Identification of the Non-Volatile Taste-Active Components in Crab Sauce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Crab Sauce
2.3. Free Amino Acid Analysis
2.4. Nucleotide Analysis
2.5. Organic Acids Analysis
2.6. Inorganic Ion Analysis
2.7. Taste Activity Value (TAV)
2.8. Equivalent Umami Concentration (EUC)
2.9. Taste Profile Analysis
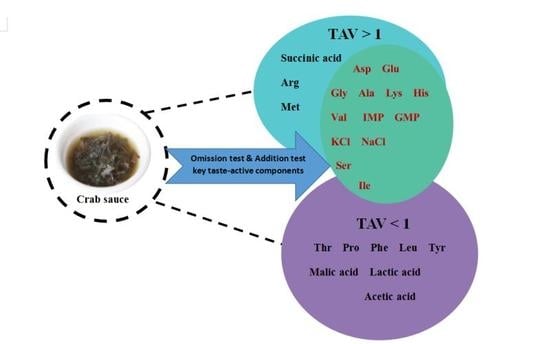
2.10. Omission Test and Addition Test
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Chen, D.; Ye, Y.; Chen, J.; Yan, X. Evolution of metabolomics profile of crab paste during fermentation. Food Chem. 2016, 192, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.G.S.; Narayan, B.; Gopal, S. Bacteriological properties and health-related biochemical components of fermented fish sauce: An overview. Food Rev. Int. 2016, 32, 203–229. [Google Scholar] [CrossRef]
- Yoshida, Y. Umami taste and traditional seasoning. Food Rev. Int. 1998, 14, 213–246. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gaenzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lopetcharat, K.; Choi, Y.J.; Park, J.W.; Daeschel, M.A. Fish sauce products and manufacturing: A review. Food Rev. Int. 2001, 17, 65–88. [Google Scholar] [CrossRef]
- Haseleu, G.; Lubian, E.; Mueller, S.; Shi, F.; Koenig, T. Quantitative Studies and Taste Reconstitution Experiments of the Sour and Lingering Mouthful Orosensation in a Debittered Extract of Traditional Japanese Dried and Fermented Skipjack Tuna (Hongarebushi). J. Agric. Food Chem. 2013, 61, 3205–3211. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; He, X.; Wang, D.; Hu, S.; Li, S.; Jiang, W. Reduction of salt content of fish sauce by ethanol treatment. J. Food Sci. Technol. 2017, 54, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Hajeb, P.; Jinap, S. Umami Taste Components and Their Sources in Asian Foods. Crit. Rev. Food Sci. 2015, 55, 778–791. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K. Microbiological and chemical changes of shrimp Acetes vulgaris during Kapi production. J. Food Sci. Technol. 2017, 54, 3473–3482. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the relative taste intensity of some L-α-amino acid and 5′-nucleotides. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Xu, X.; You, M.; Song, H.; Gong, L.; Pan, W. Investigation of umami and kokumi taste-active components in bovine bone marrow extract produced during enzymatic hydrolysis and Maillard reaction. Int J. Food Sci. Technol. 2018, 53, 2465–2481. [Google Scholar] [CrossRef]
- Gong, J.; Shen, H.; Zheng, J.Y.; Tao, N.P.; Gu, S.Q.; Huang, Y.; Wang, M. Identification of key umami-related compounds in Yangtze Coilia ectenes by combining electronic tongue analysis with sensory evaluation. RSC Adv. 2016, 6, 45689–45695. [Google Scholar] [CrossRef]
- Liu, T.; Liang, Z.; Fan, S.; Xia, N.; Chen, D. Analysis of Characteristic Taste Components of Soldier Crab (Mictyris brevidactylus). Food Sci. 2018, 14, 236–241. [Google Scholar]
