossRef]
- Masters, K. Atomization. In Spray Drying in Practice; SprayDryConsult International ApS: Charlottenlund, Denmark, 2002; pp. 129–179. [Google Scholar]
- Masters, K. Spray-air contact, particle formation and drying. In Spray Drying in Practice; SprayDryConsult International ApS: Charlottenlund, Denmark, 2002; pp. 180–215. [Google Scholar]
- Ortega-Rivas, E. Bulk Properties of Food Particulate Materials: An Appraisal of their Characterisation and Relevance in Processing. Food Bioprocess Technol. 2009, 2, 28–44. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; Barringer, S.A.; Iqbal, T. Flow property measurement of food powders and sensitivity of Jenike’s hopper design methodology to the measured values. J. Food Eng. 2004, 61, 399–405. [Google Scholar] [CrossRef]
- Kim, E.H.J.; Chen, X.D.; Pearce, D. Effect of surface composition on the flowability of industrial spray-dried dairy powders. Colloids Surf. B Biointerfaces 2005, 46, 182–187. [Google Scholar] [CrossRef]
- O’Donoghue, L.T.; Haque, M.K.; Kennedy, D.; Laffir, F.R.; Hogan, S.A.; O’Mahony, J.A.; Murphy, E.G. Influence of particle size on the physicochemical properties and stickiness of dairy powders. Int. Dairy J. 2019, 98, 54–63. [Google Scholar] [CrossRef]
- Fu, X.; Huck, D.; Makein, L.; Armstrong, B.; Willen, U.; Freeman, T. Effect of particle shape and size on flow properties of lactose powders. Particuology 2012, 10, 203–208. [Google Scholar] [CrossRef]
- Kim, E.H.J.; Chen, X.D.; Pearce, D. Surface characterization of four industrial spray-dried dairy powders in relation to chemical composition, structure and wetting property. Colloids Surf. B Biointerfaces 2002, 26, 197–212. [Google Scholar] [CrossRef]
- Vignolles, M.L.; Jeantet, R.; Lopez, C.; Schuck, P. Free fat, surface fat and dairy powders: Interactions between process and product. A review. Lait 2007, 87, 187–236. [Google Scholar] [CrossRef]
- Toikkanen, O.; Outinen, M.; Malafronte, L.; Rojas, O.J. Formation and structure of insoluble particles in reconstituted model infant formula powders. Int. Dairy J. 2018, 82, 19–27. [Google Scholar] [CrossRef]
- Chobert, J.M.; Bertrand-Harb, C.; Nicolas, M.G. Solubility and emulsifiying properties of caseins and whey proteins modified enzymatically by trypsin. J. Agric. Food Chem. 1988, 36, 883–892. [Google Scholar] [CrossRef]
- Agboola, S.O.; Dalgleish, D.G. Enzymatic Hydrolysis of Milk Proteins Used for Emulsion Formation. 1. Kinetics of Protein Breakdown and Storage Stability of the Emulsions. J. Agric. Food Chem. 1996, 44, 3631–3636. [Google Scholar] [CrossRef]
- Kelly, G.M.; O’Mahony, J.A.; Kelly, A.L.; O’Callaghan, D.J. Effect of hydrolyzed whey protein on surface morphology, water sorption, and glass transition temperature of a model infant formula. J. Dairy Sci. 2016, 99, 6961–6972. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.J.; Cronin, K.; O’Sullivan, C.; Fenelon, M.A.; O’Mahony, J.A.; Byrne, E.P. Effect of composition on the mechanical response of agglomerates of infant formulae. J. Food Eng. 2011, 107, 71–79. [Google Scholar] [CrossRef]
- Murphy, E.G.; Tobin, J.T.; Roos, Y.H.; Fenelon, M.A. The effect of high velocity steam injection on the colloidal stability of concentrated emulsions for the manufacture of infant formulations. Procedia Food Sci. 2011, 1, 1309–1315. [Google Scholar] [CrossRef][Green Version]
- Henihan, L.E.; O’Donnell, C.P.; Esquerre, C.; Murphy, E.G.; O’Callaghan, D.J. Quality assurance of model infant milk formula using a front-face fluorescence process analytical tool. Food Bioprocess Technol. 2018, 11, 1402–1411. [Google Scholar] [CrossRef]
- Wang, X.; Esquerre, C.; Downey, G.; Henihan, L.; O’Callaghan, D.; O’Donnell, C. Assessment of infant formula quality and composition using Vis-NIR, MIR and Raman process analytical technologies. Talanta 2018, 183, 320–328. [Google Scholar] [CrossRef]
- GEA-Niro. Analytical Methods for Dry Milk Products. Available online: https://www.gea.com/en/products/analytical-methods-dry-milk-products.jsp (accessed on 2 December 2019).
