Extraction of Dairy Phospholipids Using Switchable Solvents: A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dairy Byproducts
2.2. Compositional Analysis
2.3. Conventional Extraction
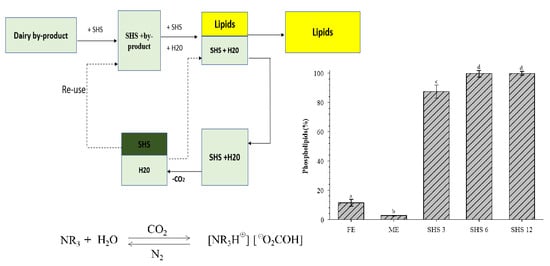
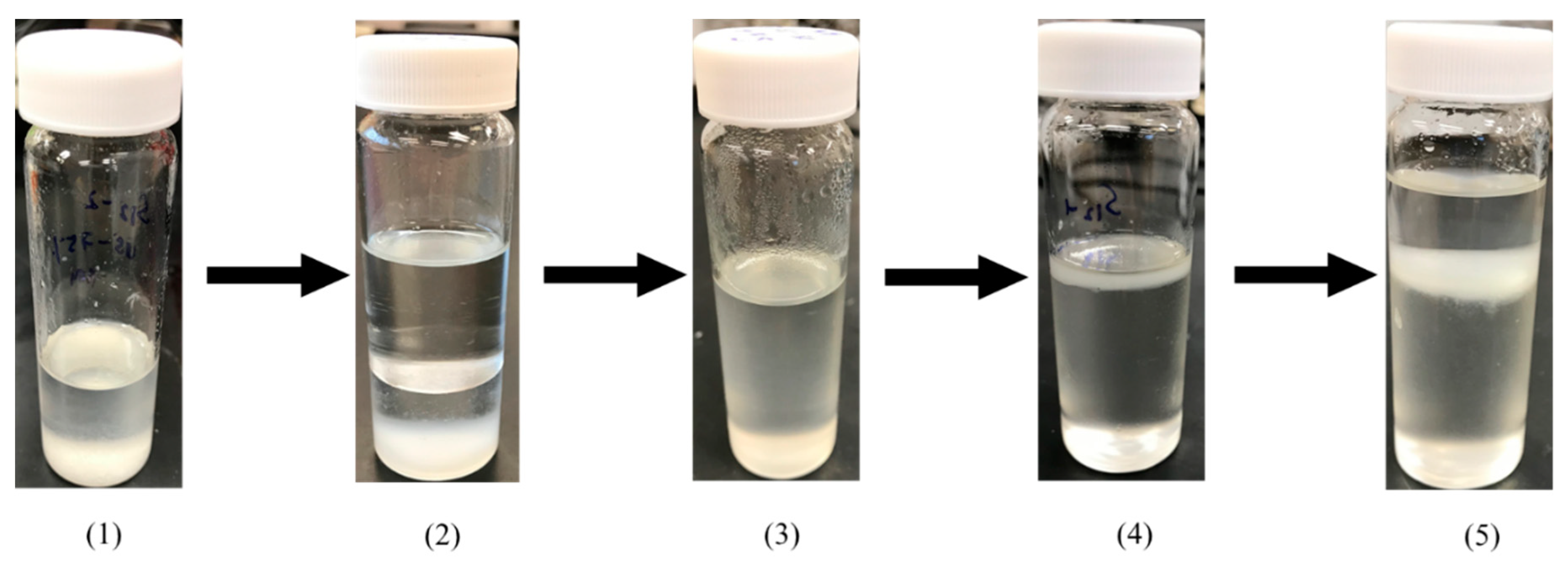
2.4. Switchable Solvent Extraction
2.5. Fractionation of Extracted Lipids
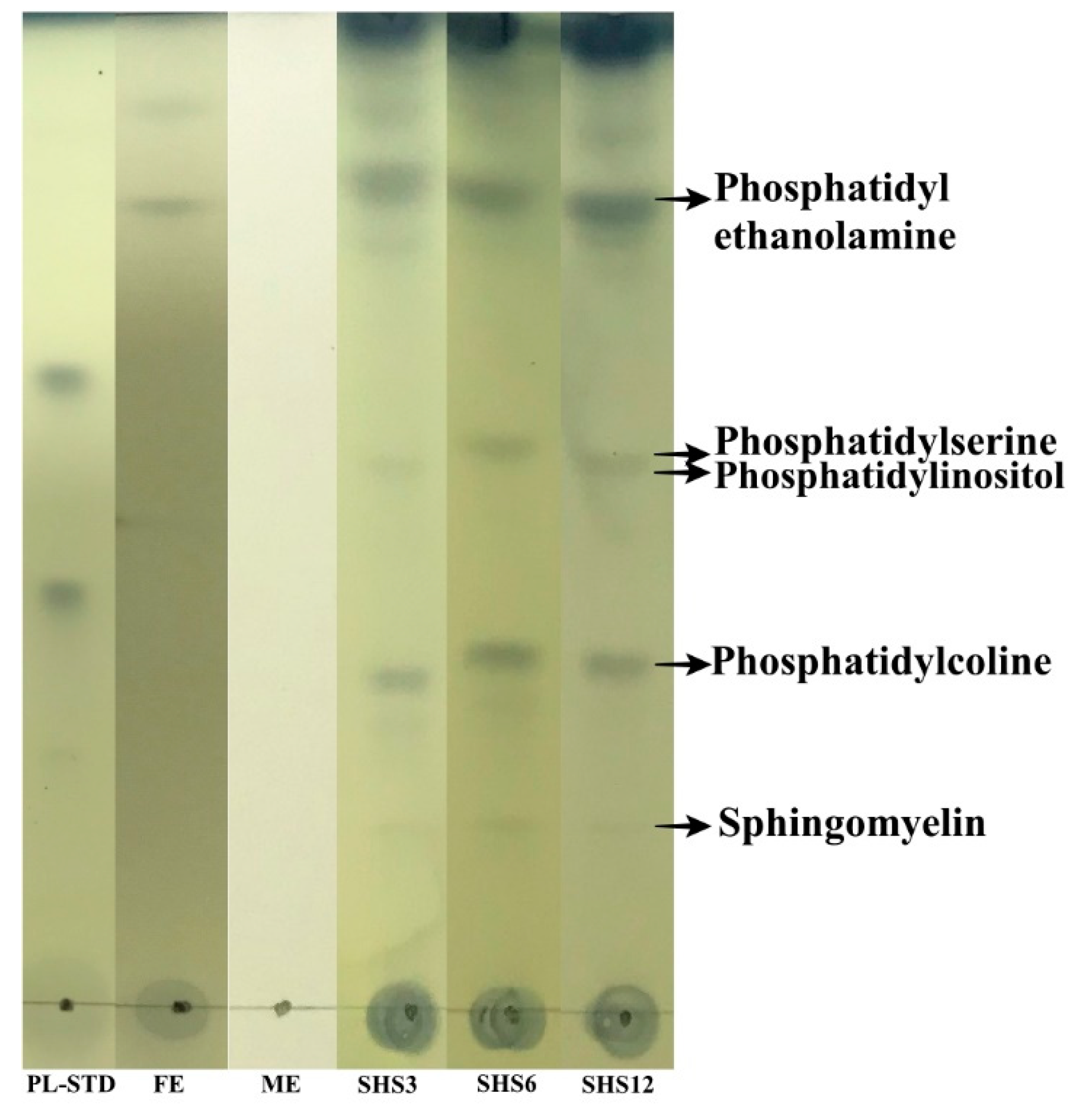
2.6. Thin-Layer Chromatography (TLC)
2.7. Statistical Analysis
3. Results
3.1. Compositional Analysis
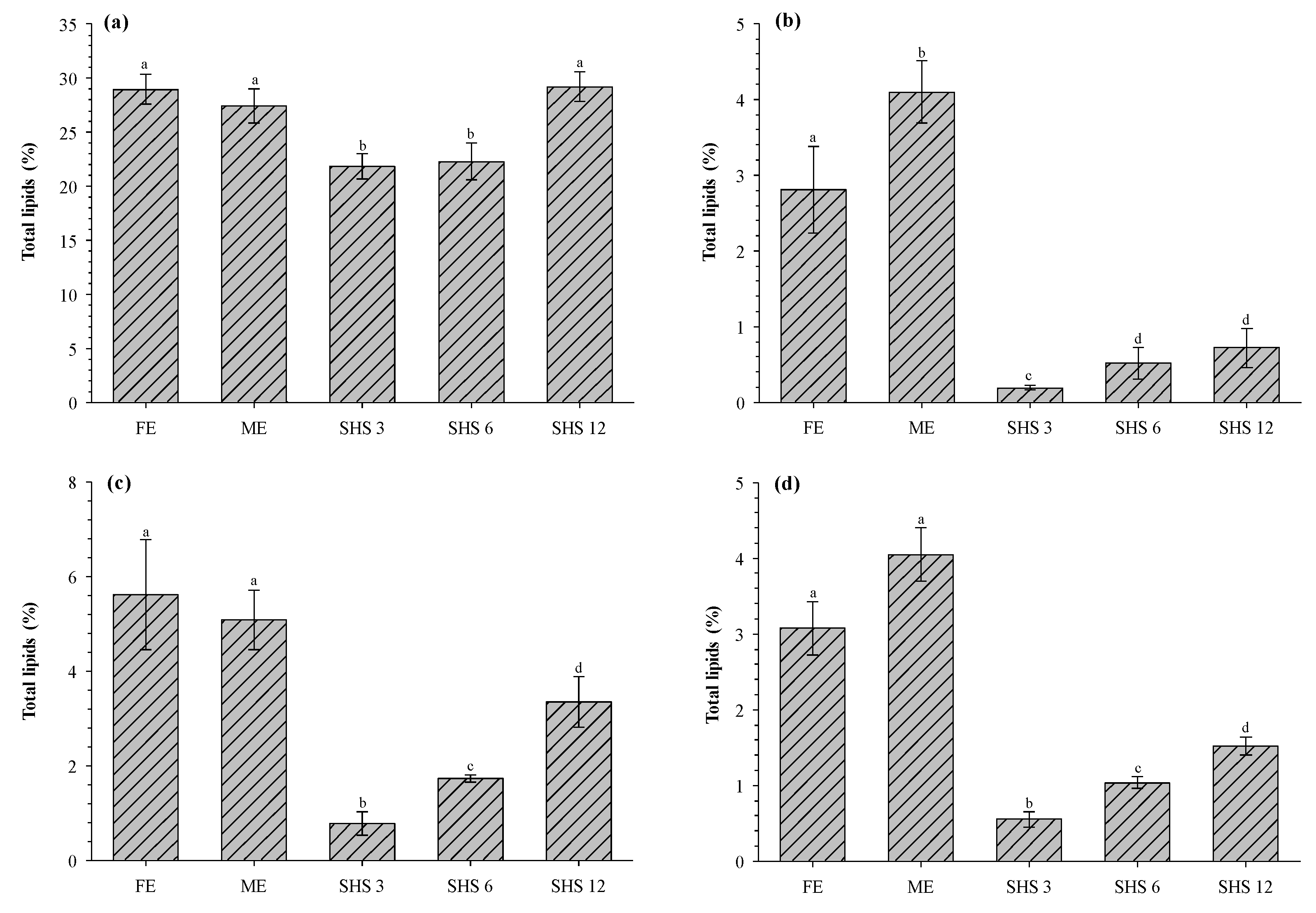
3.2. Total Lipid Extraction
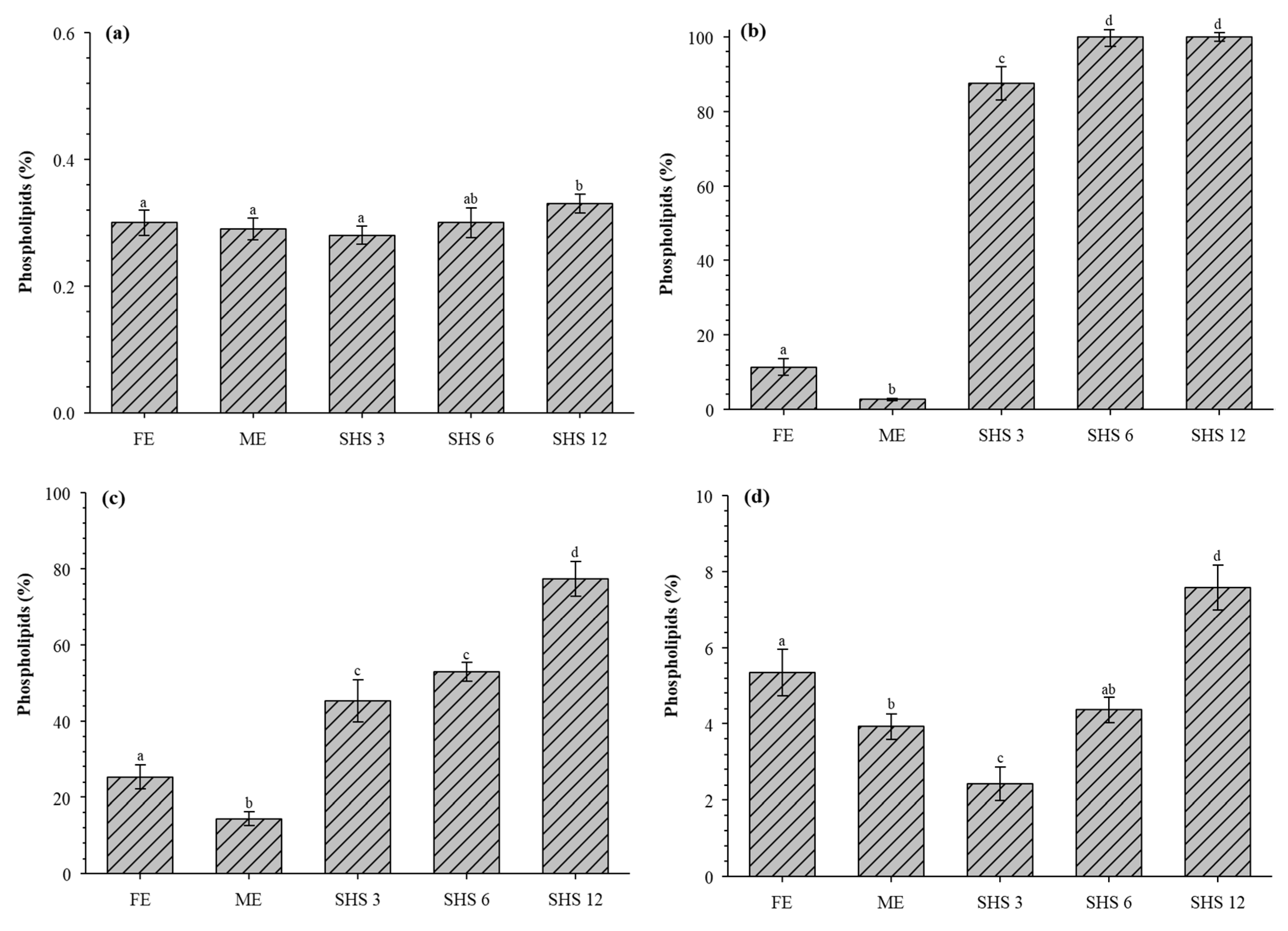
3.3. Phospholipids Recovered
3.4. PLs TLC Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Contarini, G.; Povolo, M. Phospholipids in milk fat: Composition, biological and technological significance, and analytical strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, L.; Gomes, A.; Pintado, M.; Rodríguez-Alcalá, L.M. Isolation and analysis of phospholipids in dairy foods. J. Anal. Methods Chem. 2016, 12. [Google Scholar] [CrossRef]
