Chemical-Sensory Traits of Fresh Cheese Made by Enzymatic Coagulation of Donkey Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk and Cheesemaking Trials
2.2. Chemical and Sensory Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langlois, B. The history, ethnology and social importance of mare’s milk consumption in Central Asia. J. Life Sci. 2011, 5, 863–872. [Google Scholar]
- Cunsolo, V.; Muccilli, V.; Fasoli, E.; Saletti, R.; Righetti, P.G.; Foti, S. Poppea’s bath liquor: The secret proteome of she-donkey’s milk. J. Proteom. 2011, 74, 2083–2099. [Google Scholar] [CrossRef] [PubMed]
- Salimei, E.; Fantuz, F. Equid milk for human consumption. Int. Dairy J. 2012, 24, 130–142. [Google Scholar] [CrossRef]
- Camillo, F.; Rota, A.; Biagini, L.; Tesi, M.; Fanelli, D.; Panzani, D. The current situation and trend of donkey industry in Europe. J. Equine Vet. Sci. 2018, 65, 44–49. [Google Scholar] [CrossRef]
- Giacometti, F.; Bardasi, L.; Merialdi, G.; Morbarigazzi, M.; Federici, S.; Piva, S.; Serraino, A. Shelf life of donkey milk subjected to different treatment and storage conditions. J. Dairy Sci. 2016, 99, 4291–4299. [Google Scholar] [CrossRef]
- Chiavari, C.; Coloretti, F.; Nanni, M.; Sorrentino, E.; Grazia, L. Use of donkey’s milk for a fermented beverage with lactobacilli. Le Lait 2005, 85, 481–490. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Donkey milk for manufacture of novel functional fermented beverages. J. Food Sci. 2005, 80, S1352–S1359. [Google Scholar] [CrossRef]
- Turchi, B.; Pedonese, F.; Torracca, B.; Fratini, F.; Mancini, S.; Galiero, A.; Montalbano, B.; Cerri, D.; Nuvoloni, R. Lactobacillus plantarum and Streptococcus thermophilus as starter cultures for a donkey milk fermented beverage. Int. J. Food Microbiol. 2017, 256, 54–61. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T. Studies on Equine Milk and Comparative Studies on Equine and Bovine Milk Systems. Ph.D. Thesis, University College Cork, Eire, Ireland, 2011. [Google Scholar]
- Tidona, F.; Criscione, A.; Devold, T.G.; Bordonaro, S.; Marletta, D.; Vegarud, G.E. Protein composition and micelle size of donkey milk with different protein patterns: Effects on digestibility. Int. Dairy J. 2014, 35, 57–62. [Google Scholar] [CrossRef]