- Crowley, S.V.; Gazi, I.; Kelly, A.L.; Huppertz, T.; O’Mahony, J.A. Influence of protein concentration on the physical characteristics and flow properties of milk protein concentrate powders. J. Food Eng. 2014, 135, 31–38. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Masum, A.; Huppertz, T.; Chandrapala, J.; Adhikari, B.; Zisu, B. Physicochemical properties of spray-dried model infant milk formula powders: Influence of whey protein-to-casein ratio. Int. Dairy J. 2020, 100, 104565. [Google Scholar] [CrossRef]
- Shaffer, K.; Paterson, A.H.J.; Davies, C.E.; Hebbink, G. Stokes shape factor for lactose crystals. Adv. Powder Technol. 2011, 22, 454–457. [Google Scholar] [CrossRef]
- Vignolles, M.L.; Lopez, C.; Madec, M.N.; Ehrhardt, J.J.; Méjean, S.; Schuck, P.; Jeantet, R. Fat properties during homogenization, spray-drying, and storage affect the physical properties of dairy powders. J. Dairy Sci. 2009, 92, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Euston, S.R.; Finnigan, S.R.; Hirst, R.L. Heat induced destabilisation of oil-in-water emulsions formed from hydrolyzed whey protein. J. Agric. Food Chem. 2001, 49, 5576–5583. [Google Scholar] [CrossRef] [PubMed]
- Montagne, D.H.; Van Dael, P.; Skanderby, M.; Hugelshofer, W. Infant formulae - powders and liquids. In Dairy Powders and Concentrated Products; Tamime, A.Y., Ed.; Wiley-Blackwell: Chichester, UK, 2009; pp. 294–331. [Google Scholar]
