Effect of Sugar Content on Quality Characteristics and Shelf-Life of Probiotic Dry-Fermented Sausages Produced by Free or Immobilized Lactobacillus casei ATCC 393
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Cell Immobilization
2.2. Production of Probiotic Dry-Fermented Sausages
2.3. Physicochemical Analysis
2.4. Microbiological Analysis
2.5. Determination of L. casei ATCC 393 Levels
2.6. Preliminary Sensory Evaluation
2.7. Experimental Design and Statistical Analysis
3. Results
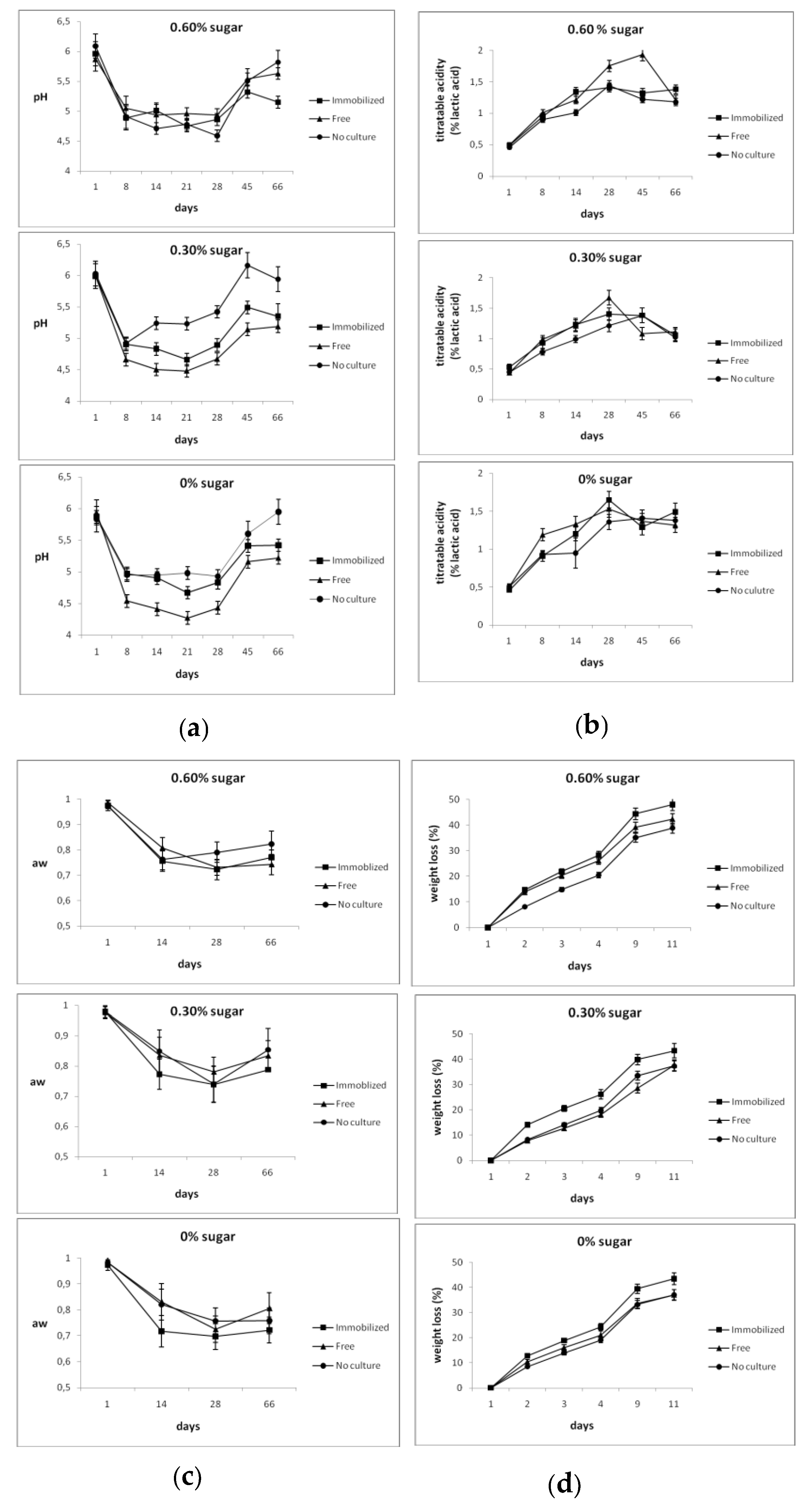
3.1. Physicochemical Analysis
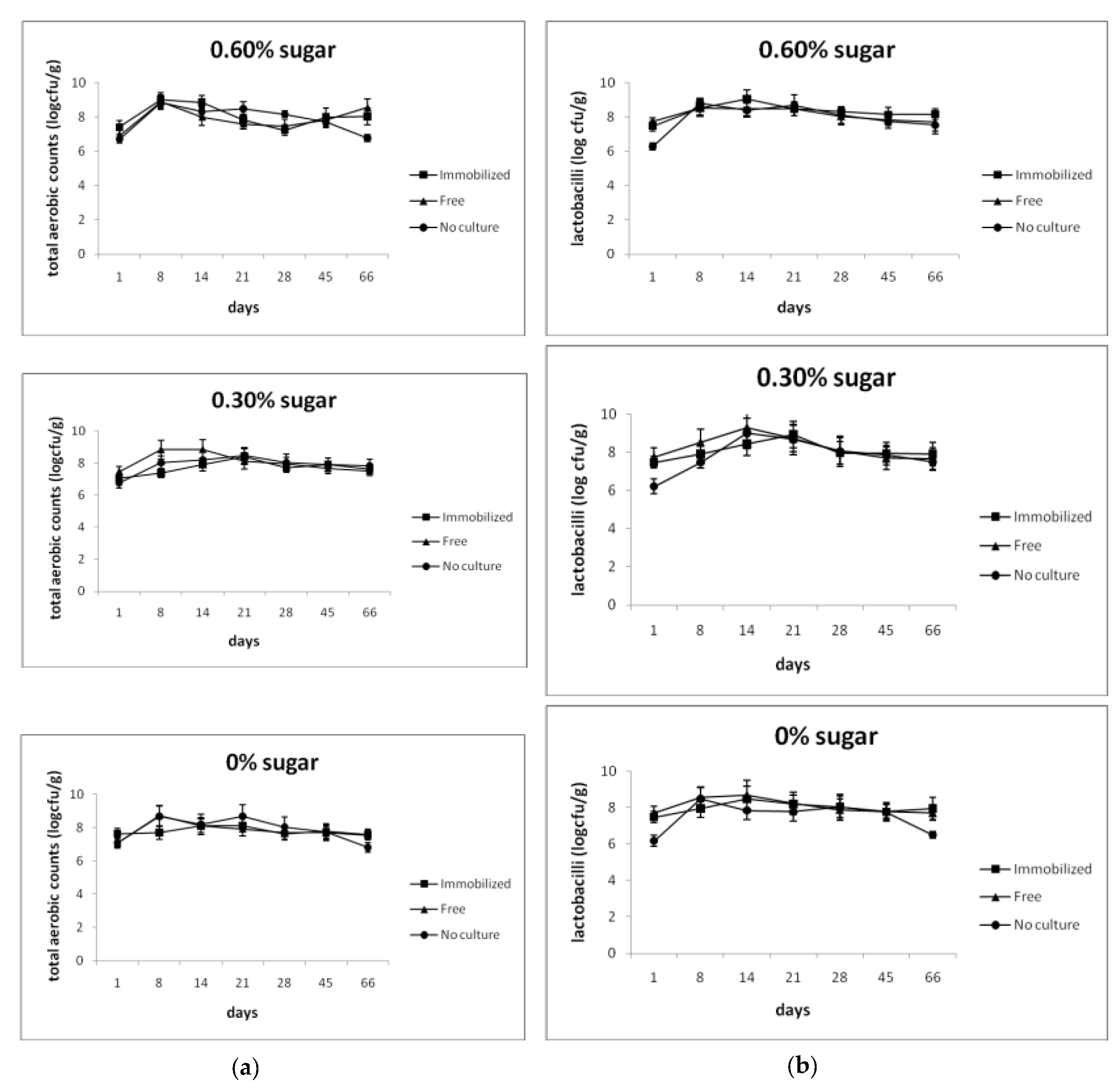
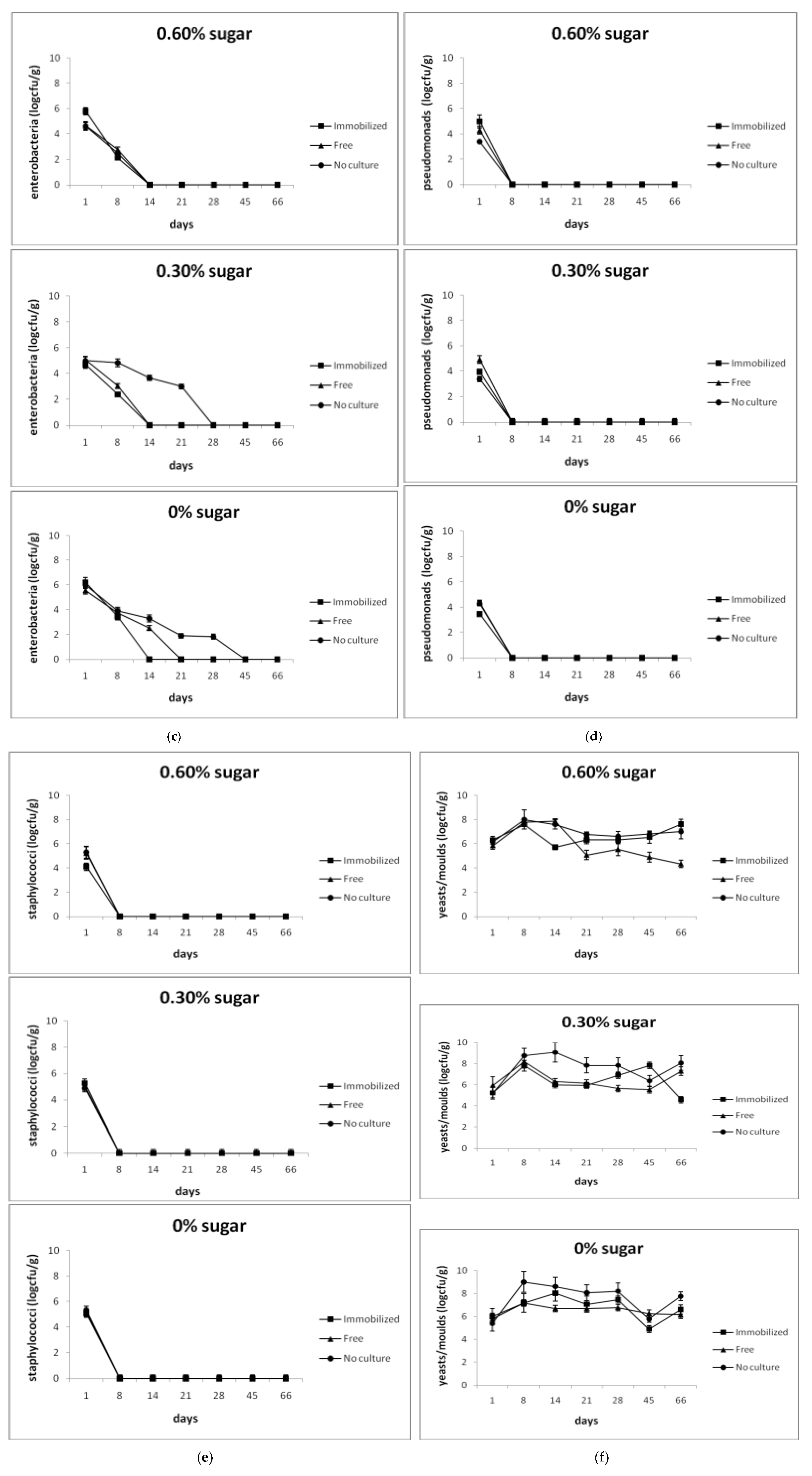
3.2. Shelf Life and Microbiological Analysis
3.3. Determination of L. casei ATCC 393 Levels
3.4. Preliminary Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization technologies in probiotic food production. J. Nutr. Metab. 2013. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Kim, Y.; Han, K.S.; You, S.; Oh, S.; Kim, S.H. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 2006, 42, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Boylston, T.D.; Vinderola, C.G.; Ghoddusi, H.B.; Reinheimer, J.A. Incorporation of bifidobacteria into cheeses: Challenges and rewards. Int. Dairy J. 2004, 19, 315–387. [Google Scholar] [CrossRef]
- Ranadheera, R.D.C.S.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy vs non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Mattila-Sandholm, T.; Myllarinen, P.; Crittenden, R.; Mogensen, G.; Fonden, R.; Saarela, M. Technological challenges for future probiotic foods. Int. Dairy J. 2002, 12, 173–182. