Determining the Arrhenius Kinetics of Avocado Oil: Oxidative Stability under Rancimat Test Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Avocado Oil
2.2. Kinetic Data Analysis
2.3. Determination of Basic Physical and Physicochemical Properties of Avocado Oil
2.3.1. Density of Avocado Oil
2.3.2. Refractive Index
2.3.3. Free Fatty Acid
2.4. Determining the Chemical Properties of Avocado Oil
2.4.1. Determination of Fatty Acid Composition of Avocado Oil
2.4.2. Determining the Total Phenolic Content of Avocado Oil
2.4.3. Determination of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
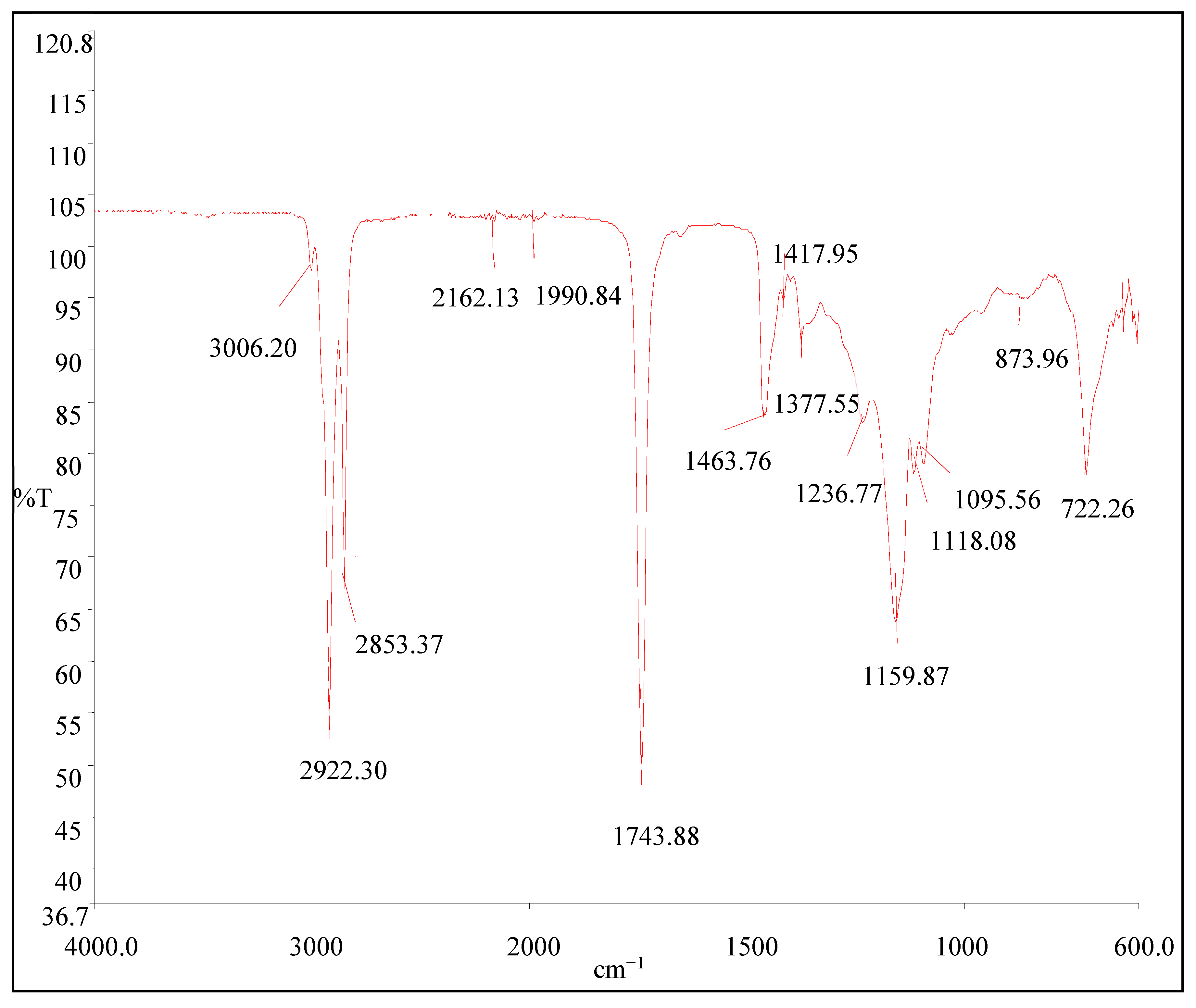
2.4.4. Component Analysis of Avocado Oil
2.5. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, M.; Requejo-Jackman, C.; Woolf, A. What is unrefined, extra virgin cold-pressed avocado oil? J. Am. Oil Chem. Soc. 2010, 87, 198–202. [Google Scholar]
- Foudjo, B.U.S.; Kansci, G.; Lazar, I.M.; Fokou, E.; Etoa, F.X. ATR-FTIR Characterization and Classification of Avocado Oils from Five Cameroon Cultivars Extracted with a Friendly Environmental Process. Environ. Eng. Manag. J. 2013, 12, 97–103. [Google Scholar]
- Qin, X.; Zhong, J. A review of extraction techniques for avocado oil. J. Oleo Sci. 2015, 65, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Farines, M.; Soulier, J.; Rancurel, A.; Montaudoin, M.G.; Leborgne, L. Influence of avocado oil processing on the nature of some unsaponifiable constituents. J. Am. Oil Chem. Soc. 1995, 72, 473–476. [Google Scholar] [CrossRef]
- Southwell, K.H.; Harris, R.V.; Swetman, A.A. Extraction and refining of oil obtained from dried avocado fruit using a small expeller. Trop. Sci. 1990, 30, 121–131. [Google Scholar]
- Bora, P.S.; Narain, N.; Rocha, R.V.M.; Paulo, M.Q. Characterization of the oils from the pulp and seeds of avocado (cultivar: Fuerte) fruits. Grasas y Aceites 2001, 52, 171–174. [Google Scholar]