- Schuck, P.; Dolivet, A.; Jeantet, R. Dehydration processes and powder properties. In Analytical Methods for Food and Dairy Powders; Wiley-Blackwell: Chichester, UK, 2012; pp. 1–43. [Google Scholar]
- Yazdanpanah, N.; Langrish, T.A.G. Egg-shell like structure in dried milk powders. Food Res. Int. 2011, 44, 39–45. [Google Scholar] [CrossRef]
- Schuck, P.; Dolivet, A.; Jeantet, R. Determination of flowability anf floodability indices. In Analytical Methods for Food and Dairy Powders; Wiley-Blackwell: Chichester, UK, 2012; pp. 129–143. [Google Scholar]
- Lowe, E.K.; Paterson, A.H.J. A mathematical model for lactose dissolution, part II. Dissolution below the alpha lactose solubility limit. J. Food Eng. 1998, 38, 15–25. [Google Scholar] [CrossRef]
- Fang, Y.; Selomulya, C.; Chen, X.D. On Measurement of Food Powder Reconstitution Properties. Dry. Technol. 2008, 26, 3–14. [Google Scholar] [CrossRef]
- Snoeren, T.H.M.; Brinkhuis, J.A.; Damman, A.J.; Klok, H.J. Viscosity and age thickening of skim milk concentrate. Neth. Milk Dairy J. 1984, 38, 43–53. [Google Scholar]
- McCarthy, O.J.; Singh, H. Physico-chemical properties of milk. In Advanced Dairy Chemistry Volume 3: Lactose, Water, Salts and Minor Constituents; McSweeney, P.L.H., Fox, P.F., Eds.; Springer Verlag: New York City, NY, USA, 2009; pp. 691–758. [Google Scholar]