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Xolias, V.; Kallis, M.; Bezirtzoglou, E.; Kanellaki, M. Lactobacillus casei immobilization on fruit pieces for probiotic additive, fermented milk and lactic acid production. Process Biochem. 2005, 40, 411–416. [Google Scholar] [CrossRef]
- Bosnea, L.; Kourkoutas, Y.; Albantaki, N.; Tzia, C.; Koutinas, A.A.; Kanellaki, M. Functionality of freeze-dried L. casei cells immobilized on wheat grains. LWT Food Sci. Technol. 2009, 42, 1696–1702. [Google Scholar] [CrossRef]
- Chen, K.N.; Chen, M.J.; Liu, J.R.; Lin, C.W.; Chiu, H.Y. Optimization of incorporated prebiotics as coating materials for probiotic microencapsulation. J. Food Sci. 2005, 70, M260–M266. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Knez, M.; Stangoulis, J.C.R. The effect of wheat prebiotics on the gut bacterial population and iron status of iron deficient broiler chickens. Nutr. J. 2014, 13, 58. [Google Scholar] [CrossRef]
- FAO: Cereals and Other Starch-Based Staples: Are Consumption Patterns Changing? Available online: http://www.fao.org/docrep/meeting/007/j1183e/j1183e00.htm (accessed on 10 February 2004).
- Marotti, I.; Bregola, V.; Aloisio, I.; Di Gioia, D.; Bosi, S.; Di Silvestro, R.; Quinn, R.; Dinelli, G. Prebiotic effect of soluble fibers from modern and old durum-type wheat varieties on Lactobacillus and Bifidobacterium strains. J. Sci. Food Agric. 2012, 92, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Delzenne, N.M. Potential interest of gut microbial changes induced by non-digestible carbohydrates of wheat in the management of obesity and related disorders. