Alpha-Cyclodextrin Attenuates the Glycemic and Insulinemic Impact of White Bread in Healthy Male Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment and Ethics
2.2. Preparation of Test Meals
2.2.1. Bread Preparation
2.2.2. Test Product
2.3. Study Design
2.4. Blood Sampling and Analytical Methods
2.5. Statistical Analyses
3. Results
3.1. Participants and Compliance
3.2. Postprandial Glucose and Insulin Response
3.3. Side-Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- US Food and Drug Administration. The Declaration of Certain Isolated or Synthetic Non-Digestible Carbohydrates as Dietary Fiber on Nutrition and Supplement Facts Labels: Guidance for Industry. June 2018; p. 9. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-declaration-certain-isolated-or-synthetic-non-digestible-carbohydrates-dietary (accessed on 28 November 2019).
- US Food and Drug Administration. Scientific Evaluation of the Evidence on the Beneficial Physiological Effects of Isolated or Synthetic Non-Digestible Carbohydrates Submitted as a Citizen Petition (21 CFR 10.30): Guidance for Industry. February 2018; p. 19. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-scientific-evaluation-evidence-beneficial-physiological-effects-isolated-or (accessed on 28 November 2019).
- Buckley, J.D.; Thorp, A.A.; Murphy, K.J.; Howe, P.R.C. Dose-dependent inhibition of the post-prandial glycaemic response to a standard carbohydrate meal following incorporation of alpha-cyclodextrin. Ann. Nutr. Metab. 2006, 50, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Bolognesi, C. Source and amount of carbohydrate affect postprandial glucose and insulin in normal subjects. J. Nutr. 1996, 126, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- FAO. Carbohydrates in human nutrition. Chapter 4: The role of the glycemic index in food choice. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr. Paper 1998, 66, 25–30. [Google Scholar]
- WHO Technical Report Series 909. In Proceedings of the 57th Report of the Joint FAO/WHO Expert Committee on Food Additives, Rome, Italy, 5–14 June 2001; pp. 40–42.
- WHO Technical Report Series 928. In Proceedings of the 63rd Report of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland, 8–17 June 2005; pp. 16–20.
- Opperman, A.; Venter, C.; Oosthuizen, W.; Thomson, R.; Vorster, H. Meta-analysis of the health effects of using the glycemic index in meal-planning. Br. J. Nutr. 2004, 92, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C.; Holt, S.H.; Pawlak, D.B.; Mc-Millan, J. Glycemic index and obesity. Am. J. Clin. Nutr. 2002, 76, 281–285. [Google Scholar] [CrossRef]
- Jovanovski, E.; Zurbau, A.; Vuksan, V. Carbohydrates and endothelial function: Is a low-carbohydrate diet or a low-glycaemic index diet favourable for vascular health? Clin. Nutr. Res. 2015, 4, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Koukiekolo, R.; Desseaux, V.; Moreau, Y.; Marchis-Mouren, G.; Santimone, M. Mechanism of porcine pancreatic α-amylase. Inhibition of amylose and maltopentaose hydrolysis by α-, β- and γ-cyclodextrins. Eur. J. Biochem. 2001, 268, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Desseaux, V.; Koukiekolo, R.; Moreau, Y.; Santimone, M.; Marchis-Mouren, G. Mechanism of porcine pancreatic α-amylase: Inhibition of amylose and maltopentaose hydrolysis by various inhibitors. Biol. Bratisl. 2002, 57, 163–170. Available online: http://biologia.savba.sk/section_c/Suppl_11/Desseaux.pdf (accessed on 28 November 2019).
- Oudjeriouat, N.; Moreau, Y.; Santimone, M.; Svennson, B.; Marchis-Mouren, G.; Desseaux, V. On the mechanism of α-amylase. Acarbose and cyclodextrin inhibition of barely amylase isozymes. Eur. J. Biochem. 2003, 270, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.B.; Greenwood, A.; Cascio, D.; Day, J.; McPherson, A. Refined molecular structure of pig pancreatic α-amylase at 2.1 Å resolution. J. Mol. Biol. 1994, 235, 1560–1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jin, Z.; Liu, L.; Wang, Z.; Li, F.; Sun, W.; Cai, H.; Chen, X.; Shen, W.; Zhu, Z.; et al. Inhibition of cyclodextrins on the activity of α-amylase. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 351–356. [Google Scholar] [CrossRef]
- Fletcher, E.N. The effect of alpha-cyclodextrin on acute blood lipid and glycemic responses to a fat containing meal. Wayne State Univ Thesis 2013, 231, 1–29. Available online: https://digitalcommons.wayne.edu/oa_theses (accessed on 28 November 2019).
