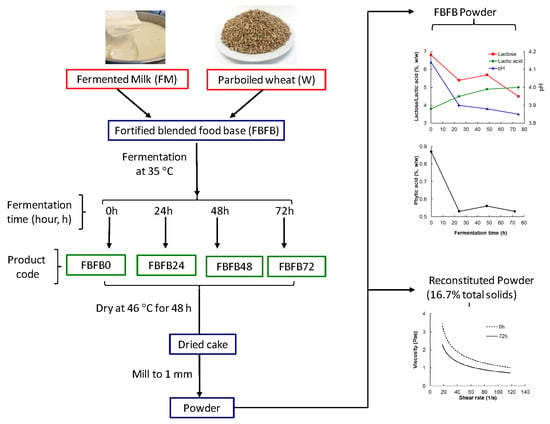
Fortified Blended Food Base: Effect of Co-Fermentation Time on Composition, Phytic Acid Content and Reconstitution Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Ingredients
2.2. Preparation of Fermented Milk (FM) and Parboiled Wheat (PW)
2.3. Preparation of Fortified Blended Food Base (FBFB)
2.4. Composition of FBFB Powders
2.5. Microbiology of the FBFB Wet Blend and Powders
2.6. Analysis of Reconstituted FBFB Powder (R-FBFB)
2.6.1. Gelatinization temperature
2.6.2. Water Holding Capacity (WHC)
2.6.3. Soluble Starch (SSC)
2.6.4. Pasting Behaviour
2.6.5. Rheology
2.6.6. Flowability
2.7. Statistics
3. Results
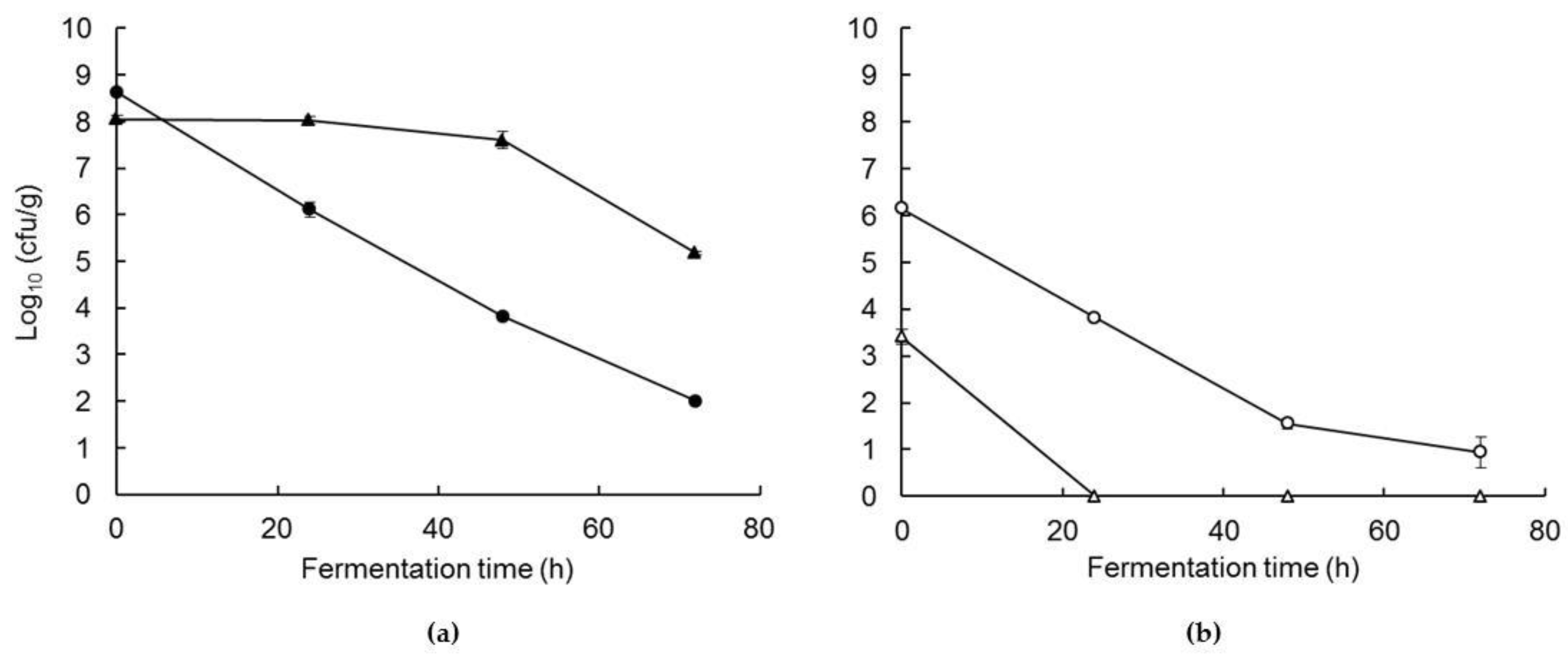
3.1. Changes in Starter Bacteria During Co-Feremntation and Drying
3.2. Properties of the FBFB Powders
Composition and pH
3.3. Properties of Reconstituted Fortified Blended Food Base (R-FBFB)
3.3.1. Gelatinization, Water holding Capacity and Starch Solubilisation on Cooking and Cooling
3.3.2. Pasting Behaviour
3.3.3. Rheology and Flowability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; ESA Working Paper; FAO: Rome, Italy, 2012; Available online: http://www.fao.org/fileadmin/templates/esa/Global_persepctives/world_ag_2030_50_2012_rev.pdf/ (accessed on 31 July 2019).
- Michaelsen, K.F.; Hoppe, C.; Roos, N.; Kaestel, P.; Stougaard, M.; Lauritzen, L.; Mølgaard, C.; Girma, T.; Friis, H. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr. Bull. 2009, 30, S343–S404. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef] [PubMed]
- World Food Programme. Technical Specifications for the Manufacture of: Super Cereal Plus—Wheat Soya Blend. Version 15.1. Available online: https://documents.wfp.org/stellent/groups/public/documents/manual_guide_proced/wfp281208.pdf (accessed on 31 July 2019).