- Liu, Y.; Qiu, C. Calculated Taste Activity Values and Umami Equivalences Explain Why Dried Sha-chong (Sipunculus nudus) Is a Valuable Condiment. J. Aquat. Food Prod. Technol. 2016, 25, 177–184. [Google Scholar] [CrossRef]
- Kani, Y.; Yoshikawa, N.; Okada, S.; Abe, H. Taste-active components in the mantle muscle of the oval squid Sepioteuthis lessoniana and their effects on squid taste. Food Res. Int. 2008, 41, 371–379. [Google Scholar] [CrossRef]
- Park, J.N.; Watanabe, T.; Endoh, K.; Watanabe, K.; Abe, H. Taste-active components in a Vietnamese fish sauce. Fish. Sci. 2002, 68, 913–920. [Google Scholar] [CrossRef]
- Shallenberger, R.S. Taste of Amino Acids. In Taste Chemistry; Blackie Academic and Professional: London, UK, 1993; pp. 226–233. [Google Scholar]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste. In Proceedings of the ACS Symposium Series-American Chemical Society, Washington, DC, USA, 31 October 1989. [Google Scholar]
- Spurvey, S.; Pan, B.S.; Shahidi, F. Flavour of shellfish. In Flavor of Meat, Meat Products, and Seafoods, 2nd ed.; Shahidi, F., Ed.; Blackie Academic and Professional: London, UK, 1998; pp. 159–196. [Google Scholar]
- Yoshikawa, S.; Kurihara, H.; Kawai, Y.; Yamazaki, K.; Tanaka, A.; Nishikiori, T.; Ohta, T. Effect of Halotolerant Starter Microorganisms on Chemical Characteristics of Fermented Chum Salmon (Oncorhynchus keta) Sauce. J. Agric. Food Chem. 2010, 58, 6410–6417. [Google Scholar] [CrossRef]
- Skonberg, D.I.; Perkins, B.L. Nutrient composition of green crab (Carcinus maenus) leg meat and claw meat. Food Chem. 2002, 77, 401–404. [Google Scholar] [CrossRef]
- Rotzoll, N.; Dunkel, A.; Hofmann, T. Quantitative studies, taste reconstitution, and omission experiments on the key taste compounds in Morel mushrooms (Morchella deliciosa fr.). J. Agric. Food Chem. 2006, 54, 2705–2711. [Google Scholar] [CrossRef]
- Fuke, S.; Konosu, S. Taste-active components in some foods: A review of Japanese research. Physiol. Behav. 1991, 49, 863–868. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Q.; Xu, Y.; Xia, W. Effect of mixed kojis on physiochemical and sensory properties of rapid-fermented fish sauce made with freshwater fish by-products. Int. J. Food. Sci. Technol. 2017, 52, 2088–2096. [Google Scholar] [CrossRef]
- Ninomiya, K. Umami: A universal taste. Food Rev. Int. 2002, 18, 23–38. [Google Scholar] [CrossRef]
- Chen, D.W.; Su, J.; Liu, X.L.; Yan, D.M.; Lin, Y.; Jiang, W.M.; Chen, X.H. Amino Acid Profiles of Bivalve Mollusks from Beibu Gulf, China. J. Aquat. Food Prod. Technol. 2012, 21, 369–379. [Google Scholar] [CrossRef]
- Normah, I.; Diyana, N.M.R. Evaluation of umaminess in green mussel hydrolysate (Perna viridis) produced in the presence of sodium tripolyphosphate and NaCl. Int. Food Res. J. 2018, 25, 2524–2530. [Google Scholar]
