Optimization of Emulsifier and Stabilizer Concentrations in a Model Peanut-Based Beverage System: A Mixture Design Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Model Peanut-Based Beverages
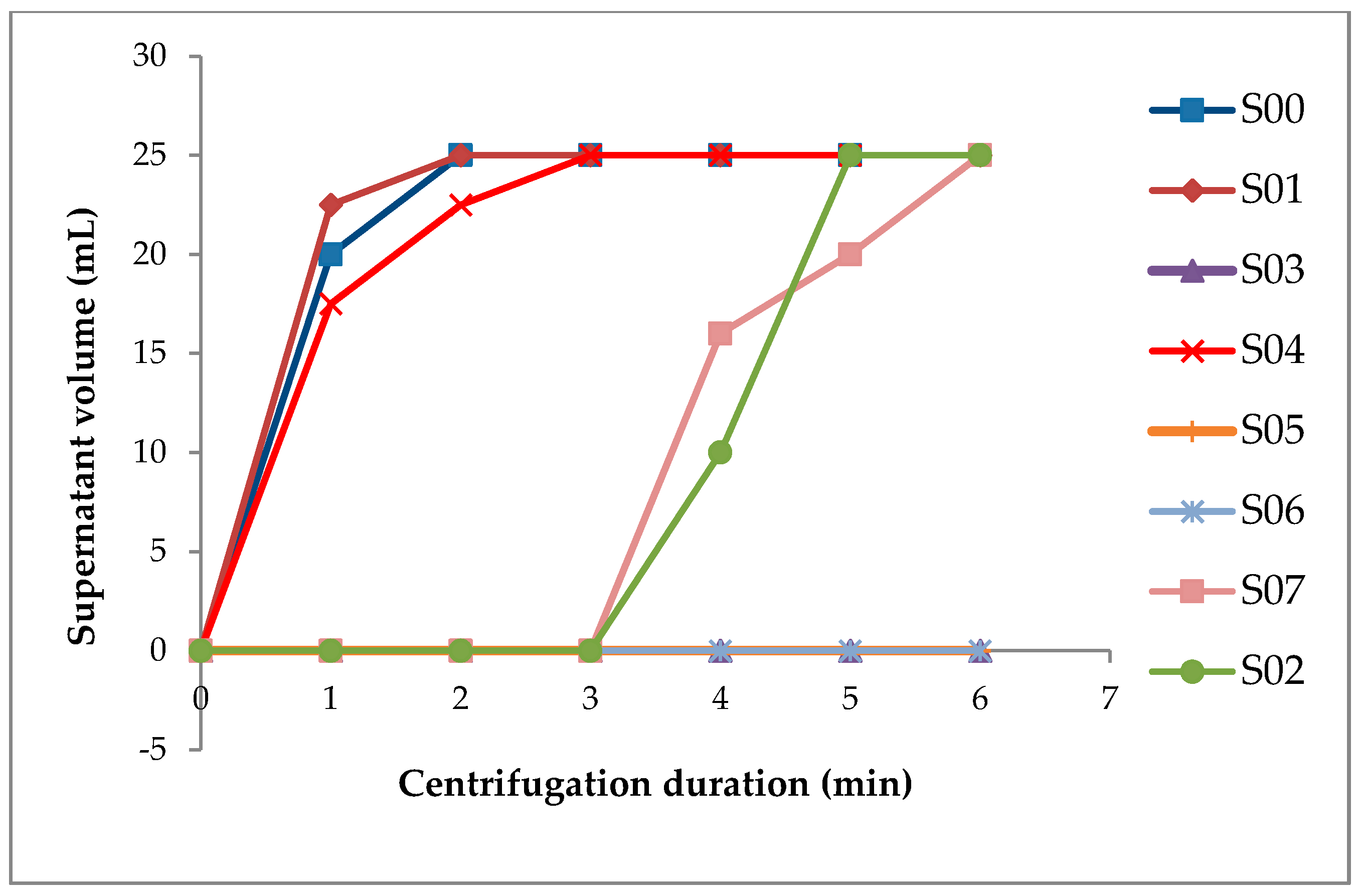
2.2. Determination of Stability Indices
2.3. Measurement of Viscosity and Flow Behavior
2.4. Measurement of Color, pH, Water Activity, and Soluble Solids
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Effect on Physicochemical Properties
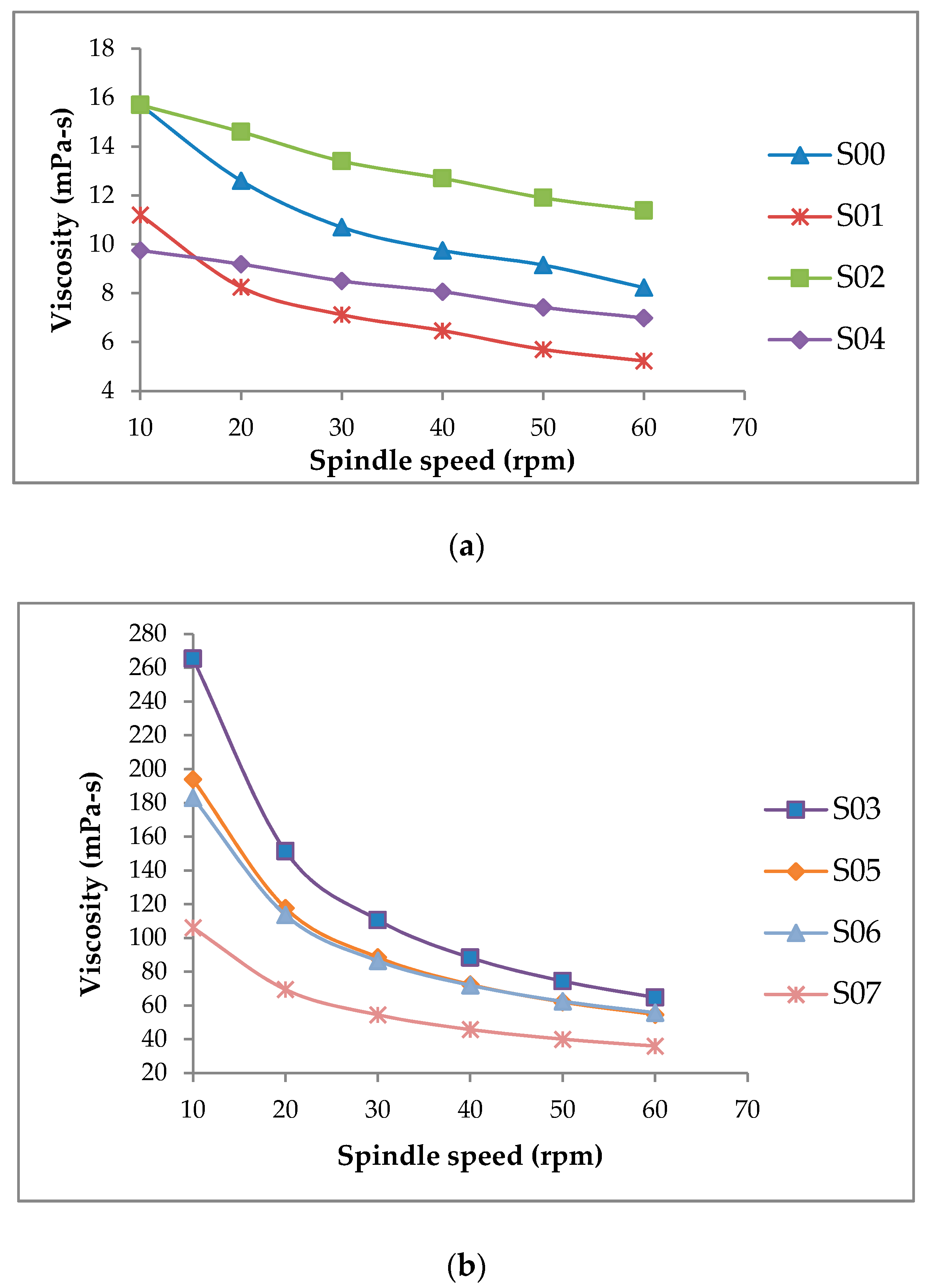
3.2. Effect on Flow Properties
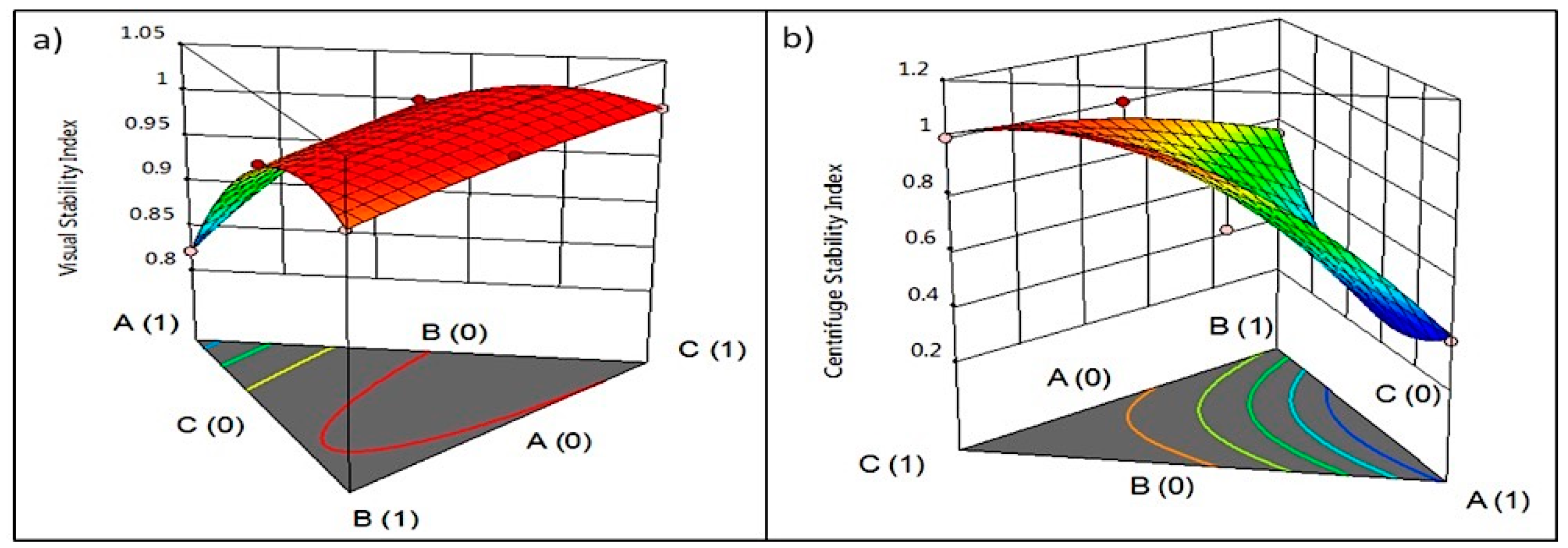
3.3. Effect on Viscosity and Colloidal Stability
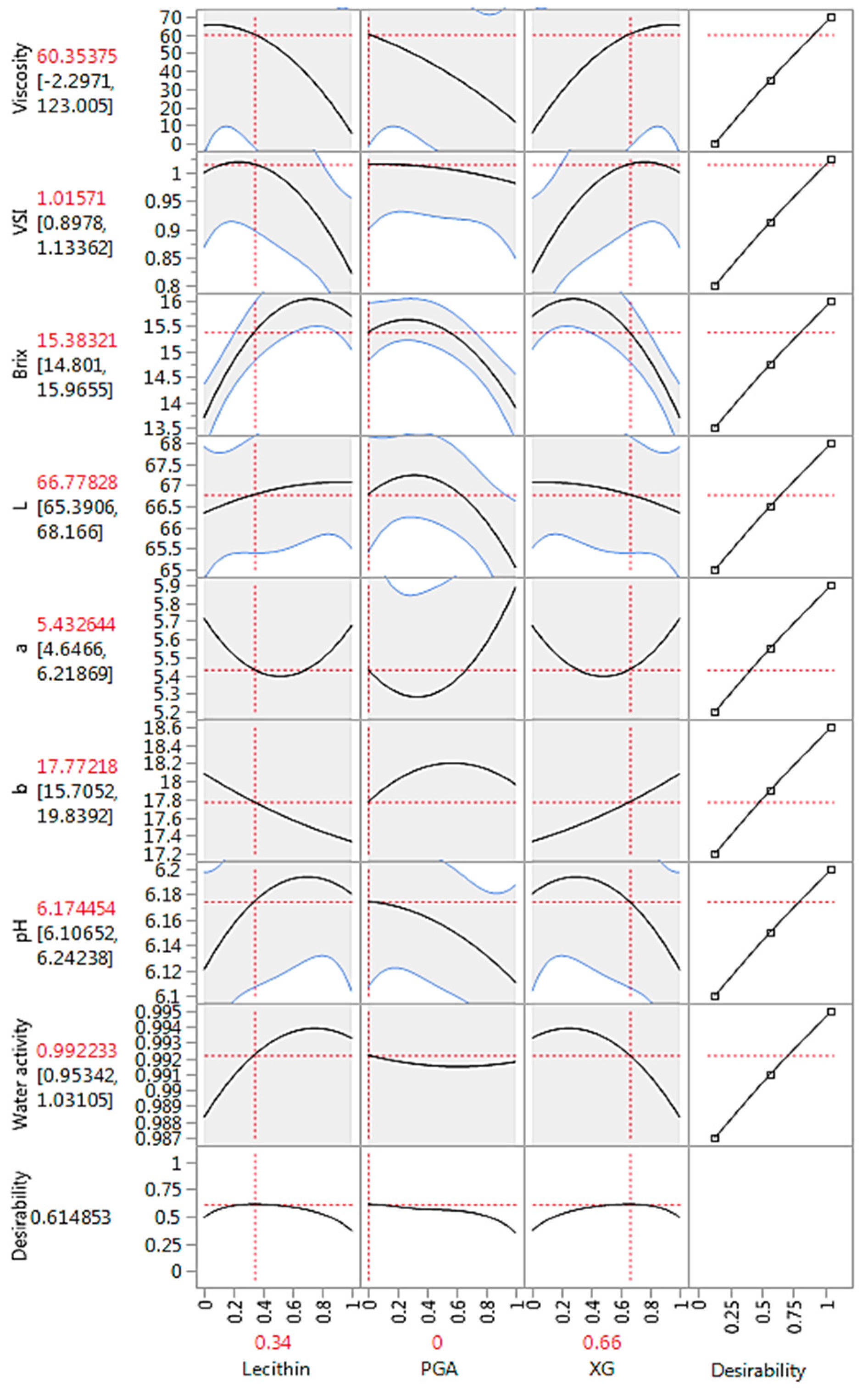