- Luo, J.; Jian, S.; Wang, P.; Ren, F.; Wang, F.; Chen, S.; Guo, H. Thermal instability and characteristics of donkey casein micelles. Food Res. Int. 2019, 119, 436–443. [Google Scholar] [CrossRef]
- Chianese, L.; Calabrese, M.G.; Ferranti, P.; Mauriello, R.; Garro, G.; De Simone, C.; Quarto, M.; Addeo, F.; Cosenza, G.; Ramunno, L. Proteomic characterization of donkey milk “caseome”. J. Chromatogr. A 2010, 1217, 4834–4840. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G. Donkey cheese made through pure camel chymosin. Afr. J. Food Sci. 2015, 9, 421–425. [Google Scholar]
- Faccia, M.; Gambacorta, G.; Martemucci, G.; Natrella, G.; D’Alessandro, A.G. Technological attempts at producing cheese from donkey milk. J. Dairy Res. 2018, 85, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Faccia, M.; Trani, A.; Gambacorta, G.; Loizzo, P.; Cassone, A.; Caponio, F. Production technology and characterization of Fiordilatte cheese from ovine and caprine milk. J. Dairy Sci. 2015, 98, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, G.; Trani, A.; Fasciano, C.; Paradiso, V.M.; Faccia, M. Effects of prefermentative cold soak on polyphenols and volatiles of Aglianico, Primitivo and Nero di Troia red wines. Food Sci. Nutr. 2019, 7, 483–491. [Google Scholar] [CrossRef]
- Andrews, A.T. Proteinases in normal bovine milk and their action on caseins. J. Dairy Res. 1983, 50, 45–55. [Google Scholar] [CrossRef]
- Harper, J.; Meyer, M.; Knighton, D.; Lelievre, J. Effects of whey proteins on the proteolysis of Cheddar cheese slurries. (A model for the maturation of cheese made from ultrafiltered milk). J. Dairy Sci. 1989, 72, 333–341. [Google Scholar] [CrossRef]
- Egito, A.S.; Miclo, L.; Lopez, C.; Adam, A.; Girardet, J.-M.; Gaillard, J.-L. Separation and characterization of mares’ milk αs1-, β-, κ-caseins, γ-casein-like, and proteose peptone component 5-like peptides. J. Dairy Sci. 2002, 85, 697–706. [Google Scholar] [CrossRef]
- Egito, A.S.; Girardet, J.-M.; Poirson, C.; Mollè, D.; Humbert, G.; Miclo, L.; Gaillard, J.-L. Action of plasmin on equine β-casein. Int. Dairy J. 2003, 13, 813–820. [Google Scholar] [CrossRef]