- Michihata, T.; Sado, Y.; Yano, T.; Enomoto, T. Free amino acids, oligopeptides, organic acids and nucleotides of ISHIRU (fish sauce). J. Jpn. Soc. Food Sci. 2000, 47, 241–248. [Google Scholar] [CrossRef]
- Lee, H.; Choi, Y.; Hwang, I.M.; Hong, S.W.; Lee, M. Relationship between chemical characteristics and bacterial community of a Korean salted-fermented anchovy sauce, Myeolchi-Aekjeot. LWT-Food Sci. Technol. 2016, 73, 251–258. [Google Scholar]
- Istiqamah, A.; Lioe, H.N.; Adawiyah, D.R. Umami compounds present in low molecular umami fractions of asam sunti—A fermented fruit of Averrhoa bilimbi L. Food Chem. 2019, 270, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Lioe, H.N.; Apriyantono, A.; Takara, K.; Wada, K.; Yasuda, M. Umami taste enhancement of MSG/NaCl mixtures by subthreshold L-alpha-aromatic amino acids. J. Food Sci. 2005, 70, S401–S405. [Google Scholar] [CrossRef]
- Wise, P.M.; Damani, S.; Breslin, P.A.S. Sodium, but not potassium, blocks bitterness in simple model chicken broths. J. Food Sci. Technol. 2019, 56, 3151–3156. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M.; Hrynkiewicz, M. Food protein-originating peptides as tastants-Physiological, technological, sensory, and bioinformatic approaches. Food Res. Int. 2016, 89, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Lin, L.; Zhao, M.; Dong, Y.; Sun-Waterhouse, D.; Chen, H.; Qiu, C.; Su, G. Sequence, taste and umami-enhancing effect of the peptides separated from soy sauce. Food Chem. 2016, 206, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Park, J.N.; Ishida, K.; Watanabe, T.; Endoh, K.I.; Watanabe, K.; Murakami, M.; Abe, H. Taste effects of oligopeptides in a Vietnamese fish sauce. Fish. Sci. 2002, 68, 921–928. [Google Scholar] [CrossRef]

| Components | Taste Attribute 1 | Threshold Value 2 (mg/100 mL) | Content (mg/100 mL) | TAV |
|---|---|---|---|---|
| Umami amino acids | ||||
| Asp | Umami and sweet (+) | 100 | 401.8 ± 0.9 | 4 |
| Glu | Umami (+) | 30 | 758.7 ± 2.9 | 25.3 |
| Total umami amino acids | 1160.5 (33%) | |||
| Sweet amino acids | ||||
| Ser | Sweet (+) | 150 | 148.6 ± 0.7 | 1 |
| Gly | Sweet (+) | 130 | 422.9 ± 2.6 | 3.3 |
| Thr | Sweet (+) | 260 | 138.8 ± 3.3 | 0.5 |
| Ala | Sweet (+) | 60 | 340.9 ± 1.0 | 5.7 |
| Lys | Sweet and bitter (−) | 50 | 258.2 ± 0.8 | 5.2 |
| Pro | Sweet and bitter (+) | 300 | 152.8 ± 1.6 | 0.5 |
| Total sweet amino acids | 1462.2 (42%) | |||
| Bitter amino acids | ||||
| Arg | Sweet and bitter (+) | 50 | 301.3 ± 4.7 | 6 |
| His | Bitter (−) | 20 | 99.2 ± 1.7 | 5 |
| Tyr | Bitter (−) | / | 67.2 ± 0.4 | / |
| Val | Sweet and bitter (+) | 40 | 57.8 ± 2.1 | 1.5 |
| Phe | Bitter (−) | 90 | 61.4 ± 0.2 | 0.7 |
| Ile | Bitter (−) | 90 | 70.4 ± 1.5 | 0.8 |
| Leu | Bitter (−) | 190 | 142.9 ± 1.3 | 0.8 |
| Met | Sweet and bitter (−) | 30 | 56.4 ± 0.1 | 1.9 |
| Total bitter amino acids | 856.6 (25%) | |||
| Total free amino acids | 3479.3 | |||
| Flavor nucleotides | ||||