- Tan, C.P.; Man, Y.B.C.; Selamat, J.; Yusoff, M.S.A. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J. Am. Oil Chem. Soc. 2001, 78, 1133. [Google Scholar] [CrossRef]
- Tan, C.P.; Man, Y.B.C. Differential scanning calorimetric analysis for monitoring the oxidation of heated oils. Food Chem. 1999, 67, 177–184. [Google Scholar] [CrossRef]
- Boekel, M. Statistical aspects of kinetic modeling for food science problems. J. Food Sci. 1996, 61, 477–486. [Google Scholar] [CrossRef]
- Labuza, T.P.; Dugan, L.R. Kinetics of lipid oxidation in foods. Crit. Rev. Food Sci. Nutr. 1971, 2, 355–405. [Google Scholar] [CrossRef]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasarán, I. Stability of avocado oil during heating: Comparative study to olive oil. Food Chem. 2012, 132, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.O.; Dorantes, L.; Galíndez, J.; Guzmán, R.I. Effect of different extraction methods on fatty acids, volatile compounds, and physical and chemical properties of avocado (Persea americana Mill.) oil. J. Agric. Food Chem. 2003, 51, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.; Wong, M.; Eyres, L.; Mcghie, T.; Lund, C.; Olsson, S.; Wang, Y.; Bulley, C.; Wang, M.Y.; Friel, E.; et al. Avocado oil. In Gourmet and Health-Promoting Specialty Oils; Elsevier: Kidlington, UK, 2009; pp. 73–125. [Google Scholar]
- AOCS. Official Methods of Analysis of AOCS International; AOCS: Arlington, TX, USA, 1995. [Google Scholar]
- Arranz, S.; Cert, R.; Pérez-Jiménez, J.; Cert, A.; Arranz, S. Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chem. 2008, 110, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 200, 661–676. [Google Scholar] [CrossRef]
- Román Falcó, I.P.; Grané Teruel, N.; Prats Moya, S.; Martín Carratalá, M.L. Kinetic study of olive oil degradation monitored by Fourier transform infrared spectrometry. Application to oil characterization. J. Agric. Food Chem. 2012, 60, 11800–11810. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Lipid Oxidation; Elsevier: Kidlington, UK, 2014. [Google Scholar]
