Valorizations of Sweet Cherries Skins Phytochemicals by Extraction, Microencapsulation and Development of Value-Added Food Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sweet Cherries Fruits and Anthocyanins Extraction
2.3. Microencapsulation of Anthocyanins
2.4. Powder Characterization
2.5. Confocal Laser Scanning Microscopy
2.6. Scanning Electron Microscopy
2.7. Formulation of Value-Added Food Products
2.8. Phytochemical Analysis of Value-Added Food Products
2.9. Statistical Analysis of Data
3. Results and Discussion
3.1. Sweet Cherries Extract Characterization
3.2. Encapsulation Efficiency
3.3. Structure and Morphology of the Microencapsulated Powder
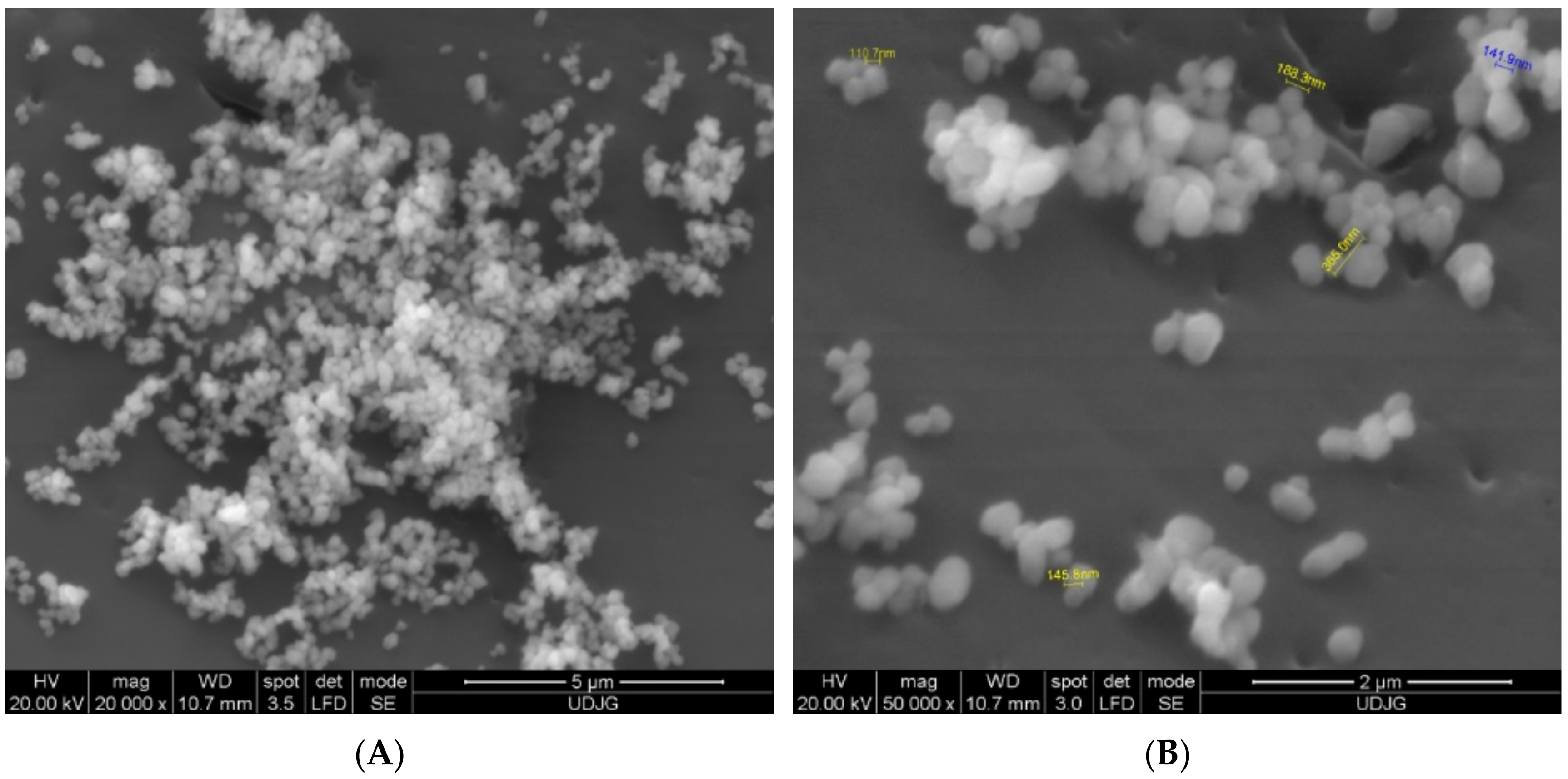
3.4. Scanning Electron Microscopy
3.5. Phytochemical Characterization of Microencapsulated Powder
3.6. The Influence of Microencapsulated Anthocyanins on the Growth of L. casei 431®
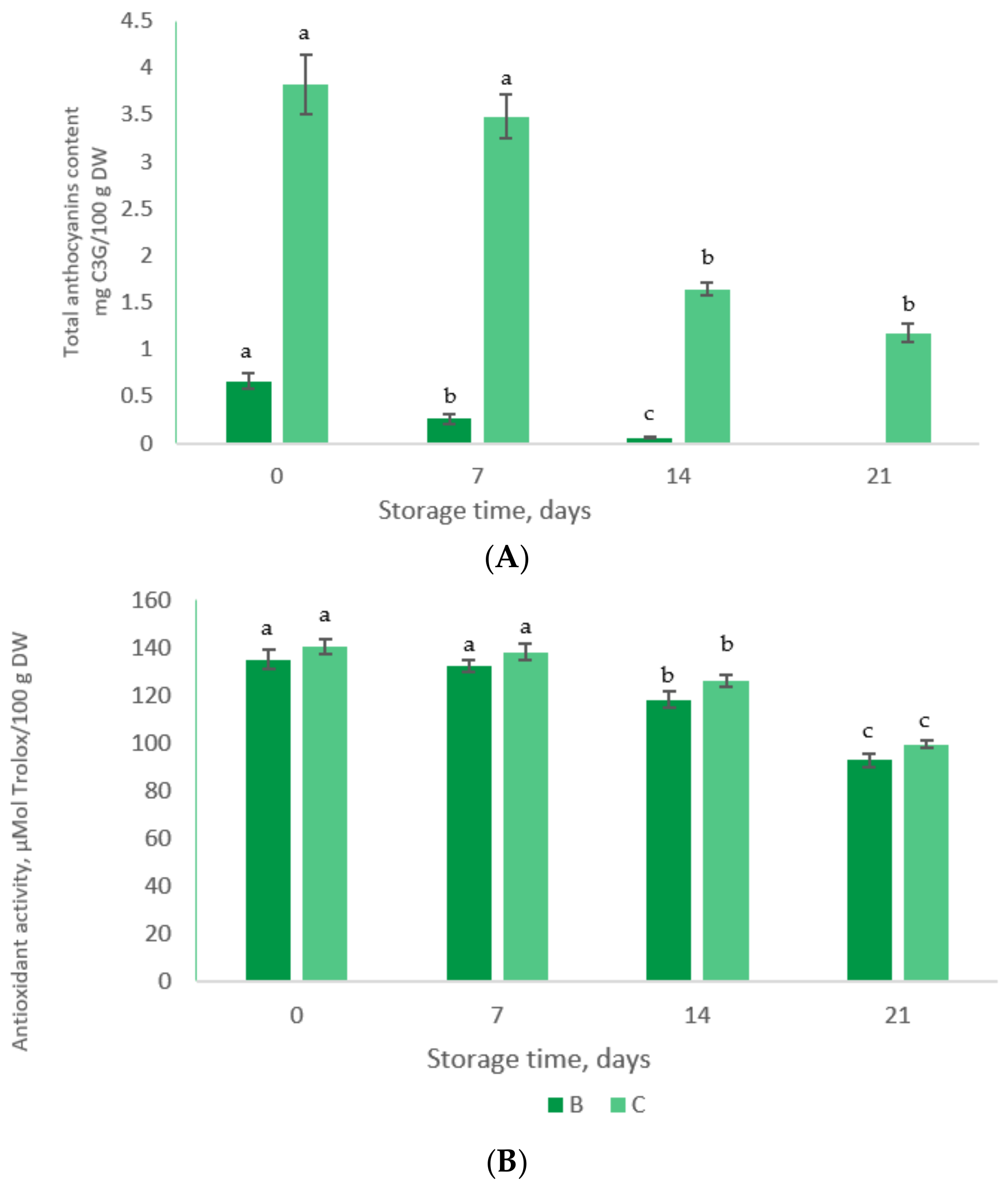
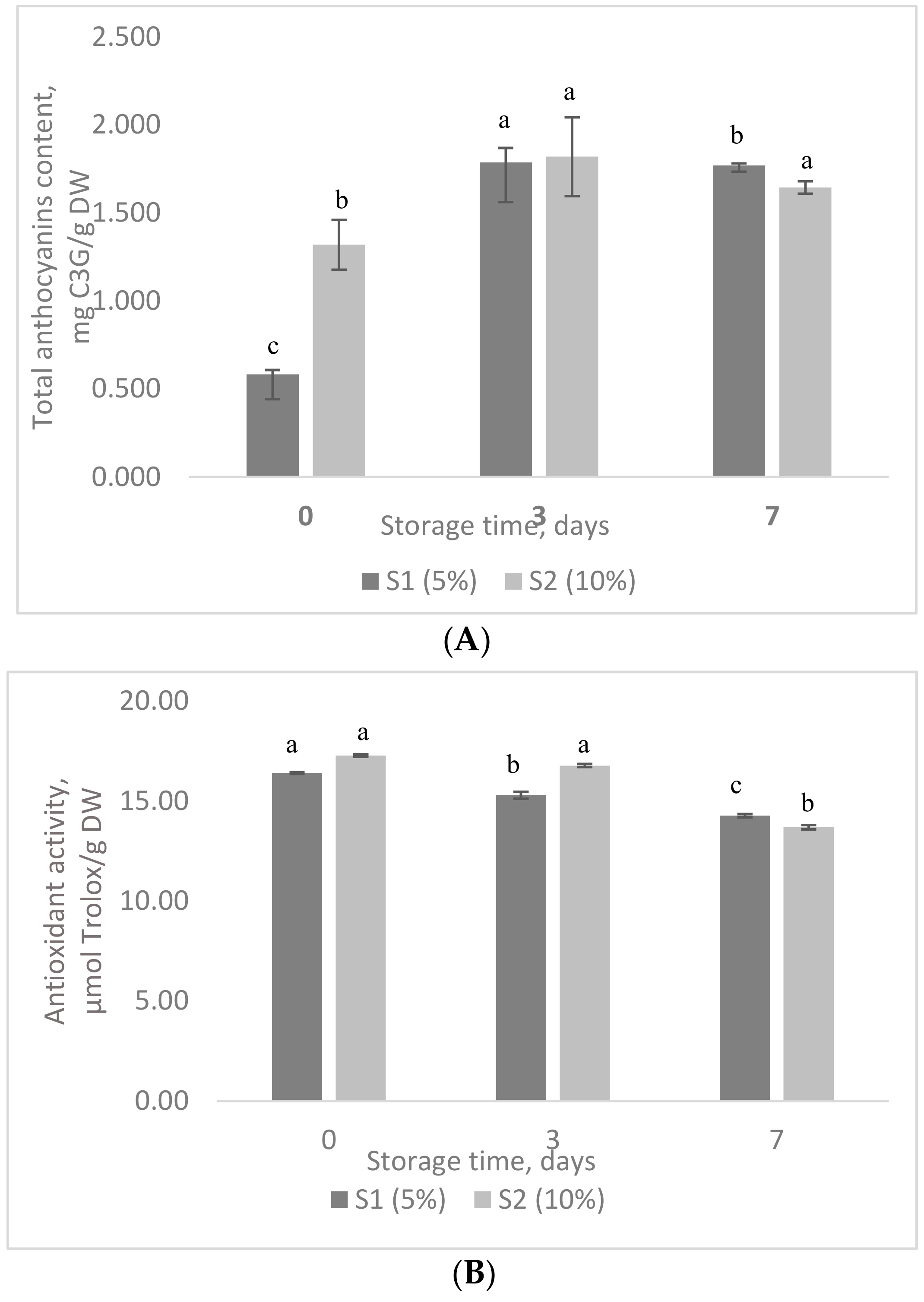
3.7. The Stability of Phytochemicals in Value-Added Food Products during Storage Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helkar, P.B.; Sahoo, A.K.; Patil, N.J. Food industry by-products used as a functional food ingredient. Int. J. Waste Resour. 2016, 6, 248. [Google Scholar]
- Oliviera, D.A.; Angonese, M.; Ferreira, R.S.; Gomes, C.L. Nanoencapsulation of passion fruit by-products extracts for enhanced antimicrobial activity. Food Bioprod. Process. 2017, 104, 137–146. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Anthocyanin content and antioxidant activity of various red fruit juices. Dtsch. Lebensmitt. Rundsch. 2007, 103, 58–64. [Google Scholar]