- Tamime, A.Y.; O’Connor, T.P. Kishk—A dried fermented milk/cereal mixture. Int. Dairy J. 1995, 5, 109–128. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Muir, D.D.; Khaskheli, M.; Barclay, M.N.I. Effect of processing conditions and raw materials on the properties of kishk 1. Compositional and microbiological qualities. LWT-Food Sci. Technol. 2000, 33, 444–451. [Google Scholar] [CrossRef]
- Simsek, S.; Martinez, M.O.; Daglioglu, O.; Guner, K.G.; Gecgel, U. Physicochemical properties of starch from a cereal-based fermented food (tarhana). J. Nutr. Food Sci. 2014, 4, 263. [Google Scholar]
- Nassar, K.S.; Shamsia, S.M.; Attia, I.A. Improvement of the nutritional value of cereal fermented milk: 1. Soft kishk like. J. Food Process. Technol. 2016, 7, 619. [Google Scholar]
- Toufeili, I.; Melki, C.; Shadarevian, S.; Robinson, R.K. Some nutritional and sensory properties of bulgur and whole wheatmeal kishk (a fermented milk-wheat mixture). Food Qual. Prefer. 1998, 10, 9–15. [Google Scholar] [CrossRef]
- Rahmawati, I.S.; Suntornsuk, W. Effects of fermentation and storage on bioactive activities in milks and yoghurts. Procedia Chem. 2016, 18, 53–62. [Google Scholar] [CrossRef]
- Graulet, B.; Martin, B.; Agabriel, C.; Girard, C.L. Vitamins in milks. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; Wiley-Blackwell, John Wiley and Sons Ltd.: West Sussex, UK, 2013; pp. 172–199. ISBN 978-0-470-67418-5. [Google Scholar]
- Heyman, M.B. Lactose intolerance in infants, children, and adolescents. Pediatrics 2006, 118, 1279–1286. [Google Scholar] [CrossRef]
- Greiner, R.; Konietzny, U. Phytase for food application. Food Technol. Biotechnol. 2006, 44, 125–140. [Google Scholar]
- Hoff-Jørgensen, E.; Andersen, O.; Nielsen, G. The effect of phytic acid on the absorption of calcium and phosphorus: 3. In children. Biochem. J. 1946, 40, 555. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Sandberg, A.