- Anema, S.G.; Li, Y. Association of denatured whey proteins with casein micelles in heated reconstituted skim milk and its effect on casein micelle size. J. Dairy Res. 2003, 70, 73–83. [Google Scholar] [CrossRef]
- Snoeren, T.H.M.; Damman, A.J.; Klok, H.J. The viscosity of skim-milk concentrates. Neth. Milk Dairy J. 1982, 36, 305–316. [Google Scholar]
- Biliaderis, C.G.; Maurice, T.J.; Vose, J.R. Starch gelatinisation phenomena studied by differential scanning calorimetry. J. Food Sci. 1980, 45, 1669–1674. [Google Scholar] [CrossRef]
- Kett, A.P.; Chaurin, V.; Fitzsimons, S.M.; Morris, E.R.; O’Mahony, J.A.; Fenelon, M.A. Influence of milk proteins on the pasting behaviour and microstructural characteristics of waxy maize starch. Food Hydrocoll. 2013, 30, 661–671. [Google Scholar] [CrossRef]
- Leman, J.; Haque, Z.; Kinsella, J.E. Creaming stability of fluid emulsions containing different milk protein preparations. Milchwissenschaft 1988, 43, 286–289. [Google Scholar]
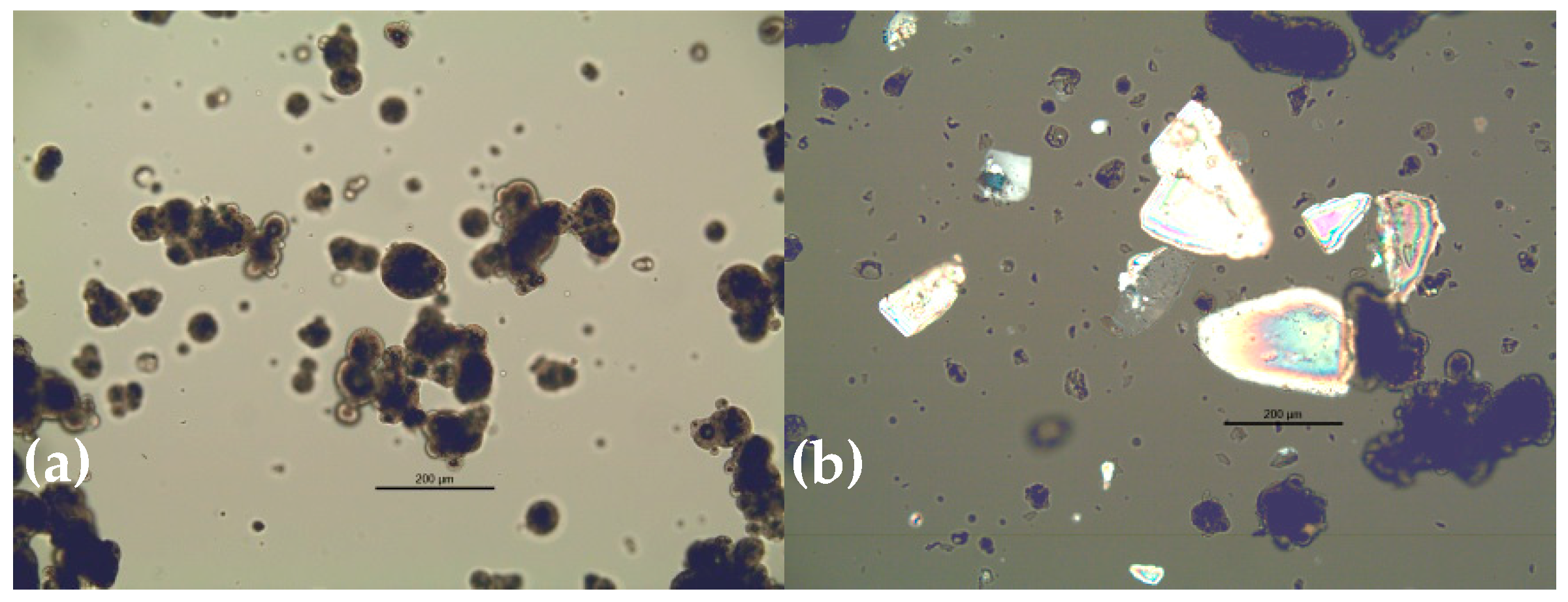
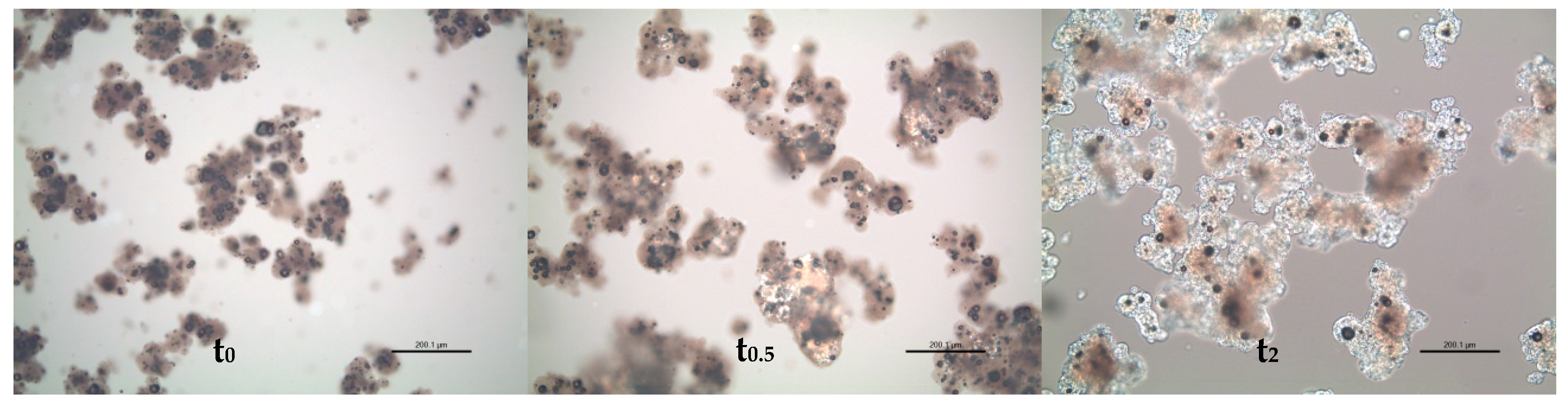
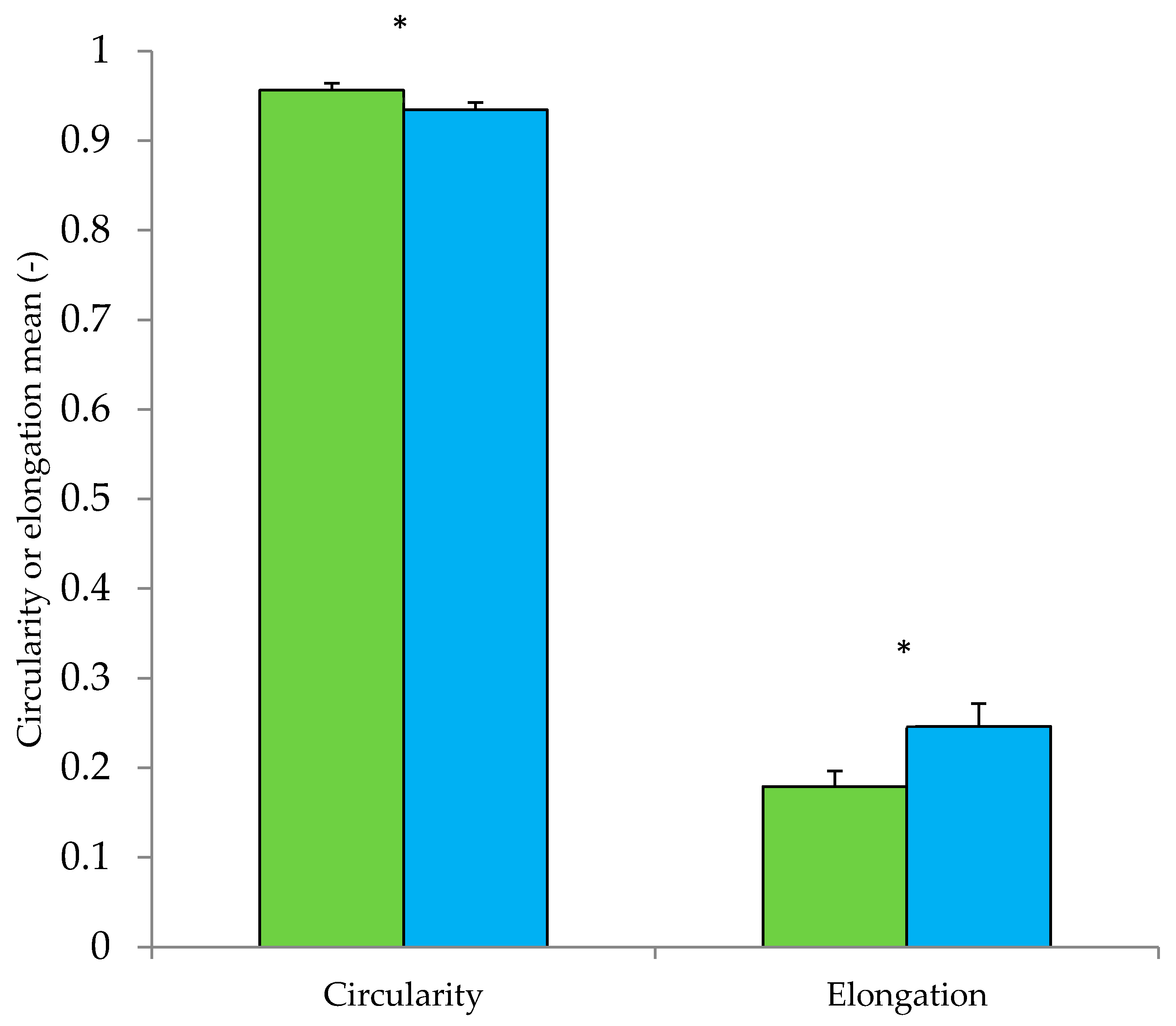
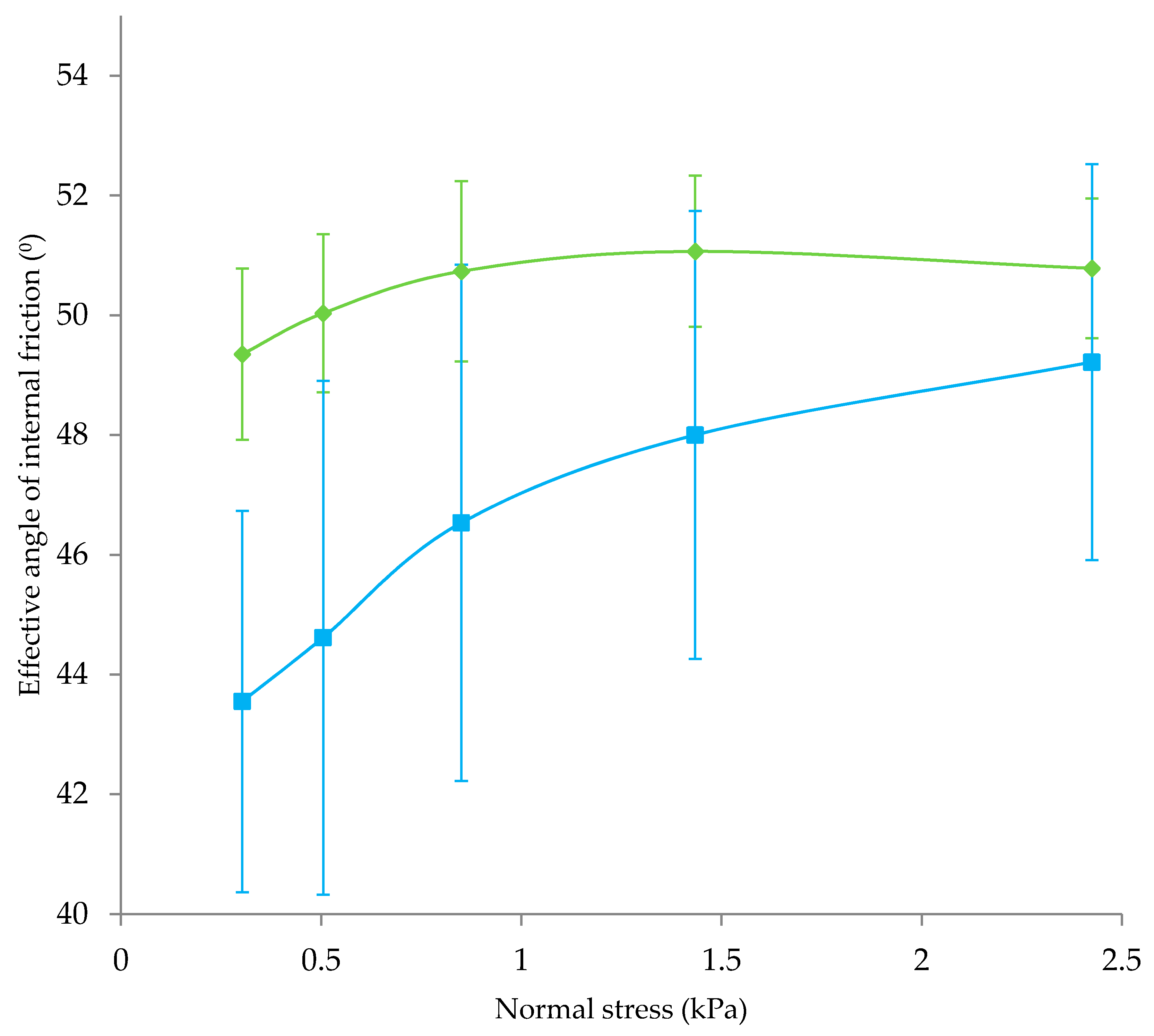
| Powder No. | Stage | Comment on Formulation */Structure ** | Powder Type |
|---|---|---|---|
| 1 | From birth | Intact milk protein source; no lactose crystals | I |
| 2 | 6 months + | Intact milk protein source; no lactose crystals | I |
| 3 | 1 year + | Intact milk protein source; no lactose crystals | I |
| 4 | From birth | Intact milk protein source; no lactose crystals | I |