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 722–728. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Changes in biogenic amine and polyamine contents in slightly fermented sausages manufactured with and without sugar. Meat Sci. 2001, 57, 215–221. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Santos, E.M.; Jaime, I.; Rovira, J. Influence of starter cultures and sugar concentrations on biogenic amine contents in chorizo dry sausage. Food Microbiol. 2003, 20, 275–284. [Google Scholar] [CrossRef]
- Saucier, L.; Champagne, C.P. Immobilised cell technology and meat processing. In Applications of Cell Immobilisation Biotechnology; Nédovic, V., Willaert., R., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume FOBI 8P, pp. 337–353. ISBN 978-1-4020-3363-6. [Google Scholar]
- Drosinos, E.H.; Mataragas, M.; Xiraphi, N.; Moschonas, G.; Gaitis, F.; Metaxopoulos, J. Characterization of the microbial flora from a traditional Greek fermented sausage. Meat Sci. 2005, 69, 307–317. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Santos, E.M.; Rovira, J.; Jaimi, I. The effect of sugar concentration and starter culture on instrumental and sensory textural properties of chorizo-Spanish dry cured sausage. Meat Sci. 2006, 74, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sidira, M.; Karapetsas, A.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effective survival of immobilized Lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of microbial dynamics. Meat Sci. 2014, 96, 948–955. [Google Scholar] [CrossRef]