-S.; Sandström, B.; Kunz, C. Inhibitory effects of phytic acid and other inositol phosphates on zinc and calcium absorption in suckling rats. J. Nutr. 1989, 119, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Konietzny, U.; Coppola, R.; Sorrentino, E.; Greiner, R. The importance of lactic acid bacteria for phytate degradation during cereal dough fermentation. J. Agric. Food Chem. 2007, 55, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, M.; Certel, M.; Uslu, M.K. Microbiological and chemical properties of tarhana during fermentation and storage as wet—Sensorial properties of tarhana soup. LWT-Food Sci. Technol. 2005, 38, 409–416. [Google Scholar] [CrossRef]
- Ekinci, R. The effect of fermentation and drying on the water-soluble vitamin content of tarhana, a traditional Turkish cereal food. Food Chem. 2005, 90, 127–132. [Google Scholar] [CrossRef]
- Bilgiçli, N.; İbanoğlu, Ş. Effect of wheat germ and wheat bran on the fermentation activity, phytic acid content and colour of tarhana, a wheat flour-yoghurt mixture. J. Food Eng. 2007, 78, 681–686. [Google Scholar] [CrossRef]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. The impact of food viscosity on eating rate, subjective appetite, glycemic response and gastric emptying rate. PLoS ONE 2013, 8, e67482. [Google Scholar] [CrossRef]
- Day, L.; Golding, M. Food structure, rheology, and texture. In Reference Module in Food Science, Encylopedia of Food Chemistry; Smithers, G.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 125–129. Available online: http://dx.doi.org/10.1016/B978-0-08-100596-5.03412-0/ (accessed on 14 June 2019).
- Campbell, C.L.; Wagoner, T.B.; Foegeding, E.A. Designing foods for satiety: The roles of food structure and oral processing in satiation and satiety. Food Struct. 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Shevade, A.V.; O’Callaghan, Y.C.; O’Brien, N.M.; O’Connor, T.P.; Guinee, T.P. The proportion of fermented milk in dehydrated fermented milk− parboiled wheat composites significantly affects their composition, pasting behaviour, and flow properties on reconstitution. Foods 2018, 7, 113. [Google Scholar] [CrossRef]