| 5 | 6 months + | Intact milk protein source; no lactose crystals | I |
| 6 | 1 year + | Intact milk protein source; no lactose crystals | I |
| 7 | From birth | Intact milk protein source; lactose crystals | II |
| 8 | 6 months + | Intact milk protein source; lactose crystals | II |
| 9 | 1 year + | Intact milk protein source; lactose crystals | II |
| 10 | From birth | Intact milk protein source; lactose crystals | II |
| 11 | 6 months + | Intact milk protein source; lactose crystals | II |
| 12 | 1 year + | Intact milk protein source; lactose crystals | II |
| 13 | From birth | Hydrolysed whey protein source; lactose crystals; starch | III |
| 14 | From birth | Hydrolysed whey protein source; lactose crystals; starch | III |
| 15 | From birth | Hydrolysed whey protein source; no lactose crystals | III |
| Flowability | Hardened | Very Cohesive | Cohesive | Easy-Flow | Free-Flowing |
|---|---|---|---|---|---|
| i | <1 | <2 | <4 | <10 | >10 |
| Powder Type | Powder No. | D [3,2] (μm) | D (v, 0.1) (μm) | Span of Particles | Surface Free Fat (% w/w of Powder) |
|---|---|---|---|---|---|
| I | 1 | 183.0 ± 6.0 | 103.3 ± 1.5 | 1.5 ± 0.0 | 1.5 ± 0.0 |
| I | 2 | 139.0 ± 0.1 | 77.4 ± 0.1 | 1.5 ± 0.0 | 0.6 ± 0.0 |
| I | 3 | 166.2 ± 0.1 | 90.8 ± 0.3 | 1.6 ± 0.0 | 1.1 ± 0.0 |
| I | 4 | 181.4 ± 7.1 | 129.0 ± 3.8 | 1.1 ± 0.0 | 0.8 ± 0.0 |
| I | 5 | 170.0 ± 0.1 | 112.0 ± 0.6 | 1.3 ± 0.0 | 1.0 ± 0.0 |