- Avalli, A.; Contarini, G. Determination of phospholipids in dairy products by SPE/HPLC/ELSD. J. Chromatogr. A 2005, 1071, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.R.; Elias-Argote, X.E.; Jiménez-Flores, R.; Gigante, M.L. Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. Int. Dairy J. 2010, 20, 598–602. [Google Scholar] [CrossRef]
- Ali, A.H.; Wei, W.; Abed, S.M.; Korma, S.A.; Mousa, A.H.; Hassan, H.M.; Wang, X. Impact of technological processes on buffalo and bovine milk fat crystallization behavior and milk fat globule membrane phospholipids profile. LWT 2018, 90, 424–432. [Google Scholar] [CrossRef]
- Rombaut, R.; Camp, J.V.; Dewettinck, K. Analysis of phospho- and sphingolipids in dairy products by a new HPLC method. J. Dairy Sci. 2005, 88, 482–488. [Google Scholar] [CrossRef]
- Astaire, J.C.; Ward, R.; German, J.; Jiminez-Flores, R. Concentration of polar MFGM lipids from buttermilk by microfiltration and supercritical fluid extraction. J. Dairy Sci. 2003, 86, 2297–2307. [Google Scholar] [CrossRef]
- Barry, K.M.; Dinan, T.G.; Kelly, P.M. Pilot scale production of a phospholipid-enriched dairy ingredient by means of an optimised integrated process employing enzymatic hydrolysis, ultrafiltration and super-critical fluid extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 301–306. [Google Scholar] [CrossRef]
- Spence, A.J.; Jimenez-Flores, R.; Qian, M.; Goddik, L. Phospholipid enrichment in sweet and whey cream buttermilk powders using supercritical fluid extraction. J. Dairy Sci. 2009, 92, 2373–2381. [Google Scholar] [CrossRef]
- Price, N.; Fei, T.; Clark, S.; Wang, T. Extraction of phospholipids from a dairy by-product (whey protein phospholipid concentrate) using ethanol. J. Dairy Sci. 2018, 101, 8778–8787. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012, 5, 7240–7253. [Google Scholar] [CrossRef]
- Jessop, P.G.; Phan, L.; Carrier, A.; Robinson, S.; Dürr, C.J.; Harjani, J.R. A solvent having switchable hydrophilicity. Green Chem. 2010, 12, 809–814. [Google Scholar] [CrossRef]
- Boyd, A.R.; Champagne, P.; McGinn, P.J.; MacDougall, K.M.; Melanson, J.E.; Jessop, P.G. Switchable hydrophilicity solvents for lipid extraction from microalgae for biofuel production. Bioresour. Technol. 