- Farhoosh, R.; Niazmand, R.; Rezaei, M.; Sarabi, M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur. J. Lipid Sci. Technol. 2008, 110, 587–592. [Google Scholar] [CrossRef]
- Aurand, L.W. Food Composition and Analysis; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- IUPAC (International Union of Pure and Applied Chemistry). Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Castello, J.; Van Hecke, E.; Lanoisellé, J.L. Optimization of oil yield and oil total phenolic content during grape seed cold screw pressing. Ind. Crops. Prod. 2015, 63, 26–33. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Some of the most significant changes in the Fourier transform infrared spectra of edible oils under oxidative conditions. J. Sci. Food Agric. 2000, 80, 2028–2036. [Google Scholar] [CrossRef]
- Ostrowska-Ligeza, E.; Bekas, W.; Kowalska, D.; Lobacz, M.; Wroniak, M.; Kowalski, B. Kinetics of commercial olive oil oxidation: Dynamic differential scanning calorimetry and Rancimat studies. Eur. J. Lipid Sci. Technol. 2010, 112, 268–274. [Google Scholar] [CrossRef]
- Kochhar, S.P.; Henry, C.J.K. Oxidative stability and shelf-life evaluation of selected culinary oils. Int. J. Food Sci. Nutr. 2009, 60, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R. The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J. Am. Oil Chem. Soc. 2007, 84, 205–209. [Google Scholar] [CrossRef]
- Hasenhuettl, G.L.; Wan, P.J. Temperature effects on the determination of oxidative stability with the Metrohm Rancimat. J. Am. Oil Chem. Soc. 1992, 69, 525–527. [Google Scholar] [CrossRef]