- ISO/IDF. Yoghurt-Enumeration of Characteristic Microorganisms: Colony-Count Technique at 37 °C; SO 7889:2003 (IDF 117: 2003); International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Shevade, A.V.; O’Callaghan, Y.C.; Kennedy, D.; O’Brien, N.M.; O’Connor, T.P.; Guinee, T.P. Cereal type significantly affects the composition and reconstitution characteristics of dried fermented milk-cereal composites. J. Sci. Food Agric. 2019, 99, 3097–3105. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide. Version 9.3; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Adamberg, K.; Kask, S.; Laht, T.-M.; Paalme, T. The effect of temperature and ph on the growth of lactic acid bacteria: A ph-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef]
- Bielecka, M.; Majkowska, A. Effect of spray drying temperature of yoghurt on the survival of starter cultures, moisture content and sensoric properties of yoghurt powder. Food/Nahrung 2000, 44, 257–260. [Google Scholar] [CrossRef]
- Santos, G.D.; Nogueira, R.I.; Rosenthal, A. Powdered yoghurt produced by spray drying and freeze drying: A review. Braz. J. Food Technol. 2018, 21, e2016127. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Tamime and Robinson’s Yoghurt: Science and Technology; Woodhead Publishing Ltd.: Cambridge, UK, 2007. [Google Scholar]
- Erbaş, M.; Uslu, M.K.; Erbaş, M.O.; Certel, M. Effects of fermentation and storage on the organic and fatty acid contents of tarhana, a turkish fermented cereal food. J. Food Compos. Anal. 2006, 19, 294–301. [Google Scholar] [CrossRef]
- O’Callaghan, Y.C.; Shevade, A.V.; Guinee, T.P.; O’Connor, T.P.; O’Brien, N.M. Comparison of the nutritional composition of experimental fermented milk: Wheat bulgur blends and commercially available kishk and tarhana products. Food Chem. 2019, 278, 110–118. [Google Scholar] [CrossRef]
- Leenhardt, F.; Levrat-Verny, M.-A.; Chanliaud, E.; Rémésy, C. Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J. Agric. Food Chem. 2005, 53, 98–102. [Google Scholar] [CrossRef]