| I | 6 | 220.1 ± 10.1 | 140.5 ± 6.93 | 1.2 ± 0.0 | 0.8 ± 0.0 |
| Average I | 176.5 ± 25.3 a | 108.8 ± 22.3 a | 1.4 ± 0.2 a | 1.0 ± 0.3 a | |
| II | 7 | 160.0 ± 7.5 | 92.4 ± 4.1 | 1.6 ± 0.0 | 0.9 ± 0.0 |
| II | 8 | 162.0 ± 1.7 | 100.9 ± 2.1 | 1.5 ± 0.0 | 0.9 ± 0.0 |
| II | 9 | 117.0 ± 0.1 | 62.1 ± 0.1 | 1.8 ± 0.0 | 0.4 ± 0.0 |
| II | 10 | 149.3 ± 1.5 | 87.6 ± 0.7 | 1.6 ± 0.0 | 1.1 ± 0.0 |
| II | 11 | 143.0 ± 1.4 | 82.3 ± 0.1 | 1.6 ± 0.0 | 0.8 ± 0.0 |
| II | 12 | 126.1 ± 1.7 | 68 ± 0.8 | 1.8 ± 0.0 | 0.7 ± 0.0 |
| Average II | 142.9 ± 17.3 b | 82.2 ± 14.0 b | 1.7 ± 0.1 b | 0.8 ± 0.2 a | |
| III | 13 | 125.5 ± 0.7 | 67.2 ± 0.2 | 1.6 ± 0.0 | 1.3 ± 0.1 |
| III | 14 | 112.3 ± 0.6 | 59.6 ± 0.2 | 1.7 ± 0.0 | 1.2 ± 0.1 |
| III | 15 | 134.3 ± 0.6 | 73.3 ± 0.3 | 1.5 ± 0.0 | 2.2 ± 0.1 |
| Average III | 124.1 ± 9.6 b | 66.7 ± 5.9 c | 1.6 ± 0.1 b | 1.5 ± 0.5 b | |
| Powder Type | Powder No. | Flow Index (i) | Drum Flow (g min−1) |
|---|---|---|---|
| I | 1 | 6.5 ± 0.3 | 32.9 ± 1.0 |
| I | 2 | 4.6 ± 0.3 | 11.2 ± 0.2 |
| I | 3 | 4.7 ± 0.2 | 17.4 ± 1.7 |
| I | 4 | 7.7 ± 0.8 | 38.5 ± 1.2 |
| I | 5 | 7.7 ± 0.1 | 38.5 ± 1.5 |
| I | 6 | 8.7 ± 0.5 | 38.2 ± 1.2 |
| Average I | 6.6 ± 1.7 a | 29.4 ± 11.6 a | |
| II | 7 | 8.7 ± 3.4 | 39.0 ± 0.3 |
| II | 8 | 8.3 ± 0.1 | 29.0 ± 1.3 |
| II | 9 | 4.4 ± 0.3 | 24.8 ± 0.1 |
| II | 10 | 6.9 ± 0.3 | 35.1 ± 0.1 |
| II | 11 | 5.6 ± 0.4 | 34.6 ± 1.0 |
| II | 12 | 4.7 ± 0.2 | 20.2 ± 0.4 |