- Piggott, J.R.; Simpson, S.J.; Williams, S.A.R. Sensory analysis. Int. J. Food Sci. Technol. 1998, 33, 7–18. [Google Scholar] [CrossRef]
- Drake, M.A.; Civille, G.V. Flavor lexicon. Compr. Rev. Food Sci. Food Saf. 2002, 2, 33–40. [Google Scholar] [CrossRef]
- ISO 11035. Sensory Analysis—Identification and Selection of Descriptors for Establishing A Sensory Profile by A Multidimensional Approach; International Organization for Standardization: Geneva, Switzerland, 1994. [Google Scholar]
- Trani, A.; Gambacorta, G.; Loizzo, P.; Cassone, A.; Faccia, M. Short communication: Chemical and sensory characteristics of Canestrato di Moliterno cheese manufactured in spring. J. Dairy Sci. 2016, 99, 6080–6085. [Google Scholar] [CrossRef]
- Jacob, M.; Jaros, D.; Rohm, H. Recent advances in milk clotting enzymes. Int. J. Dairy Technol. 2011, 64, 14–33. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T.; Chevalier, F.; Hem, S.; Fox, P.F.; Mulvihill, D.M. Proteomic comparison of equine and bovine milks on renneting. J. Agric. Food Chem. 2013, 61, 2839–2850. [Google Scholar] [CrossRef]
- Martemucci, G.; D’Alessandro, A.G. Fat content, energy value and fatty acid profile of donkey milk during lactation and implications for human nutrition. Lipids Health Dis. 2012, 11, 113. [Google Scholar] [CrossRef]
- Gastaldi, D.; Bertino, E.; Monti, G.; Baro, C.; Fabris, C.; Lezo, A.; Medana, C.; Baiocchi, C.; Mussap, M.; Galvano, F.; et al. Donkey’s milk detailed lipid composition. Front. Biosci. 2010, E2, 537–546. [Google Scholar]
- Martini, M.; Altomonte, I.; Salari, F. Amiata donkeys: Fat globule characteristics, milk gross composition and fatty acids. Ital. J. Anim. Sci. 2014, 13, 123–126. [Google Scholar] [CrossRef]
- Briard, V.; Leconte, N.; Michel, F.; Michalski, M.C. The fatty acid composition of small and large naturally occurring milk fat globules. Eur. J. Lipid Sci. Technol. 2003, 105, 677–682. [Google Scholar] [CrossRef]
- Fauquant, C.; Briard, V.; Leconte, N.; Michalski, M.C. Differently sized native milk fat globules separated by microfiltration: Fatty acid composition of the milk fat globule membrane and triglyceride core. Eur. J. Lipid Sci. Technol. 2005, 107, 80–86. [Google Scholar] [CrossRef]