| IMP | Umami (+) | 25 | 29.7 ± 0.9 | 1.2 |
| GMP | Umami (+) | 12.5 | 49.1 ± 2.2 | 3.9 |
| Organic acid | ||||
| Tartaric acid | Sour and thrill (+) | 1.5 | ND | / |
| Malic acid | Sour and bitter (+) | 50 | 13.16 ± 0.78 | 0.3 |
| Lactic acid | Sour and bitter (+) | 126 | 71.02 ± 0.21 | 0.6 |
| Citric acid | Sour and mild (+) | 45 | ND | / |
| Succinic acid | Sour and umami (+) | 10.6 | 16.17 ± 1.01 | 1.5 |
| Acetic acid | Sour and thrill (+) | 10.6 | 9.84 ± 0.52 | 0.9 |
| Inorganic ion | ||||
| KCl | Salt (+) | 130 | 205.4 ± 4.4 | 1.6 |
| NaCl | Salt (+) | 150 | 2228.9 ± 15 | 15.9 |
| Omitted Components | N1 | Sig 2 | Description of Taste Difference |
|---|---|---|---|
| A (Glu, Gly, Asp, Ala, Arg, Lys) | 20 | *** | Less sweet, less umami |
| B (Ser, Thr, Leu, Pro, His) | 17 | *** | Less sweet |
| C (Val, Met, Phe, Ile, Tyr) | 15 | *** | Less bitter |
| D (GMP, IMP) | 16 | *** | Less sweet, less umami |
| E (malic acid, succinic acid, lactic acid, acetic acid) | 6 | ns | |
| F (NaCl, KCl) | 20 | *** | Less sweet, less umami, less salt |
| Omitted Components | N1 | Sig 2 | Description of Taste Difference |
|---|---|---|---|
| Asp | 19 | *** | Less sweet, less umami |
| Glu | 20 | *** | Less sweet, less umami |
| Gly | 15 | *** | Less sweet |
| Ala | 15 | *** | Less sweet, less umami |
| Arg | 10 | ns | |
| Lys | 15 | *** | Less sweet, less bitter |
| B1 (Thr, Pro, Leu) | 9 | ns | |
| B2 (Ser) | 10 | ns | |
| B3 (His) | 14 | ** | Less bitter |
| C1 (Phe, Ile) | 4 | ns | |
| C2 (Val) | 9 | ns | |
| C3 (Tyr) | 8 | ns | |
| C4 (Met) | 5 | ns | |
| IMP | 14 | ** | Less sweet, less umami |
| GMP | 18 | *** | Less sweet, less umami |
| KCl | 14 | ** | Less umami, less salt |
| NaCl | 20 | *** | Less sweet, less umami, less salt, more bitter |
| Added Components | N 1 | Sig 2 | Description of Taste Difference |
|---|---|---|---|
| Arg | 10 | ns | |
| Leu | 8 | ns | |
| Ser | 16 | *** | More sweet, less bitter |
| Pro | 10 | ns | |
| Tyr | 6 | ns | |
| Ile | 16 | *** | Less sweet, more bitter |
| Phe | 2 | ns | |
| Val | 16 | *** | Less sweet, more bitter |
| Tyr | 10 | ns | |
| Met | 10 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-T.; Xia, N.; Wang, Q.-Z.; Chen, D.-W. Identification of the Non-Volatile Taste-Active Components in Crab Sauce. Foods 2019, 8, 324. https://doi.org/10.3390/foods8080324
Liu T-T, Xia N, Wang Q-Z, Chen D-W. Identification of the Non-Volatile Taste-Active Components in Crab Sauce. Foods. 2019; 8(8):324. https://doi.org/10.3390/foods8080324
Chicago/Turabian StyleLiu, Tian-Tian, Ning Xia, Qin-Zhi Wang, and De-Wei Chen. 2019. "Identification of the Non-Volatile Taste-Active Components in Crab Sauce" Foods 8, no. 8: 324. https://doi.org/10.3390/foods8080324
APA StyleLiu, T.-T., Xia, N., Wang, Q.-Z., & Chen, D.-W. (2019). Identification of the Non-Volatile Taste-Active Components in Crab Sauce. Foods, 8(8), 324. https://doi.org/10.3390/foods8080324