| Average II | 6.4 ± 2.1 a | 30.5 ± 6.8 a | |
| III | 13 | 4.2 ± 0.0 | 20.0 ± 0.9 |
| III | 14 | 4.5 ± 0.4 | 16.5 ± 0.5 |
| III | 15 | 3.9 ± 0.4 | 14.5 ± 0.3 |
| Average III | 4.2 ± 0.4 b | 17.0 ± 2.5 b | |
| Type I | Type II | |||
|---|---|---|---|---|
| Drum Flowability | Flow Index | Drum Flowability | Flow Index | |
| Compressibility by tapping | −0.94 | −0.90 | 0.35 | 0.33 |
| Compressibility by flow index | −0.98 | −0.93 | −0.88 | −0.75 |
| Powder Type | Powder No. | Wettability (s) | Viscosity * 12.5% (w/w) (mPa s) | pH 12.5% (w/w); 40 °C | Emulsion Particle Size D [4,3] (μm) | Creaming Rate (mm day−1) |
|---|---|---|---|---|---|---|
| I | 1 | 18.6 ± 0.6 | 2.22 ± 0.01 | 7.01 | 0.70 ± 0.01 | 0.35 ± 0.06 |
| I | 2 | 10.8 ± 0.3 | 2.37 ± 0.06 | 6.99 | 0.60 ± 0.01 | 0.15 ± 0.03 |
| I | 3 | 15.6 ± 0.6 | 2.47 ± 0.05 | 6.89 | 1.17 ± 0.12 | 0.03 ± 0.00 |
| I | 4 | 16.3 ± 0.9 | 2.33 ± 0.04 | 6.65 | 0.57 ± 0.02 | 0.22 ± 0.01 |
| I | 5 | 16.9 ± 0.5 | 2.33 ± 0.03 | 6.61 | 0.56 ± 0.06 | 0.15 ± 0.01 |
| I | 6 | 16.6 ± 0.8 | 2.31 ± 0.01 | 6.55 | 0.67 ± 0.02 | 0.21 ± 0.03 |
| Average I | 15.7 ± 2.6 a | 2.33 ± 0.08 a | 6.78 ± 0.20 a | 0.71 ± 0.24 a | 0.18 ± 0.11 a | |
| II | 7 | 24.7 ± 0.6 | 2.21 ± 0.06 | 6.79 | 0.38 ± 0.01 | 0.25 ± 0.02 |
| II | 8 | 32.0 ± 0.8 | 2.29 ± 0.08 | 6.81 | 0.80 ± 0.08 | 0.07 ± 0.01 |
| II | 9 | 14.9 ± 0.5 | 2.30 ± 0.03 | 6.76 | 0.54 ± 0.01 | 0.22 ± 0.03 |
| II | 10 | 16.9 ± 0.3 | 2.36 ± 0.04 | 6.77 | 0.41 ± 0.01 | 0.18 ± 0.04 |