- Usenik, V.; Fabcic, J.; Stampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Goncalves, B.; Landbo, A.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Gul, K.; Wani, M.H.; Langowski, H.C. Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packag. Shelf 2014, 1, 86–99. [Google Scholar] [CrossRef]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Seeram, N.P.; Bourquin, L.D.; Nair, M.G. Tart cherry anthocyanins inhibit tumour development in ApcMin mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef]
- James, T.; Belanger, N.D. Perillyl alcohol: Applications in oncology. Altern. Med. Rev. 1998, 3, 448–457. [Google Scholar]
- Mamani-Matsuda, M.; Kauss, T.; Al-Kharrat, A.; Rambert, J.; Fawaz, F.; Thiolat, D.; Moynet, D.; Coves, S.; Malvy, D.; Mossalayi, M.D. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decrease macrophage inflammatory mediators. Biochem. Pharmacol. 2006, 72, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.A.J.; McHugh, M.P.; Padilla-Zakour, O.I. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Traustadtir, T.; Davies, S.S.; Tian, S.P.; Jiang, A.L.; Xu, Y.; Wang, Y.S. Responses of physiology and quality of sweet cherry fruit to different atmosphere in storage. Food Chem. 2004, 87, 43–49. [Google Scholar]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Aprodu, I.; Râpeanu, G.; Bahrim, G.; Stănciuc, N. Binding mechanism of anthocyanins from sour cherries (Prunus cerasus L.) skins to bovine β-lactoglobulin: A fluorescence and in silico based approach. Int. J. Food Prop. 2017, 20, S3096–S3111. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT Food. Sci. Technol. 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Turturică, M.