- Magallanes-López, A.M.; Hernandez-Espinosa, N.; Velu, G.; Posadas-Romano, G.; Ordoñez-Villegas, V.M.G.; Crossa, J.; Ammar, K.; Guzmán, C. Variability in iron, zinc and phytic acid content in a worldwide collection of commercial durum wheat cultivars and the effect of reduced irrigation on these traits. Food Chem. 2017, 237, 499–505. [Google Scholar] [CrossRef]
- Majzoobi, M.; Pashangeh, S.; Farahnaky, A.; Eskandari, M.H.; Jamalian, J. Effect of particle size reduction, hydrothermal and fermentation treatments on phytic acid content and some physicochemical properties of wheat bran. J. Food Sci. Technol. 2014, 51, 2755–2761. [Google Scholar] [CrossRef]
- Himmelsbach, D.S.; Manful, J.T.; Coker, R.D. Changes in rice with variable temperature parboiling: Thermal and spectroscopic assessment. Cereal Chem. 2008, 85, 384–390. [Google Scholar] [CrossRef]
- Sittipod, S.; Shi, Y.-C. Changes of starch during parboiling of rice kernels. J. Cereal Sci. 2016, 69, 238–244. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice starch diversity: Effects on structural, morphological, thermal, and physicochemical properties—A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Kett, A.P.; Chaurin, V.; Fitzsimons, S.M.; Morris, E.R.; O’Mahony, J.A.; Fenelon, M.A. Influence of milk proteins on the pasting behaviour and microstructural characteristics of waxy maize starch. Food Hydrocoll. 2013, 30, 661–671. [Google Scholar] [CrossRef]
- Chiotelli, E.; Pilosio, G.; Le Meste, M. Effect of sodium chloride on the gelatinization of starch: A multimeasurement study. Biopolymers 2002, 63, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Samutsri, W.; Suphantharika, M. Effect of salts on pasting, thermal, and rheological properties of rice starch in the presence of non-ionic and ionic hydrocolloids. Carbohydr. Polym. 2012, 87, 1559–1568. [Google Scholar] [CrossRef]