- Conte, F.; Rapisarda, T.; Belvedere, G.; Carpino, S. Shelf-life del latte d’asina: Batteriologia e componente volatile. Ital. J. Food Saf. 2010, 7, 25–29. [Google Scholar] [CrossRef][Green Version]
- Tidona, F.; Charfi, I.; Povolo, M.; Pelizzola, V.; Carminati, D.; Contarini, G.; Giraffa, G. Fermented beverage emulsion based on donkey milk with sunflower oil. Int. J. Food Sci. Technol. 2015, 50, 2644–2652. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Cecchi, T.; Perinelli, D.R.; Pucciarelli, S.; Polzonetti, V.; Bonacucina, G.; Ariani, A.; Parrocchia, L.; Spera, D.M.; Ferretti, E.; et al. Effects of freeze-drying and spray-drying on donkey milk volatile compounds and whey proteins stability. LWT 2018, 88, 189–195. [Google Scholar] [CrossRef]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Sang, J.L.R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef]
- Nielsen, B.L.; Jerôme, N.; Saint-Albin, A.; Ouali, C.; Rochut, S.; Zins, E.L.; Briant, C.; Guettier, E.; Reigner, F.; Couty, I.; et al. Oestrus odours from rats and mares: Behavioural responses of sexually naive and experienced rats to natural odours and odorants. Appl. Anim. Behav. Sci. 2016, 176, 128–135. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Hao, G.; Yua, H.; Tiana, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Wu, S.; Jin, R.; Ren, W.; Sun, T. Profiles of Volatile flavor compounds in milk fermented with different proportional combinations of lactobacillus delbrueckii subsp. bulgaricus and streptococcus thermophiles. Molecules 2017, 22, 1633. [Google Scholar]
- Picon, A.; López-Pérez, O.; Torres, E.; Garde, S.; Nuñez, M. Contribution of autochthonous lactic acid bacteria to the typical flavour of raw goat milk cheeses. Int. J. Food Microbiol. 2019, 299, 8–22. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Xiao, Z.; Song, S.; Eric, K.; Jia, C.; Yu, H.; Zhu, J. Characterization of odor-active compounds of various cherry wines by gas chromatography–mass spectrometry, gas chromatography–olfactometry and their correlation with sensory attributes. J. Chromatogr. B 2011, 879, 2287–2293. [Google Scholar] [CrossRef]