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. Phytochemicals and antioxidant activity degradation kinetics during thermal treatments of sour cherry extract. LWT Food. Sci. Technol. 2017, 82, 139–146. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunov, O.B.; Solovchenko, A.E.; Naqvi, K.R. Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J. Exp. Bot. 2008, 59, 3903–3911. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, N.S.; Carneiro, I.; da Silva, A.S.; Honjoya, E.R.; de Santana, E.H.; Aragon-Alegro, L.C.; de Souza, C.H. Viability of probiotic Lactobacillus casei in yogurt: Defining the best processing step to its addition. Arch. Latinoam. Nutr. 2013, 63, 58–63. [Google Scholar]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Investigations on sweet cherry phenolics degradation during thermal treatment based on fluorescence spectroscopy and inactivation kinetics. Food Bioprocess Technol. 2016, 9, 1706–1715. [Google Scholar] [CrossRef]
- Stănciuc, N.; Turturică, M.; Oancea, A.-M.; Barbu, V.; Ionita, E.; Aprodu, I.; Râpeanu, G. Microencapsulation of anthocyanins from grapes skins by whey proteins isolates and different polymers. Food Bioprocess Technol. 2017, 10, 1715–1726. [Google Scholar] [CrossRef]
- Li, Y.; Tang, B.; Chen, J.; Lai, P. Microencapsulation of plum (Prunus salicina Lindl.) phenolics by spray drying technology and storage stability. Int. J. Food Sci. Technol. 2018, 38, 530–536. [Google Scholar] [CrossRef]
- Akhavan Mahdavi, S.; Mahdi Jafari, S.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- TumbasŠaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S. Encapsulation of Beetroot Pomace Extract: RSM Optimization, Storage and Gastrointestinal Stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef]