- Soares, S.E.; Mancini Filho, J.; Turatti, J.M.; Tango, J.S. Caracterização física, química e avaliação da estabilidade do óleo de abacate (Persea americana, Mill.) nas diferentes etapas do processo de refinação. Rev. Farm Bioquim. Univ. Sao Paulo 1991, 27, 70–82. [Google Scholar]
- Yunus, W.M.M.; Fen, Y.W.; Yee, L.M. Refractive index and fourier transform infrared spectra of virgin coconut oil and virgin olive oil. Am. J. Appl. Sci. 2009, 6, 328. [Google Scholar] [CrossRef]
- Albi, T.; Lanzón, A.; Guinda, A.; León, M.; Pérez-Camino, M.C. Microwave and conventional heating effects on thermoxidative degradation of edible fats. J. Agric. Food Chem. 1997, 45, 3795–3798. [Google Scholar] [CrossRef]
- Fuentes, E.; Báez, M.E.; Díaz, J. Microwave-assisted extraction at atmospheric pressure coupled to different clean-up methods for the determination of organophosphorus pesticides in olive and avocado oil. J. Chromatogr. A 2009, 1216, 8859–8866. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado, sunflower and olive oils as replacers of pork back-fat in burger patties: Effect on lipid composition, oxidative stability and quality traits. Meat Sci. 2012, 90, 106–115. [Google Scholar] [CrossRef]
- Sinyida, S.; Gramshaw, J.W. Volatiles of Avocado Fruit. J. Food Chem. 1998, 62, 483–487. [Google Scholar] [CrossRef]
- Dallard, I.; Cathebras, P.; Sauron, C.; Massoubre, C. Is cocoa a psychotropic drug? Psychopathologic study of a population of subjects self-identified as chocolate addicts. Encephale 2000, 27, 181–186. [Google Scholar]
- Giua, L.; Blasi, F.; Simonetti, M.S.; Cossignani, L. Oxidative modifications of conjugated and unconjugated linoleic acid during heating. Food Chem. 2013, 140, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Cossignani, L.; Giua, L.; Simonetti, M.S.; Blasi, F. Volatile compounds as indicators of conjugated and unconjugated linoleic acid thermal oxidation. Eur. J. Lipid Sci. Technol. 2014, 116, 407–412. [Google Scholar] [CrossRef]