- Morales, P.; Calzada, J.; Juez, C.; Nunez, M. Volatile compounds in cheeses made with Micrococcus sp. INIA 528 milk culture or high enzymatic activity curd. Int. J. Dairy Technol. 2010, 63, 538–543. [Google Scholar] [CrossRef]
- Fay, M.; Brattin, W.J.; Donohue, J.M. Public health statement. Toxicol. Ind. Health 1999, 15, 652–654. [Google Scholar] [CrossRef]
- Awad, S.; Lthi-Peng, Q.-Q.; Puhan, Z. proteolytic activities of suparen and rennilase on buffalo, cow, and goat whole casein and β-casein. J. Agric. Food Chem. 1999, 47, 3632–3639. [Google Scholar] [CrossRef]
- Van Hekken, D.L.; Thompson, M.P. Application of PhastSystem® to the resolution of bovine milk proteins on urea-polyacrylamide gel electrophoresis. J. Dairy Sci. 1992, 75, 1204–1210. [Google Scholar] [CrossRef]
- Plagemann, I.; Zelena, K.; Krings, U.; Berger, R.G. Volatile flavours in raw egg yolk of hens fed on different diets. J. Sci. Food Agric. 2011, 91, 2061–2065. [Google Scholar] [CrossRef]



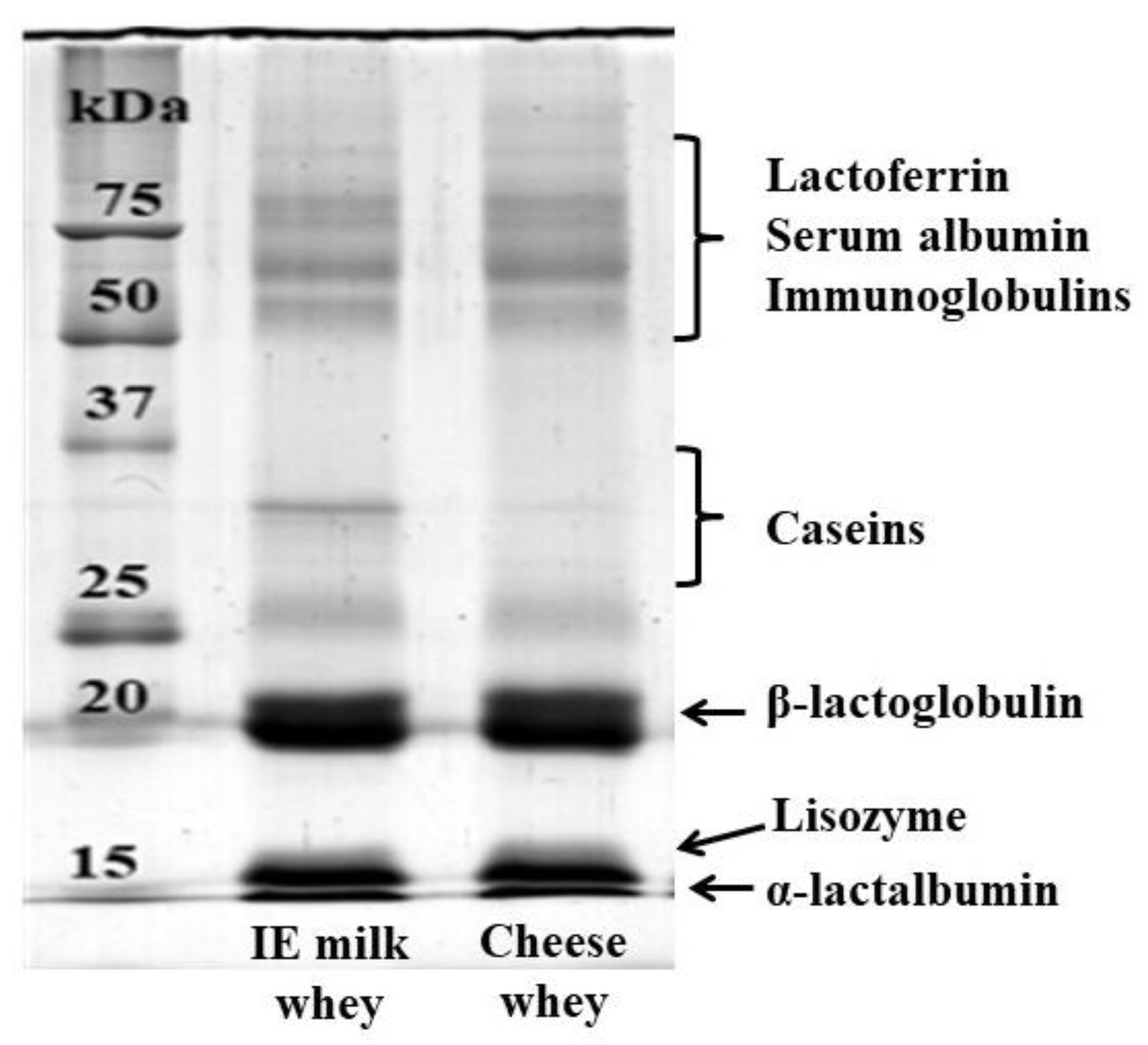
| Item | Donkey | Cow (Control) | ||
|---|---|---|---|---|
| Milk | Cheese | Milk | Cheese | |
| pH | 7.01 ± 0.03 a | 5.85 ± 0.10 c | 6.71 ± 0.02 b | 5.61 ± 0.06 d |
| Total Solids% | n.a. | 34.19 ± 2.64 b | n.a. | 49.88 ± 1.16 a |
| Protein% | 2.21 ± 0.11 d | 25.87 ± 3.37 a | 3.33 ± 0.07 c | 17.29 ± 2.96 b |
| Fat% | 0.33 ± 0.08 d | 2.90 ± 0.11 c | 3.62 ± 0.39 b | 23.76 ± 3.11 a |
| Lactose% | 6.76 ± 0.07 a | n.a. | 4.91 ± 0.02 b | n.a. |
| Ash% | 0.39 ± 0.06 d | 3.31 ± 0.30 b | 0.81 ± 0.05 c | 4.83 ± 0.44 a |
| Total processing time *, min | 42.10 ± 6.82 a | 13.20 ± 0.20 b | ||
| Cheese yield% | 7.21 ± 0.76 b | 23.52 ± 1.50 a | ||
| Item | Donkey | Cow (Control) | ||||
|---|---|---|---|---|---|---|
| Milk | Cheese | Whey | Milk | Cheese | Whey | |
| Total fat content | 0.33 ± 0.08 | 2.90 ± 0.11 | <0.1 | 3.62 ± 0.39 | 23.76 ± 3.11 | 0.28 ± 0.10 |
| Butyric Acid | 0.22 ± 0.11 | 0.19 ± 0.09 | n.d. | 4.99 ± 0.05 | 4.85 ± 0.11 | 5.19 ± 0.23 |
| Caproic Acid | 0.40 ± 0.21 | 0.72 ± 0.66 | n.d. | 2.73 ± 0.21 | 2.91 ± 0.19 | 2.03 ± 1.21 |
| Caprylic Acid | 3.69 ± 1.27 a | 1.50 ± 0.19 b | 2.71 ± 0.90 a | 1.29 ± 0.24 | 1.44 ± 0.34 | 1.51 ± 0.54 |