- Prigent, S.V.E.; Gruppen, H.; Visser, A.J.W.G.; van Koningsveld, G.A.; de Jong, G.A.H.; Voragen, A.G.J. Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J. Agric. Food Chem. 2003, 51, 5088–5095. [Google Scholar] [CrossRef]
- Glover, B.J.; Martin, C. Anthocyanins. Curr. Biol. 2012, 22, 147–150. [Google Scholar] [CrossRef]
- Osorio, C.; Acevedo, B.; Hillebrand, S.; Carriazo, J.; Winterhalter, P.; Morales, A.L. Microencapsulation by spray-drying of anthocyanin pigments from corozo (Bactris guineensis) fruit. J. Agric. Food Chem. 2010, 58, 6977–6985. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, D.A.; Hill, L.E.; Zambiazi, R.C.; Borges, C.D.; Vizzotto, M.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 2008, 240, 396–404. [Google Scholar] [CrossRef]
- Silva, L.M.; Hill, L.E.; Figueiredo, E.; Gomes, C.L. Delivery of phytochemicals of tropical fruit by-products using poly (dl-lactide-co-glycolide) (PLGA) nanoparticles: Synthesis, characterization, and antimicrobial activity. Food Chem. 2014, 165, 362–370. [Google Scholar] [CrossRef]
- Kar, A.; Kumar Mahato, D.; Patel, A.S.; Bal, L.M. The Encapsulation Efficiency and Physicochemical Characteristics of Anthocyanin from Black Carrot (Daucus Carota Ssp. Sativus) as Affected by Encapsulating Materials. Curr. Agric. Res. J. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microb. 2011, 28, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, D. Development of antioxidant rich fruit supplemented probiotic yogurts using free and microencapsulated Lactobacillus rhamnosus culture. Int. J. Food Sci. Technol. 2016, 53, 667–675. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Coisson, J.; Travaglia, F.; Piana, G.; Capasso, M.; Arlorio, M. Euterpe oleracea juice as a functional pigment for yogurt. Food Res. Int. 2005, 38, 893–897. [Google Scholar] [CrossRef]