2012, 118, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Amamcharla, J.K.; Metzger, L.E. Development of a rapid method for the measurement of lactose in milk using a blood glucose biosensor. J. Dairy Sci. 2011, 94, 4800–4809. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Monteagudo, S.I.; Leal-Dávila, M.; Curtis, J.M.; Saldaña, M.D. Oxidative stability of ultra high temperature milk enriched in conjugated linoleic acid and trans-vaccenic acid. Int. Dairy J. 2015, 43, 70–77. [Google Scholar] [CrossRef]
- Gallier, S.; Gragson, D.; Cabral, C.; Jimenez-Flores, R.; Everett, D.W. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J. Agric. Food Chem. 2010, 58, 10503–10511. [Google Scholar] [CrossRef]
- Donato, P.; Cacciola, F.; Cichello, F.; Russo, M.; Dugo, P.; Mondello, L. Determination of phospholipids in milk samples by means of hydrophilic interaction liquid chromatography coupled to evaporative light scattering and mass spectrometry detection. J. Chromatorg. A 2011, 1218, 6476–6482. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. Fractionation of lipids and purification of γ-linolenic acid (GLA) from Spirulinaplatensis. Food Chem. 2008, 109, 580–586. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K.; Van Camp, J. Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC–ELSD). J. Food Compos. Anal. 2007, 20, 308–312. [Google Scholar] [CrossRef]
- Du, Y.; Schuur, B.; Kersten, S.R.; Brilman, D.W. Opportunities for switchable solvents for lipid extraction from wet algal biomass: An energy evaluation. Algal Res. 2015, 11, 271–283. [Google Scholar] [CrossRef]
- Samorì, C.; López Barreiro, D.; Vet, R.; Pezzolesi, L.; Brilman, D.W.F.; Galletti, P.; Tagliavini, E. Effective lipid extraction from algae cultures using switchable solvents. Green Chem. 2013, 15, 353–356. [Google Scholar] [CrossRef]

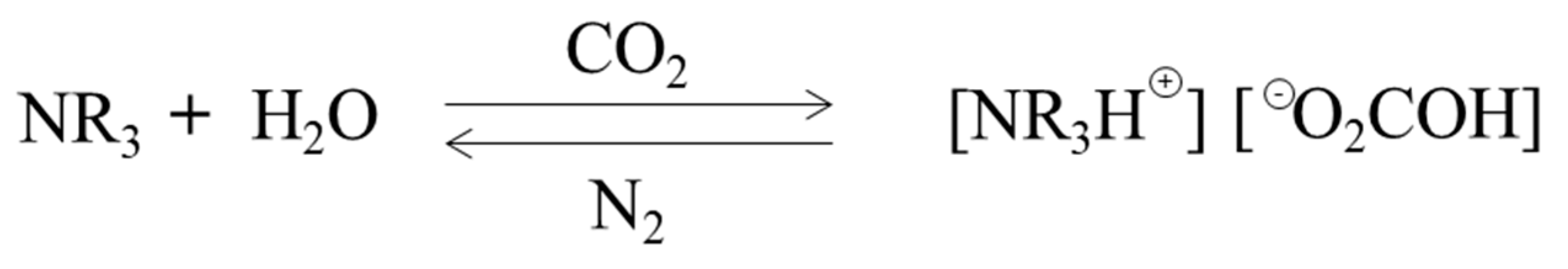
| Parameter | Dairy Matrix | |||
|---|---|---|---|---|
| Raw Cream | Butter Milk | Concentrated Butter Milk | B-Serum | |
| Total Solids (%) | 51.77 ± 0.18 | 9.63 ± 0.03 | 91.81 ± 0.06 | 8.17 ± 0.34 |
| Protein (%) | 0.24 ± 0.01 | 0.33 ± 0.01 | 1.44 ± 0.07 | 0.18 ± 0.01 |
| Fat (%) | 27.47 ± 1.60 | 4.09 ± 0.41 | 5.09 ± 0.63 | 4.05 ± 0.04 |