- Majzoobi, M.; Beparva, P. Effects of acetic acid and lactic acid on physicochemical characteristics of native and cross-linked wheat starches. Cereal Chem. 2014, 147, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Kasai, M.; Shimada, A.; Hatae, K. Effects of acetic acid on the rice gelatinization and pasting properties of rice starch during cooking. Food Res. Int. 2007, 40, 224–231. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Hirashima, M.; Takahashi, R.; Nishinari, K. Effects of adding acids before and after gelatinization on the viscoelasticity of cornstarch pastes. Food Hydrocoll. 2005, 19, 909–914. [Google Scholar] [CrossRef]
- İbanoğlu, Ş.; İbanoğlu, E. Rheological properties of cooked tarhana, a cereal-based soup. Food Res. Int. 1999, 32, 29–33. [Google Scholar] [CrossRef]
- Muir, D.D.; Tamime, A.Y.; Khaskheli, M. Effect of processing conditions and raw materials on the properties of kishk 2. Sensory profile and microstructure. LWT-Food Sci. Technol. 2000, 33, 452–461. [Google Scholar] [CrossRef]
- Flood, J.E.; Rolls, B.J. Soup preloads in a variety of forms reduce meal energy intake. Appetite 2007, 49, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Rothacker, D. Beverage viscosity is inversely related to postprandial hunger in humans. Physiol. Behav. 2001, 74, 551–557. [Google Scholar] [CrossRef]

| Item | Fermentation Time | ||||
|---|---|---|---|---|---|
| Code: | 0 FBFB0 | 24 FBFB24 | 48 FBFB48 | 72 FBFB72 | |
| Powder composition | |||||
| Dry matter (%, w/w) | 93.83 a | 93.44 a | 93.65 a | 93.21 a | |
| Moisture (%, w/w) | 6.17 a | 6.56 a | 6.35 a | 6.79 a | |
| Protein (%, w/w) | 19.05 a | 18.88 a | 19.05 a | 19.19 a | |
| Fat (%, w/w) | 6.09 a | 6.07 a | 6.23 a | 6.01 a | |
| Salt (%, w/w) | 0.56 a | 0.59 a | 0.64 a | 0.62 a | |
| Starch (%, w/w) | 37.33 a | 36.36 a | 38.33 a | 37.41 a | |
| Lactose (%, w/w) | 6.82 a | 5.43 b | 5.71 bc | 4.54 c | |
| Galactose (%, w/w) | 3.60 b | 4.18 ab | 4.21 ab | 4.80 a | |
| Lactic acid (%, w/w) | 3.75 c | 4.47 b | 4.87 ab | 5.01 a | |
| Glucose (%, w/w) | 0.09 a | 0.06 b | 0.05 b | 0.05 b | |