- Kumar, S.N. Variability in coconut (Cocos nucifera L.) germplasm and hybrids for fatty acid profile of oil. J. Agric. Food Chem. 2011, 59, 13050–13058. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Navarro, D.; Maunier, S.; Sigoillot, J.C.; Lorquin, J.; Delattre, M.; Simon, J.L.; Asther, M.; Labat, M. Simple phenolic content in olive oil residues as a function of extraction systems. Food Chem. 2001, 75, 501–507. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Pellegrini, N.; Simonetti, P.; Gardana, C.; Brenna, O.; Brighenti, F.; Pietta, P. Polyphenol content and total antioxidant activity of vini novelli (young red wines). J. Agric. Food Chem. 2000, 48, 732–735. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2, 2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Nisiotou, C.; Papageorgiou, Y.; Kremli, I.; Satravelas, N.; Zinieris, N.; Zygalaki, H. Comparison of the radical scavenging potential of polar and lipidic fractions of olive oil and other vegetable oils under normal conditions and after thermal treatment. J. Agric. Food Chem. 2004, 52, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-Islas, N.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Detection of adulterants in avocado oil by Mid-FTIR spectroscopy and multivariate analysis. Food Res. Int. 2013, 51, 148–154. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A.; Cabo, N.; Chirinos, R.; Pascual, G. Characterization of sacha inchi (Plukenetia volubilis L.) oil by FTIR spectroscopy 1, H.N.M.R. Comparison with linseed oil. J. Am. Oil Chem. Soc. 2003, 80, 755–762. [Google Scholar] [CrossRef]
- Ozen, B.F.; Mauer, L.J. Detection of hazelnut oil adulteration using FT-IR spectroscopy. J. Agric. Food Chem. 2002, 50, 3898–3901. [Google Scholar] [CrossRef] [PubMed]
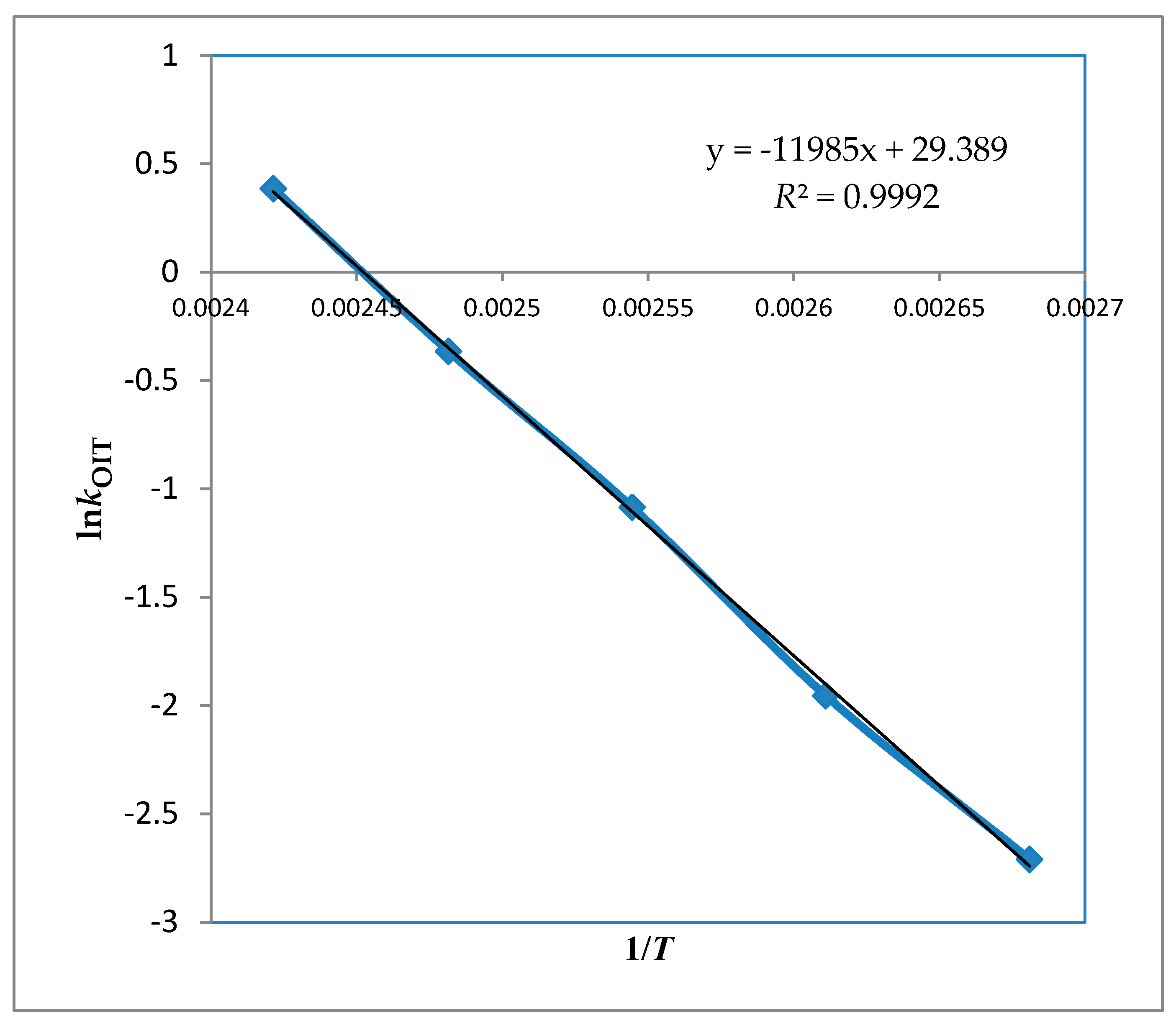
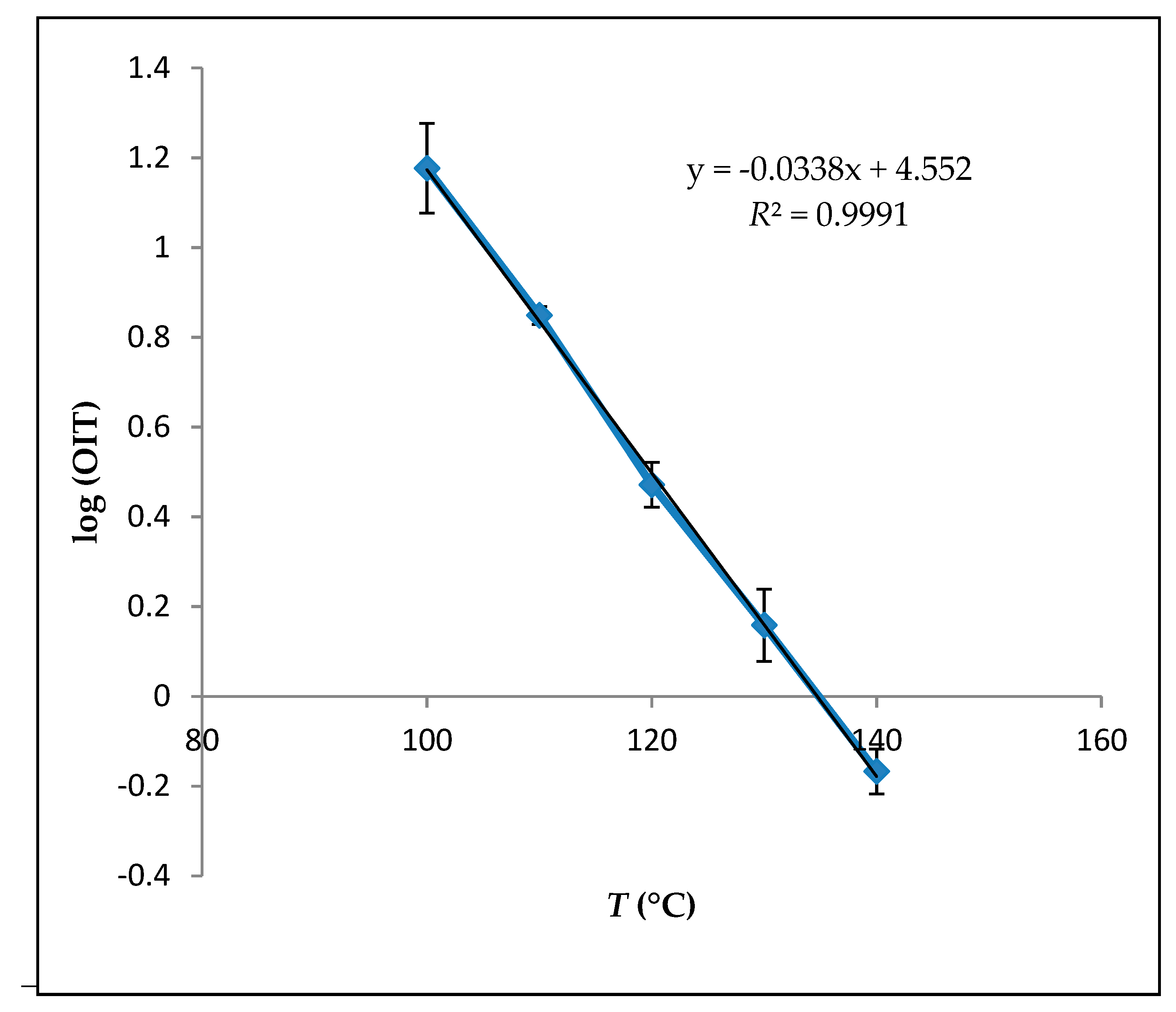
| T (°C) | OIT (h) * | kOIT (×10−3 h−1) |
|---|---|---|