| Ash (%) | 0.61 ± 0.02 | 0.94 ± 0.08 | 6.26 ± 0.17 | 1.04 ± 0.39 |
| Lactose (%) | 3.06 ± 2.73 | 3.98 ± 0.39 | 8.69 ± 0.28 | 6.91 ± 0.15 |
| pH | 6.58 ± 0.01 | 6.38 ± 0.01 | 5.05 ± 0.02 | 6.74 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Rathnakumar, K.; Martínez-Monteagudo, S.I. Extraction of Dairy Phospholipids Using Switchable Solvents: A Feasibility Study. Foods 2019, 8, 265. https://doi.org/10.3390/foods8070265
Cheng S, Rathnakumar K, Martínez-Monteagudo SI. Extraction of Dairy Phospholipids Using Switchable Solvents: A Feasibility Study. Foods. 2019; 8(7):265. https://doi.org/10.3390/foods8070265
Chicago/Turabian StyleCheng, Shouyun, Kaavya Rathnakumar, and Sergio I. Martínez-Monteagudo. 2019. "Extraction of Dairy Phospholipids Using Switchable Solvents: A Feasibility Study" Foods 8, no. 7: 265. https://doi.org/10.3390/foods8070265
APA StyleCheng, S., Rathnakumar, K., & Martínez-Monteagudo, S. I. (2019). Extraction of Dairy Phospholipids Using Switchable Solvents: A Feasibility Study. Foods, 8(7), 265. https://doi.org/10.3390/foods8070265