| Maltose (%, w/w) | 0.00 a | 0.00 a | 0.00 a | 0.00 a | |
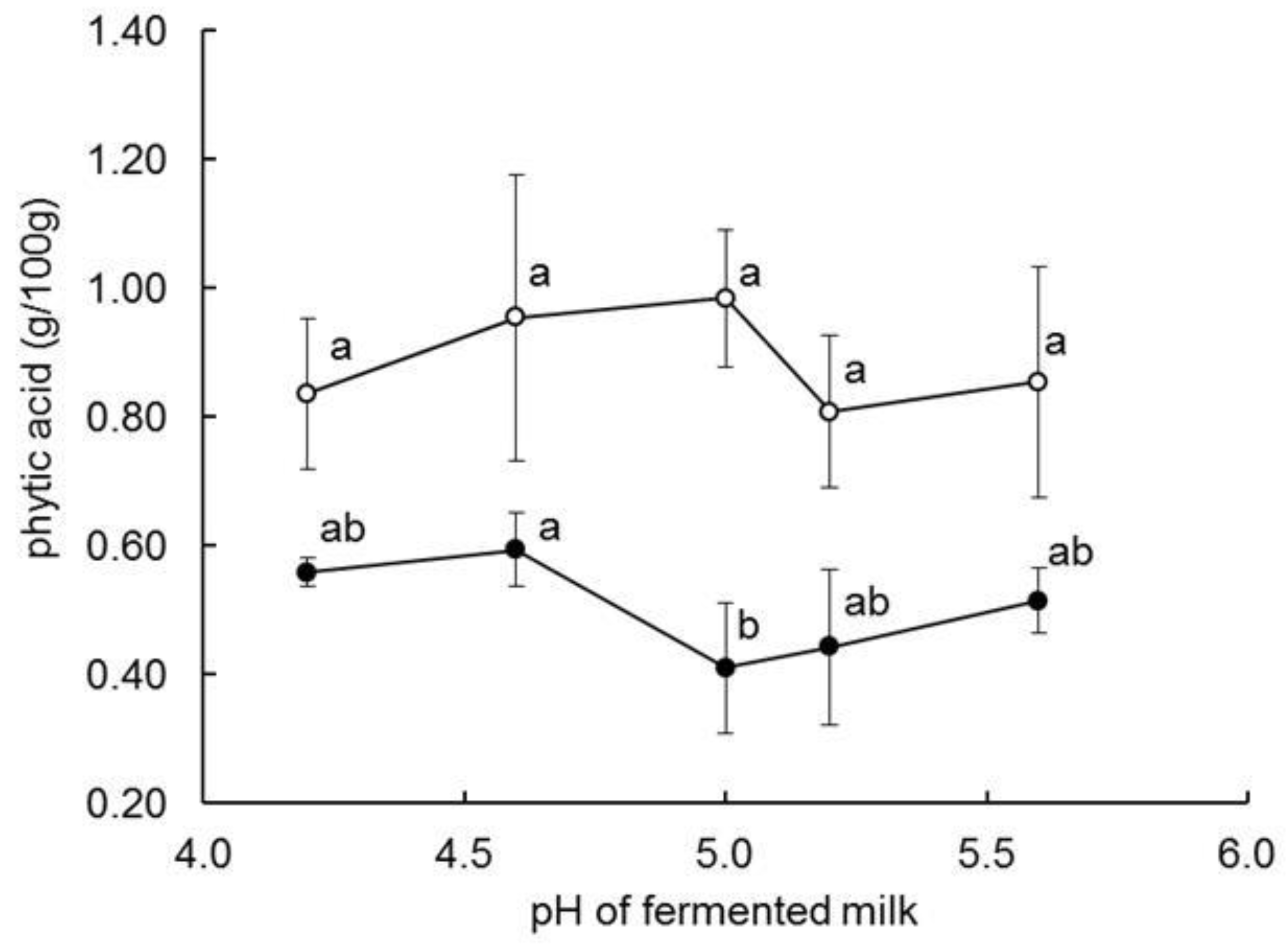
| Phytic acid (%, w/w) | 0.87 a | 0.53 b | 0.56 b | 0.53 b | |
| pH | 4.14 a | 3.94 b | 3.88 b | 3.85 b | |
| Item | Co-Fermentation Time (h) | ||||
|---|---|---|---|---|---|
| Code: | 0 | 24 | 48 | 72 | |
| R-FBFB0 | R-FBFB24 | R-FBFB48 | R-FBFB72 | ||
| Water holding capacity (WHC; g pellet/100 g) | |||||
| 0 min | 43.9 a | 43.7 a | 43.3 a | 42.9 a | |
| 10 min | 60.9 a | 60.5 a | 60.5 a | 60.3 a | |
| 35 min | 75.9 a | 75.4 a | 75.2 a | 74.8 a | |
| 45 min | 75.4 a | 75.6 a | 75.7 a | 75.3 a | |
| Soluble starch (SSC, % total starch) | |||||
| 0 min | 7.6 a | 7.4a | 7.7 a | 7.8 a | |
| 10 min | 28.9 a | 29.3a | 29.3 a | 32.0 a | |
| 35 min | 38.2 b | 38.2b | 41.0 ab | 41.1 a | |
| 45 min | 40.4 b | 42.3ab | 43.5 ab | 44.9 a | |
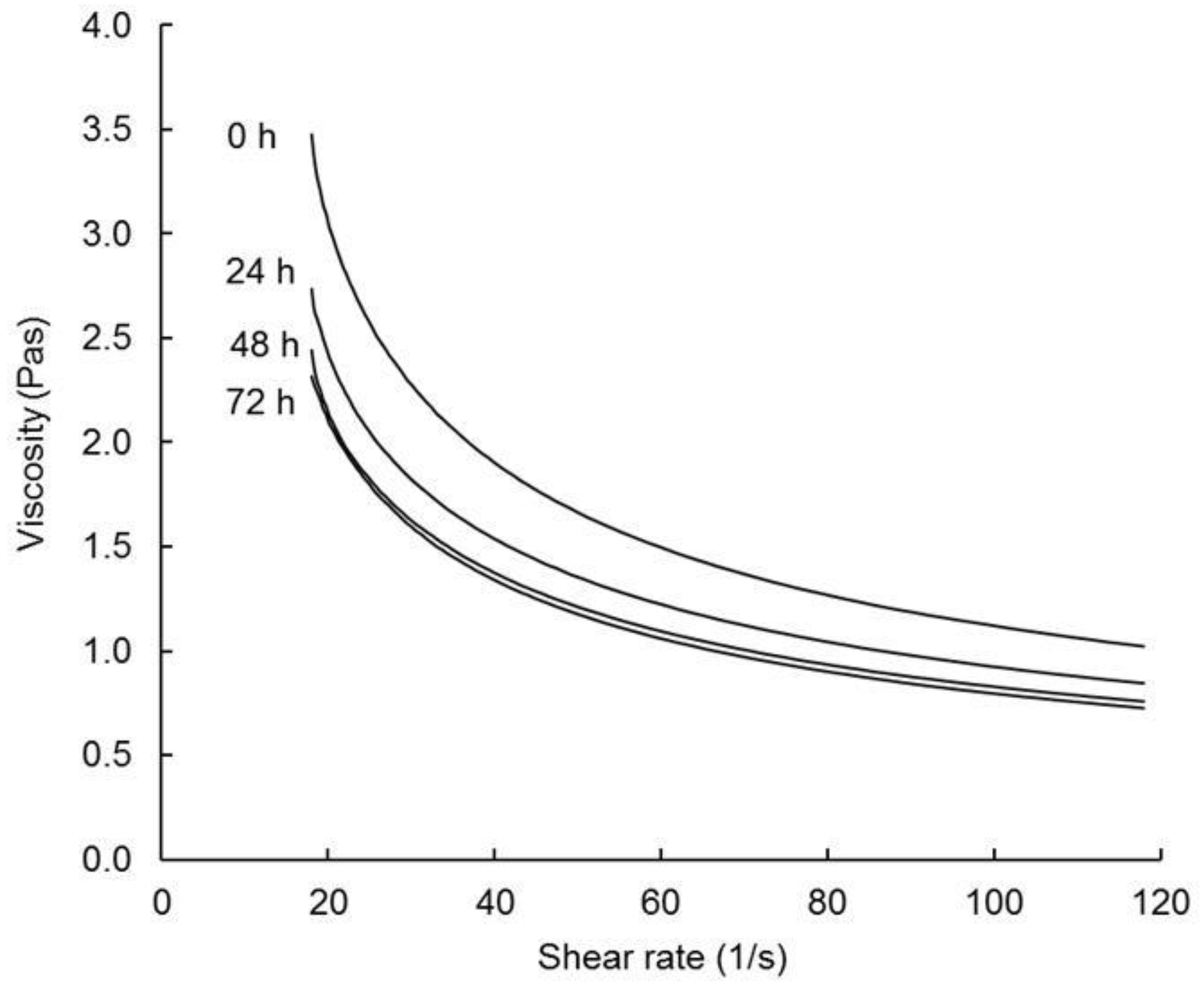
| Pasting/cooling viscosity 2 | |||||
| V95 (Pas) | 0.53 b | 0.59 ab | 0.62 ab | 0.66 a | |
| Vp (Pas) | - | 0.86 a | 0.88 a | 0.90 a | |
| Vh (Pas) | 0.90 a | 0.84 ab | 0.81 ab | 0.71 b | |
| Vc (Pas) | 2.91 a | 2.74 ab | 2.70 ab | 2.37 b | |
| BRV (Pas) | −0.10 b | 0.02 ab | 0.07 a | 0.18 a | |
| SBV (Pas) | 2.01 a | 1.91 ab | 1.89 ab | 1.66 b | |
| Rheology 2 | |||||
| σo (Pa) | 29.4 a | 22.7 b | 22.2 b | 20.5 b | |
| K (Pa·sn) | 14.3 a | 11.7 ab | 11.8 ab | 10.4 b | |
| n (-) | 0.70 a | 0.66 a | 0.65 a | 0.63 a | |
| η at 120 s−1 (Pas) | 1.1 a | 0.8 b | 0.8 b | 0.7 b | |
| Flowability (mm/30 s) | 100.2 b | 100.2 b | 110.2 a | 110.7 a | |
| Characteristic of R-FBFB | FBFB Composition | |||
|---|---|---|---|---|
| Lactose (%, w/w) | Lactic Acid (%, w/w) | Galactose (%, w/w) | pH | |
| Water holding capacity (WHC; g pellet/100 g) at | ||||
| 0 min | 0.26 | −0.19 | −0.4 | 0.80 *** |
| 10 min | 0.13 | −0.04 | −0.2 | 0.10 |
| 35 min | 0.33 | −0.28 | −0.4 | 0.10 |
| 45min | −0.02 | 0.03 | −0.1 | 0.30 |
| Soluble starch content (% total starch) at | ||||