| Capric Acid | 7.85 ± 1.22 a | 4.08 ± 0.10 b | 6.00 ± 1.73 a | 2.41 ± 0.19 | 2.55 ± 0.49 | 2.92 ± 0.55 |
| Undecanoic Acid | 1.02 ± 0.22 a | 0.30 ± 0.12 b | 1.80 ± 0.64 a | 0.81 ± 0.11 | 0.73 ± 0.15 | 0.33 ± 0.39 |
| Lauric Acid | 7.56 ± 0.40 a | 4.07 ± 0.09 b | 6.74 ± 1.78 a | 4.21 ± 0.58 | 4.40 ± 0.27 | 4.33 ± 0.61 |
| Tridecanoic Acid | 0.03 ± 0.06 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Myristic Acid | 6.07 ± 0.62 b | 12.16 ± 0.33 a | 7.77 ± 4.77 a,b | 11.94 ± 0.31 | 12.41 ± 0.77 | 13.07 ± 1.00 |
| Myristoleic Acid | 0.29 ± 0.07 b | 1.15 ± 0.46 a | n.d. | 0.49 ± 0.11 | 0.32 ± 0.21 | 0.44 ± 0.33 |
| Pentadecanoic Acid | 0.29 ± 0.37 b | 1.19 ± 0.46 a | n.d. | 0.90 ± 0.23 | 0.70 ± 0.31 | 0.61 ± 0.42 |
| Palmitic Acid | 19.08 ± 0.34 b | 33.08 ± 0.60 a | 21.37 ± 1.47 b | 28.45 ± 0.21 | 30.18 ± 0.37 | 29.41 ± 3.17 |
| Palmitoleic Acid | 2.27 ± 0.11 a | 1.97 ± 0.08 b | 2.48 ± 1.66 a,b | 1.37 ± 0.05 | 1.01 ± 0.49 | 1.78 ± 0.52 |
| Margaric Acid | 0.29 ± 0.20 | 0.71 ± 0.26 | n.d. | 0.45 ± 0.02 | 0.38 ± 0.12 | n.d. |
| Eptadecenoic Acid | 0.34 ± 0.46 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Stearic Acid | 1.87 ± 0.47 c | 8.80 ± 0.15 a | 5.18 ± 3.14 b | 10.46 ± 0.17 b | 13.09 ± 1.22 a | 9.67 ± 0.94 b |
| Elaidic Acid | n.d. | 0.95 ± 0.16 | n.d. | 0.84 ± 0.30 | 0.54 ± 0.23 | 0.89 ± 0.21 |
| Oleic Acid | 24.46 ± 1.96 | 21.84 ± 2.01 | 24.34 ± 0.84 | 23.61 ± 0.91 | 20.08 ± 1.88 | 22.57 ± 1.90 |
| Linoleic Acid | 18.27 ± 0.25 a | 5.10 ± 0.13 b | 16.54 ± 3.46 a | 3.05 ± 0.56 | 2.80 ± 0.41 | 3.56 ± 0.68 |
| Linolenic Acid | 5.32 ± 0.16 a | 1.45 ± 1.66 b | 5.07 ± 2.56 a,b | 1.21 ± 0.11 | 0.97 ± 0.05 | 1.69 ± 0.97 |
| Arachidic acid | 0.09 ± 0.05 b | 0.21 ± 0.05 a | n.d. | 0.11 ± 0.04 | 0.23 ± 0.09 | n.d. |
| Gadoleic Acid | 0.24 ± 0.16 | 0.52 ± 0.46 | n.d. | 0.30 ± 0.11 | 0.41 ± 0.15 | n.d. |
| Eicosadienoic Acid | 0.29 ± 0.07 | n.d. | n.d. | 0.38 ± 0.01 | n.d. | n.d. |
| Mean saturated | 48.46 | 67.01 | 51.57 | 70.35 | 73.87 | 68.27 |
| Mean monounsaturated | 27.60 | 26.43 | 26.82 | 26.61 | 21.95 | 25.68 |
| Mean polyunsaturated | 23.92 | 6.55 | 21.61 | 4.64 | 4.18 | 5.25 |
| Compoun.d. | Donkey | Cow (Control) | ||
|---|---|---|---|---|