| Storage Days | A | B | C |
|---|---|---|---|
| 0 | 9.41 ± 0.32 aA | 8.07 ± 0.11 aB | 6.59 ± 0.15 aB |
| 7 | 9.34 ± 0.24 aA | 8.04 ± 0.14 bB | 6.23 ± 0.11 bB |
| 14 | 9.19 ± 0.14 bA | 7.64 ± 0.17 aB | 6.17 ± 0.13 bB |
| 21 | 9.07 ± 0.11 bA | 7.55 ± 0.15 bB | 6.14 ± 0.21 bC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milea, A.Ș.; Vasile, A.M.; Cîrciumaru, A.; Dumitrașcu, L.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Valorizations of Sweet Cherries Skins Phytochemicals by Extraction, Microencapsulation and Development of Value-Added Food Products. Foods 2019, 8, 188. https://doi.org/10.3390/foods8060188
Milea AȘ, Vasile AM, Cîrciumaru A, Dumitrașcu L, Barbu V, Râpeanu G, Bahrim GE, Stănciuc N. Valorizations of Sweet Cherries Skins Phytochemicals by Extraction, Microencapsulation and Development of Value-Added Food Products. Foods. 2019; 8(6):188. https://doi.org/10.3390/foods8060188
Chicago/Turabian StyleMilea, Adelina Ștefania, Aida Mihaela Vasile, Adrian Cîrciumaru, Loredana Dumitrașcu, Vasilica Barbu, Gabriela Râpeanu, Gabriela Elena Bahrim, and Nicoleta Stănciuc. 2019. "Valorizations of Sweet Cherries Skins Phytochemicals by Extraction, Microencapsulation and Development of Value-Added Food Products" Foods 8, no. 6: 188. https://doi.org/10.3390/foods8060188
APA StyleMilea, A. Ș., Vasile, A. M., Cîrciumaru, A., Dumitrașcu, L., Barbu, V., Râpeanu, G., Bahrim, G. E., & Stănciuc, N. (2019). Valorizations of Sweet Cherries Skins Phytochemicals by Extraction, Microencapsulation and Development of Value-Added Food Products. Foods, 8(6), 188. https://doi.org/10.3390/foods8060188