| 0 min | 0.00 | 0.20 | 0.00 | −0.20 |
| 10 min | −0.20 | 0.20 | 0.20 | −0.30 |
| 35 min | −0.50 | 0.70 ** | 0.50 | −0.70 ** |
| 45 min | −0.76 ** | 0.78 ** | 0.8 *** | −0.80 *** |
| Pasting/cooling viscosity 2 | ||||
| V95 (Pas) | −0.75 ** | 0.75 ** | 0.70 ** | −0.70 ** |
| Vp (Pas) | 0.23 | 0.33 | 0.51 | 0.01 |
| Vh (Pas) | 0.75 ** | −0.66 * | −0.60 * | 0.70 ** |
| Vc (Pas) | 0.74 ** | −0.59 * | −0.60 * | 0.60 * |
| BRV (Pas) | −0.21 | 0.26 | 0.10 | −0.40 |
| SBV (Pas) | 0.72 ** | −0.55 * | −0.60 * | −0.60 * |
| Rheology 2 | ||||
| σo (Pa) | 0.86 *** | −0.78 ** | 0.8 *** | 0.80 *** |
| K (Pa·sn) | 0.65 * | −0.54 | 0.8 *** | 0.80 *** |
| n (-) | 0.64 * | −0.6 ** | 0.6 ** | 0.60 * |
| η at 120 s−1 (Pas) | 0.81 *** | −0.84 *** | 0.9 *** | 0.90 *** |
| Flowability (mm/30 s) | −0.56 * | 0.63 * | 0.56 * | −0.62 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevade, A.V.; O’Callaghan, Y.C.; O’Brien, N.M.; O’Connor, T.P.; Guinee, T.P. Fortified Blended Food Base: Effect of Co-Fermentation Time on Composition, Phytic Acid Content and Reconstitution Properties. Foods 2019, 8, 388. https://doi.org/10.3390/foods8090388
Shevade AV, O’Callaghan YC, O’Brien NM, O’Connor TP, Guinee TP. Fortified Blended Food Base: Effect of Co-Fermentation Time on Composition, Phytic Acid Content and Reconstitution Properties. Foods. 2019; 8(9):388. https://doi.org/10.3390/foods8090388
Chicago/Turabian StyleShevade, Ashwini V., Yvonne C. O’Callaghan, Nora M. O’Brien, Tom P. O’Connor, and Timothy P. Guinee. 2019. "Fortified Blended Food Base: Effect of Co-Fermentation Time on Composition, Phytic Acid Content and Reconstitution Properties" Foods 8, no. 9: 388. https://doi.org/10.3390/foods8090388
APA StyleShevade, A. V., O’Callaghan, Y. C., O’Brien, N. M., O’Connor, T. P., & Guinee, T. P. (2019). Fortified Blended Food Base: Effect of Co-Fermentation Time on Composition, Phytic Acid Content and Reconstitution Properties. Foods, 8(9), 388. https://doi.org/10.3390/foods8090388