| Milk | Cheese | Milk | Cheese | |
| Acids | ||||
| acetic | 5.65 ± 0.03 b | 0.19 ± 0.01 d | 2.69 ± 0.17 c | 66.03 ± 5.74 a |
| butanoic | n.d. | 0.36 ± 0.03 c | 5.09 ± 0.24 b | 8.77 ± 1.05 a |
| hexanoic | n.d. | 0.55 ± 0.10 c | 7.55 ± 0.87 b | 10.73 ± 0.82 a |
| heptanoic | n.d. | n.d. | n.d. | 0.68 ± 0.09 |
| octanoic | n.d. | 0.29 ± 0.07 c | 3.91 ± 0.33 b | 5.02 ± 0.22 a |
| nonanoic | n.d. | n.d. | 1..44 ± 0.03 b | 2.10 ± 0.07 a |
| decanoic | n.d. | n.d. | 3.22 ± 0.40 | 3.01 ± 0.44 |
| Alcohols | ||||
| ethanol | n.d. | n.d. | n.d. | 17.46 ± 2.07 |
| 2-ethylhexanol | n.d. | 7.16 ± 0.12 | n.d. | n.d. |
| isoamylalcohol | n.d. | 0.42 ± 0.02 | n.d. | n.d. |
| 3-methyl,1-butanol | n.d. | n.d. | n.d. | 71.84 ± 6.47 |
| 3-methyl-2-buten-1-ol | n.d. | n.d. | n.d. | 1.19 ± 0.52 |
| 1-nonanol | n.d. | 0.16 ± 0.01 | n.d. | n.d. |
| 2-octyloxy-ethanol | n.d. | n.d. | n.d. | 16.26 ± 3.62 |
| phenylethyl alcohol | n.d. | n.d. | n.d. | 1.92 ± 0.09 |
| 2-propyl-1-pentanol | n.d. | n.d. | 1.48 ± 0.39 a | 0.47 ± 0.16 b |
| Aldheydes | ||||
| diacetyl (2,3-butanedione) | n.d. | n.d. | n.d. | 27.35 ± 2.70 |
| hexanal | n.d. | n.d. | 1.04 ± 0.05 b | 5.33 ± 0.19 a |
| heptanal | n.d. | n.d. | 0.27 ± 0.07 b | 1.02 ± 0.10 a |
| octanal | n.d. | n.d. | 0.48 ± 0.13 b | 1.77 ± 0.51 a |
| nonanal | 0.02 ± 0.01 d | 0.39 ± 0.08 c | 3.22 ± 1.26 b | 17.97 ± 3.97 a |
| decanal | n.d. | n.d. | 2.05 ± 1.11 | 2.54 ± 0.33 |
| 2-methylbutanal | 0.08 ± 0.01 | 8.79 ± 0.37 | n.d. | n.d. |
| 3-methylbutanal | n.d. | 0.15 ± 0.01 | n.d. | 63.66 ± 0.29 |
| 2-ethylhexanal | 0.09 ± 0.11 | 0.71 ± 0.02 | n.d. | n.d. |
| Esters | ||||
| ethylacetate | 0.09 ± 0.01 b | 0.08 ± 0.03 b | n.d. | 17.83 ± 5.72 a |
| 1-butanol-3-methyl-acetate | n.d. | n.d. | n.d. | 1.03 ± 0.58 |
| Ketones | ||||
| acetone | 2.84 ± 0.03 c | 0.65 ± 0.01 d | 59.11 ± 9.11 a | 31.55 ± 0.29 b |
| 2-butanone | 0.03 ± 0.01 d | 0.14 ± 0.02 c | 4.21 ± 1.21 b | 13.78 ± 2.85 a |
| 2-heptanone | 0.09 ± 0.02 d | 0.37 ± 0.01 c | 4.81 ± 0.93 b | 16.09 ± 3.74 a |
| 3-heptanone | 0.07 ± 0.01 | n.d. | n.d. | n.d. |
| 2-nonanone | n.d. | 4.37 ± 0.47 | ||
| 2-un.d.ecanone | n.d. | 0.85 ± 0.23 | ||
| 3-hydroxy 2-butanone (acetoin) | 0.07 ± 0.02 c | 11.93 ± 0.04 b | n.d. | 91.71 ± 23.70 a |