| 100 | 15.02 ± 2.03 a | 66.6 |
| 110 | 7.06 ± 3.15 b | 141.6 |
| 120 | 2.96 ± 0.25 c | 337.8 |
| 130 | 1.44 ± 0.03 d | 694.4 |
| 140 | 0.68 ± 0.15 e | 1470.6 |
| Parameters | Avocado Oil |
|---|---|
| b (intercept) | 29.4 ± 0.83 |
| a (slope) | −11985 ± 311 |
| R2 | 0.99 |
| Ea (kJ/mol) | 99.6 ± 2 |
| AOIT (×1012 h−1) | 5.8 |
| Parameters | Avocado Oil |
|---|---|
| a (slope) | −0.034 ± 0.4 |
| b (intercept) | 13.8 ± 1 |
| R2 | 0.99 |
| OIT25 °C (day) | 211 ± 10 |
| Measured Property | Value |
|---|---|
| Density (g/mL) | 0.91 ± 0.001 |
| Refractive index | 1.4680 ± 0.0002 |
| Free fatty acid (% oleic acid) | 1.065 ± 0.040 |
| Fatty Acids | % |
|---|---|
| C14:0 (Myristic acid) | Not detected |
| C16:0 (Palmitic acid) | 18.29 ± 0.2 |
| C16:1 (Palmitoleic acid) | 8.36 ± 0.05 |
| C18:0 (Stearic acid) | 0.69 ± 0.01 |
| C18:1 (Oleic acid) | 54.33 ± 0.4 |
| C18:2n-6 (Linoleic acid) | 11.54 ± 0.2 |
| C18:3n-3 (Linolenic acid) | 0.78 ± 0.02 |
| Measured Property | Value |
|---|---|
| Total phenolic content (mg GAE/g oil) | 25.73 ± 2.1 |
| DPPH (IC50, mg/mL) | 32.4 ± 1.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktar, T.; Adal, E. Determining the Arrhenius Kinetics of Avocado Oil: Oxidative Stability under Rancimat Test Conditions. Foods 2019, 8, 236. https://doi.org/10.3390/foods8070236
Aktar T, Adal E. Determining the Arrhenius Kinetics of Avocado Oil: Oxidative Stability under Rancimat Test Conditions. Foods. 2019; 8(7):236. https://doi.org/10.3390/foods8070236
Chicago/Turabian StyleAktar, Tugba, and Eda Adal. 2019. "Determining the Arrhenius Kinetics of Avocado Oil: Oxidative Stability under Rancimat Test Conditions" Foods 8, no. 7: 236. https://doi.org/10.3390/foods8070236
APA StyleAktar, T., & Adal, E. (2019). Determining the Arrhenius Kinetics of Avocado Oil: Oxidative Stability under Rancimat Test Conditions. Foods, 8(7), 236. https://doi.org/10.3390/foods8070236