- Jarosz, P.A.; Fletcher, E.; Elserafy, E.; Artiss, J.D.; Jen, K.L.C. The effect of α-cyclodextrin on postprandial lipid and glycemic responses to a fat-containing meal. Metab. Clin. Exp. 2013, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Inoue, Y.; Murata, I.; Nakata, D.; Terao, K.; Kanamoto, I. Effect of cyclodextrin on postprandial blood glucose and triglycerides. Int. J. Pharm. 2016, 6, 13–19. [Google Scholar]
- Gentilcore, D.; Vanis, L.; Teng, J.C.; Wishart, J.M.; Buckley, J.D.; Rayner, C.K.; Horowitz, M.; Jones, K.L. The oligosaccharide α-cyclodextrin has modest effects to slow gastric emptying and modify the glycaemic response to sucrose in healthy older adults. Br. J. Nutr. 2011, 106, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Scazzina, F.; Siebenhandl-Ehn, S.; Pellegrini, N. The effect of dietary fibre on reducing the glycaemic index of bread. Br. J. Nutr. 2013, 109, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the substantiation of health claims related to alpha-cyclodextrin and reduction of post-prandial glycaemic responses (ID 2926, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2713. [Google Scholar]
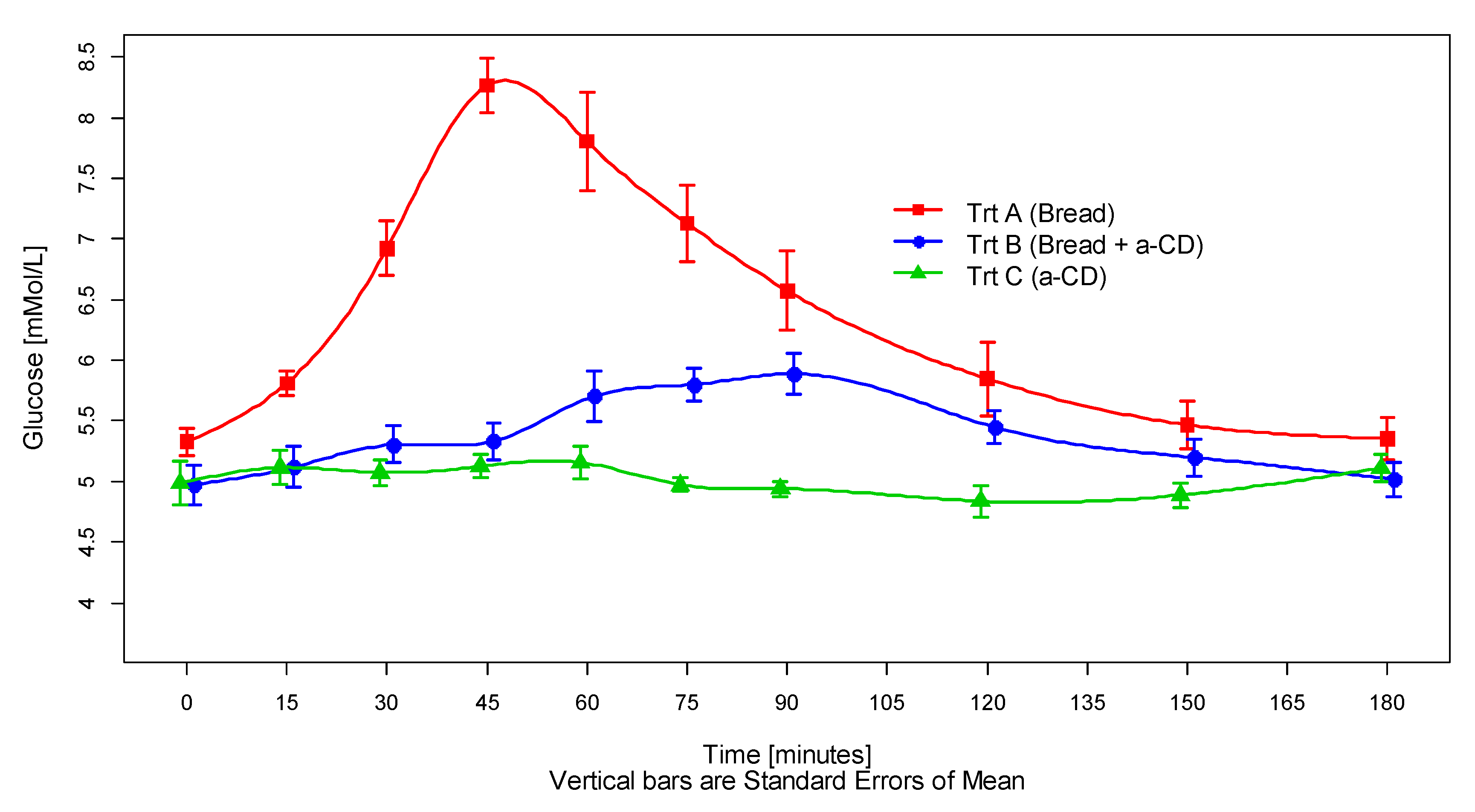
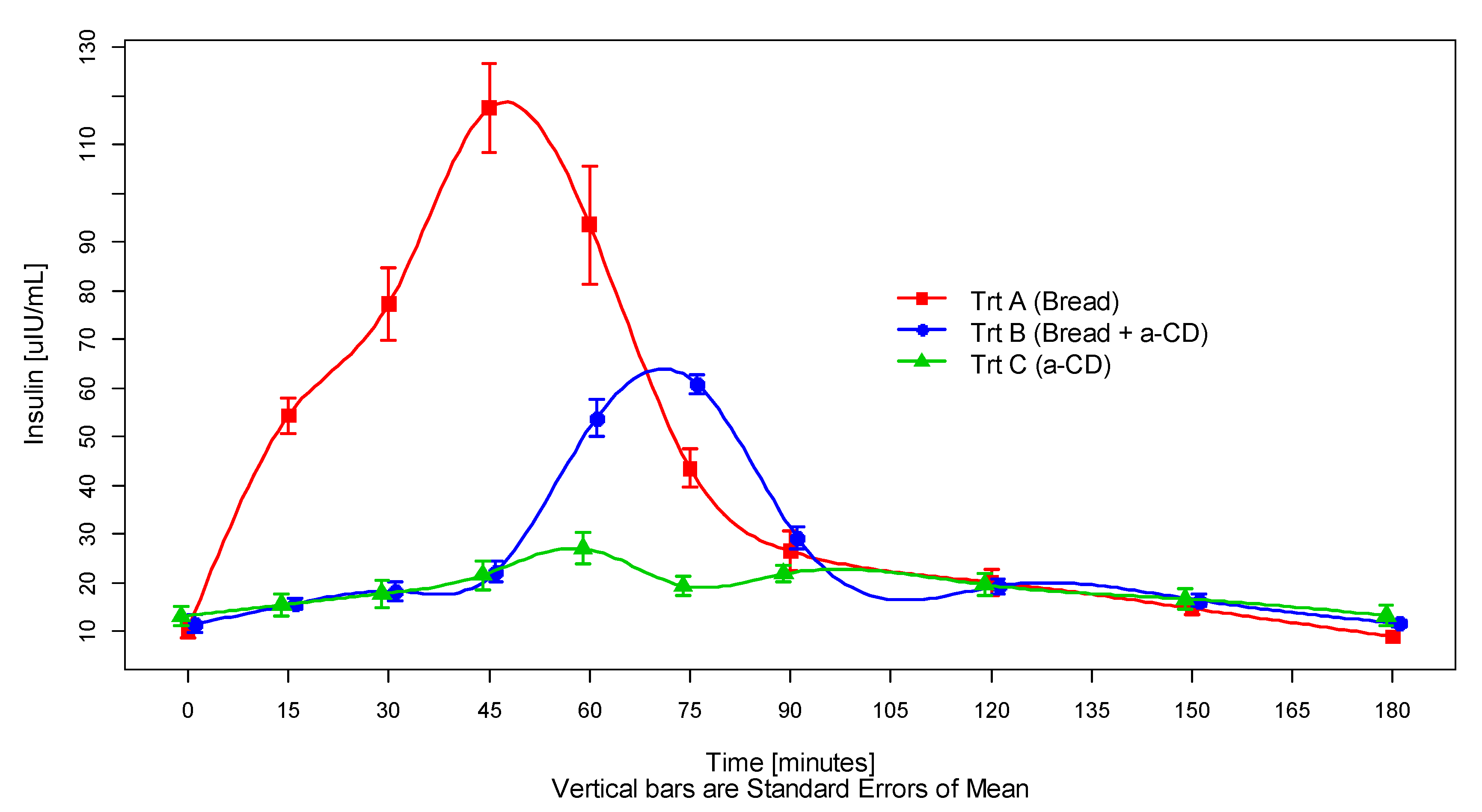
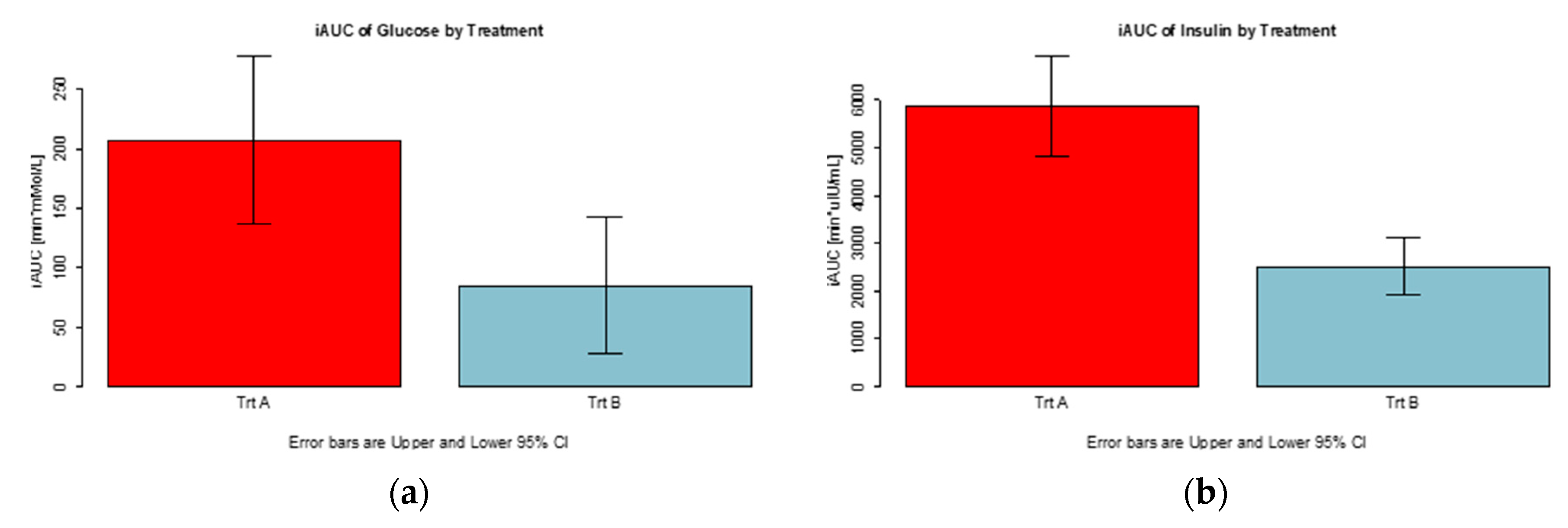
| Treatment | N | Glucose (mmol/L) | Insulin (μIU/mL) | ||||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||||
| Lower | Upper | Lower | Upper | ||||
| A | 12 | 5.33 | 5.09 | 5.57 | 10.3 | 6.5 | 13.0 |
| B | 12 | 4.97 | 4.62 | 5.33 | 11.6 | 7.6 | 15.6 |
| C | 12 | 4.99 | 4.60 | 5.38 | 13.1 | 8.6 | 17.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bär, A.; Diamantis, I.; Venetz, W.P. Alpha-Cyclodextrin Attenuates the Glycemic and Insulinemic Impact of White Bread in Healthy Male Volunteers. Foods 2020, 9, 62. https://doi.org/10.3390/foods9010062
Bär A, Diamantis I, Venetz WP. Alpha-Cyclodextrin Attenuates the Glycemic and Insulinemic Impact of White Bread in Healthy Male Volunteers. Foods. 2020; 9(1):62. https://doi.org/10.3390/foods9010062
Chicago/Turabian StyleBär, Albert, Ioannis Diamantis, and Werner P. Venetz. 2020. "Alpha-Cyclodextrin Attenuates the Glycemic and Insulinemic Impact of White Bread in Healthy Male Volunteers" Foods 9, no. 1: 62. https://doi.org/10.3390/foods9010062
APA StyleBär, A., Diamantis, I., & Venetz, W. P. (2020). Alpha-Cyclodextrin Attenuates the Glycemic and Insulinemic Impact of White Bread in Healthy Male Volunteers. Foods, 9(1), 62. https://doi.org/10.3390/foods9010062