| II | 11 | 15.6 ± 0.7 | 2.30 ± 0.04 | 6.76 | 0.54 ± 0.21 | 0.24 ± 0.02 |
| II | 12 | 19.8 ± 0.1 | 2.61 ± 0.01 | 6.70 | 0.72 ± 0.46 | 0.15 ± 0.02 |
| Average II | 20.7 ± 6.5 a | 2.34 ± 0.14 a | 6.77 ± 0.04 a | 0.57 ± 0.23 a | 0.19 ± 0.07 a | |
| III | 13 | 14.3 ± 0.2 | 6.36 ± 0.07 | 6.55 | 27.81 ± 3.37 | ** |
| III | 14 | 11.5 ± 0.1 | 10.91 ± 0.14 | 6.79 | 42.06 ± 4.40 | ** |
| III | 15 | 17.61 ± 0.7 | 2.09 ± 0.03 | 6.38 | 2.26 ± 0.14 | ** |
| Average III | 14.55 ± 3.1 a | 6.46 ± 3.94 a | 6.57 ± 0.21 a | 24.41 ± 17.45 b | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, E.G.; Regost, N.E.; Roos, Y.H.; Fenelon, M.A. Powder and Reconstituted Properties of Commercial Infant and Follow-On Formulas. Foods 2020, 9, 84. https://doi.org/10.3390/foods9010084
Murphy EG, Regost NE, Roos YH, Fenelon MA. Powder and Reconstituted Properties of Commercial Infant and Follow-On Formulas. Foods. 2020; 9(1):84. https://doi.org/10.3390/foods9010084
Chicago/Turabian StyleMurphy, Eoin G., Nicolas E. Regost, Yrjö H. Roos, and Mark A. Fenelon. 2020. "Powder and Reconstituted Properties of Commercial Infant and Follow-On Formulas" Foods 9, no. 1: 84. https://doi.org/10.3390/foods9010084
APA StyleMurphy, E. G., Regost, N. E., Roos, Y. H., & Fenelon, M. A. (2020). Powder and Reconstituted Properties of Commercial Infant and Follow-On Formulas. Foods, 9(1), 84. https://doi.org/10.3390/foods9010084