- Sidira, M.; Galanis, A.; Nikolaou, A.; Kanellaki, M.; Kourkoutas, Y. Evaluation of Lactobacillus casei ATCC 393 protective effect against spoilage of probiotic dry-fermented sausages. Food Control 2014, 42, 315–320. [Google Scholar] [CrossRef]
- De Vuyst, L.; Falony, G.; Leroy, F. Probiotics in fermented sausages. Meat Sci. 2008, 80, 75–88. [Google Scholar] [CrossRef]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bosnea, L.; Taboukos, S.; Baras, C.; Lambrou, D.; Kanellaki, M. Probiotic cheese production using Lactobacillus casei cells immobilized on fruit pieces. J. Dairy Sci. 2006, 89, 1431–1451. [Google Scholar] [CrossRef]
- Sidira, M.; Galanis, A.; Ypsilantis, P.; Karapetsas, A.; Progaki, Z.; Simopoulos, C.; Kourkoutas, Y. Effect of probiotic-fermented milk administration on gastrointestinal survival of Lactobacillus casei ATCC 393 and modulation of intestinal microbial flora. J. Mol. Microbiol. Biotechnol. 2010, 19, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Saxami, G.; Ypsilantis, P.; Sidira, M.; Simopoulos, C.; Kourkoutas, Y.; Galanis, A. Distinct adhesion of probiotic strain Lactobacillus casei ATCC 393 to rat intestinal mucosa. Anaerobe 2012, 18, 417–420. [Google Scholar] [CrossRef] [PubMed]
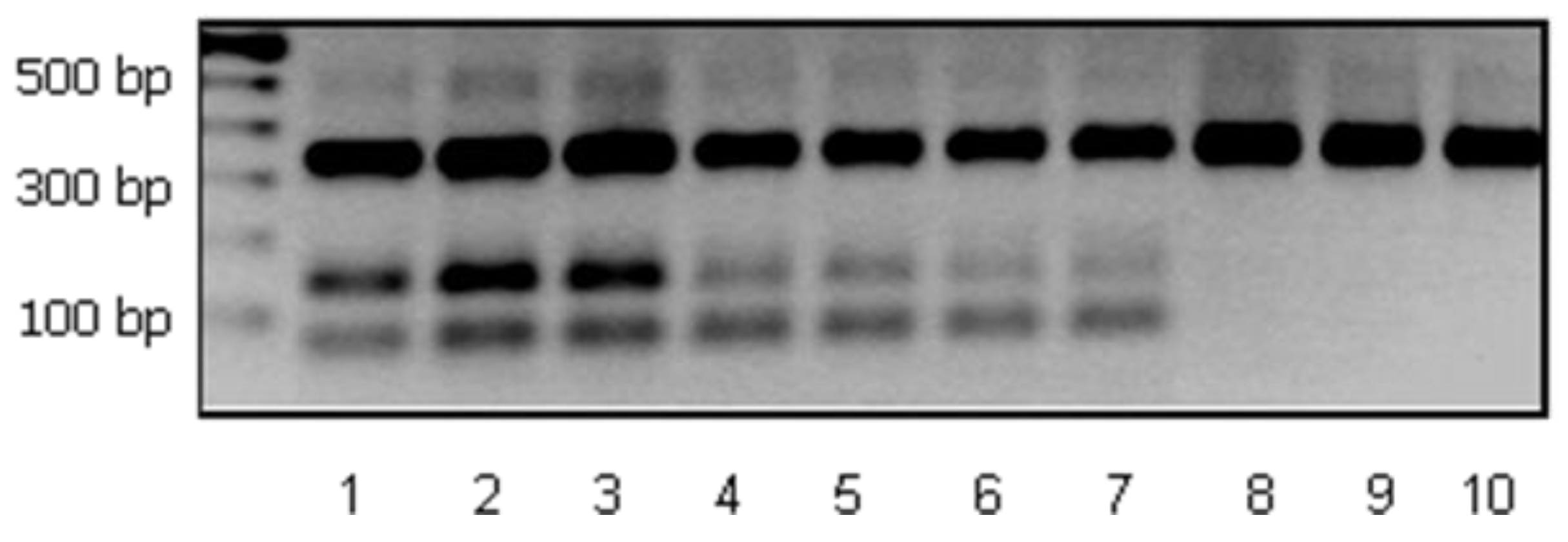