| 6-methyl-5-hepten-2-one | 0.15 ± 0.04 d | 0.38 ± 0.05 c | 0.88 ± 0.29 b | 1.83 ± 0.18 a |
| 2,3 pentanedione | n.d. | n.d. | 0.60 ± 0.38 b | 23.56 ± 6.20 a |
| Others | ||||
| dimethyl sulfide | n.d. | n.d. | 0.12 ± 0.07 b | 1.33 ± 0.40 a |
| dimethyl sulfone | n.d. | 0.26 ± 0.01 b | 1.97 ± 0.28 a | 1.83 ± 0.19 a |
| hexane | n.d. | n.d. | 1.27 ± 0.53 b | 2.98 ± 0.81 a |
| heptane | n.d. | n.d. | 0.77 ± 0.12 b | 7.20 ± 1.00 a |
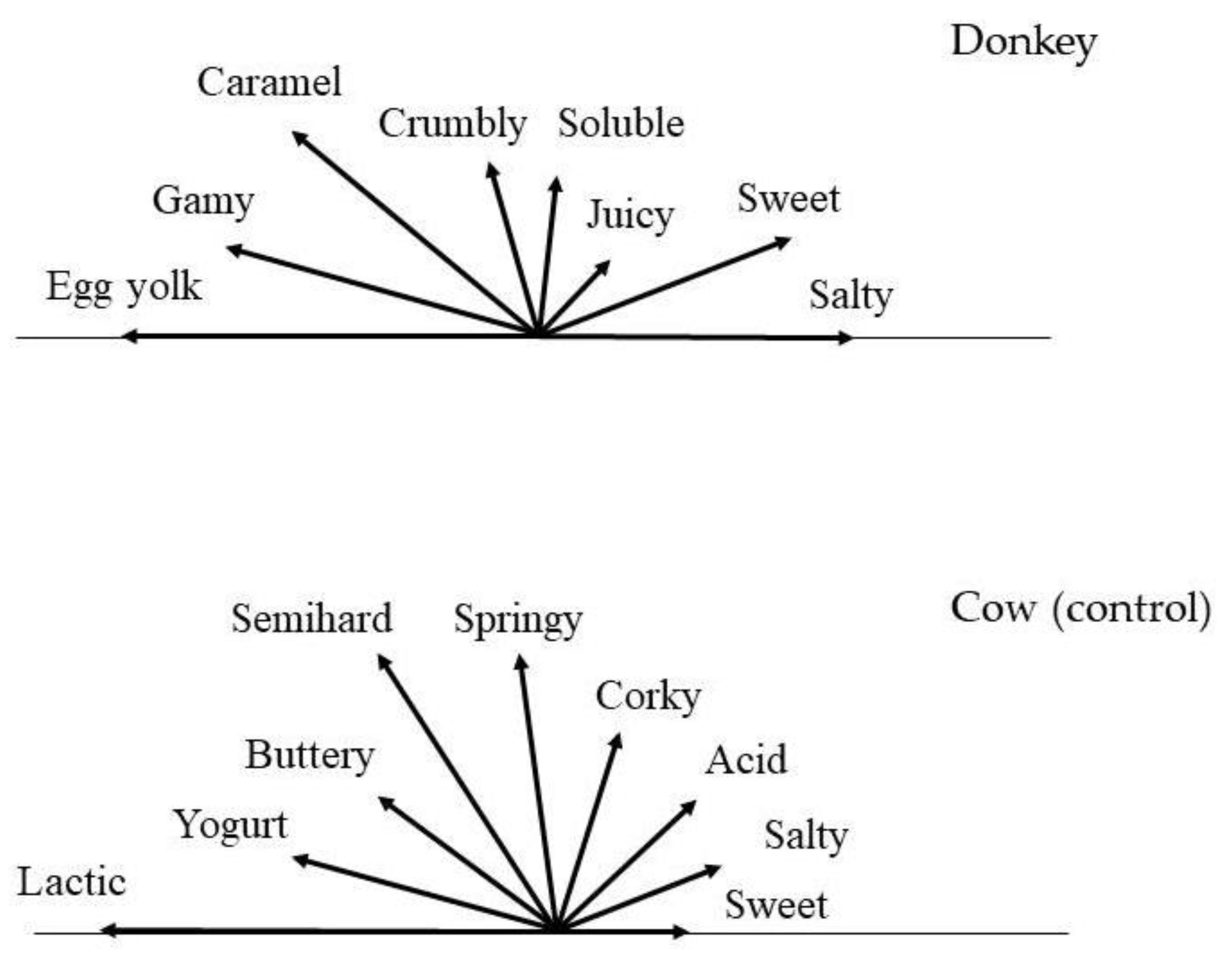
| Descriptor | Reference Product | Donkey | Cow (Control) |
|---|---|---|---|
| Colour | |||
| White | + | ||
| Ivory white | + | ||
| Texture | |||
| Corky | Rubber stopper | + | |
| Crumbly | Feta cheese | + | |
| Semi-hard | Carrot | + | |
| Soluble | Meringue | + | |
| Springy | Edam cheese | + | |
| Juicy | Water melon | + | |
| Aroma | |||
| Buttery | Butter | + | |
| Caramel | Cooked sugar | + | |
| Egg yolk | Raw egg yolk | + | |
| Gamy | Fresh donkey milk | + | |
| Lactic | Fresh uncultured milk | + | |
| Yogurt | Plain yogurt | + | |
| Taste | |||
| Salty | 0.5% NaCl in water | + | + |
| Sweet | 0.5% sucrose in water | + | + |
| Acid | 0.5% citric acid | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccia, M.; Gambacorta, G.; Martemucci, G.; Difonzo, G.; D’Alessandro, A.G. Chemical-Sensory Traits of Fresh Cheese Made by Enzymatic Coagulation of Donkey Milk. Foods 2020, 9, 16. https://doi.org/10.3390/foods9010016
Faccia M, Gambacorta G, Martemucci G, Difonzo G, D’Alessandro AG. Chemical-Sensory Traits of Fresh Cheese Made by Enzymatic Coagulation of Donkey Milk. Foods. 2020; 9(1):16. https://doi.org/10.3390/foods9010016
Chicago/Turabian StyleFaccia, Michele, Giuseppe Gambacorta, Giovanni Martemucci, Graziana Difonzo, and Angela Gabriella D’Alessandro. 2020. "Chemical-Sensory Traits of Fresh Cheese Made by Enzymatic Coagulation of Donkey Milk" Foods 9, no. 1: 16. https://doi.org/10.3390/foods9010016
APA StyleFaccia, M., Gambacorta, G., Martemucci, G., Difonzo, G., & D’Alessandro, A. G. (2020). Chemical-Sensory Traits of Fresh Cheese Made by Enzymatic Coagulation of Donkey Milk. Foods, 9(1), 16. https://doi.org/10.3390/foods9010016