- Karapetsas, A.; Vavoulidis, E.; Galanis, A.; Sandaltzopoulos, R.; Kourkoutas, Y. Rapid detection and identification of probiotic Lactobacillus casei ATCC 393 by multiplex PCR. J. Mol. Microbiol. Biotechnol. 2010, 18, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Roseiro, L.C.; Santos, C.; Sol, M.; Borges, M.J.; Anjos, M.; Conçalves, H.; Carvalho, A.S. Proteolysis in Painho de Portalegre dry-fermented sausage in relation to ripening time and salt content. Meat Sci. 2008, 79, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Castaňo, A.; García-Fontán, M.C.; Fresno, J.M.; Tornadijo, M.E.; Carballo, J. Survival of Enterobacteriaceae during processing of Chorizo de cebolla, a Spanish fermented sausage. Food Control 2002, 13, 107–115. [Google Scholar] [CrossRef]
- Holck, A.; Axelsson, L.; McLeod, A.; Rode, T.M.; Even Heir, E. Health and safety considerations of fermented sausages. J. Food Qual. 2017. [Google Scholar] [CrossRef]
- Flores, M.; Durá, M.A.; Marco, A.; Toldrá, F. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci. 2004, 68, 439–446. [Google Scholar] [CrossRef]
- Erkkilä, S.; Suihko, M.L.; Eerola, S.; Petäjä, E.; Mattila-Sandholm, T. Dry sausage fermented by Lactobacillus rhamnosus strains. Int. J. Food Microbiol. 2001, 64, 205–210. [Google Scholar] [CrossRef]
- Klingberg, T.D.; Axelsson, L.; Naterstad, K.; Elsser, D.; Budde, B.B. Identification of potential probiotic starter cultures for Scandinavian-type fermented sausages. Int. J. Food Microbiol. 2005, 105, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Muthukumarasamy, P.; Holley, R.A. Survival of Escherichia coli O157:H7 in dry-fermented sausages containing micro-encapsulated probiotic lactic acid bacteria. Food Microbiol. 2007, 24, 82–88. [Google Scholar] [CrossRef] [PubMed]
| Sugar | Starter Culture | Day of Spoilage |
|---|---|---|
| 0.60% | Immobilized | No spoilage |
| Free | No spoilage | |
| No culture | 43 | |
| 0.30% | Immobilized | 49 |
| Free | 47 | |
| No culture | 22 | |
| No sugar | Immobilized | 43 |
| Free | 43 | |
| No culture | 22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidira, M.; Mitropoulou, G.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effect of Sugar Content on Quality Characteristics and Shelf-Life of Probiotic Dry-Fermented Sausages Produced by Free or Immobilized Lactobacillus casei ATCC 393. Foods 2019, 8, 219. https://doi.org/10.3390/foods8060219
Sidira M, Mitropoulou G, Galanis A, Kanellaki M, Kourkoutas Y. Effect of Sugar Content on Quality Characteristics and Shelf-Life of Probiotic Dry-Fermented Sausages Produced by Free or Immobilized Lactobacillus casei ATCC 393. Foods. 2019; 8(6):219. https://doi.org/10.3390/foods8060219
Chicago/Turabian StyleSidira, Marianthi, Gregoria Mitropoulou, Alex Galanis, Maria Kanellaki, and Yiannis Kourkoutas. 2019. "Effect of Sugar Content on Quality Characteristics and Shelf-Life of Probiotic Dry-Fermented Sausages Produced by Free or Immobilized Lactobacillus casei ATCC 393" Foods 8, no. 6: 219. https://doi.org/10.3390/foods8060219
APA StyleSidira, M., Mitropoulou, G., Galanis, A., Kanellaki, M., & Kourkoutas, Y. (2019). Effect of Sugar Content on Quality Characteristics and Shelf-Life of Probiotic Dry-Fermented Sausages Produced by Free or Immobilized Lactobacillus casei ATCC 393. Foods, 8(6), 219. https://doi.org/10.3390/foods8060219