3.4. Optimization and Prediction Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Chompreeda, P.; Haruthaithanasan, V.; Oupadissakoon, C.; Suknak, K. Development of a chocolate flavored peanut beverage. J. Food Sci. 1989, 54, 1359–1360. [Google Scholar] [CrossRef]
- Deshpande, R.; Chinnan, M.; McWatters, K. Optimization of a chocolate-flavored, peanut–soy beverage using response surface methodology (RSM) as applied to consumer acceptability data. LWT-Food Sci. Technol. 2008, 41, 1485–1492. [Google Scholar] [CrossRef]
- Galvez, F.C.F.; Resurreccion, A.V.; Koehler, P.E. Optimization of processing of peanut beverage. J. Sens. Stud. 1990, 5, 1–17. [Google Scholar] [CrossRef]
- Hinds, M.J.; Beuchat, L.R.; Chinnan, M.S. Properties of a thermal-processed beverage prepared from roasted partially defatted peanuts. Int. J. Food Sci. Technol. 1997, 32, 203–211. [Google Scholar] [CrossRef]
- Rubico, S.; Phillips, R.; Resurreccion, A.; Beuchat, L. Nutritional, microbiological, and sensory qualities of a peanut beverage prepared using various processes. J. Food Sci. 1989, 54, 1540–1543. [Google Scholar] [CrossRef]
- Rustom, I.; López-Leiva, M.; Nair, B.M. UHT-sterilized peanut beverages: Kinetics of physicochemical changes during storage and shelf-life prediction modeling. J. Food Sci. 1996, 61, 198–203. [Google Scholar] [CrossRef]
- Howard, B.M.; Hung, Y.C.; McWatters, S.K. Analysis of ingredient functionality and formulation optimization of an instant peanut beverage mix. J. Food Sci. 2010, 75, S8–S19. [Google Scholar] [CrossRef]
- Dickinson, E. Introduction to Food Colloids; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Dickinson, E. Towards more natural emulsifiers. Trends Food Sci. Technol. 1993, 4, 330–334. [Google Scholar]
- Garti, N. What can nature offer from an emulsifier point of view: Trends and progress? Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 125–146. [Google Scholar] [CrossRef]
- Walstra, P. Physical Chemistry of Foods; Marcel Decker, Inc.: New York, NY, USA, 2003; pp. 397–450. [Google Scholar]
- Paquin, P. Functional and Speciality Beverage Technology; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Piorkowski, D.T.; McClements, D.J. Beverage emulsions: Recent developments in formulation, production, and applications. Food Hydrocoll. 2014, 42, 5–41. [Google Scholar] [CrossRef]
- Dogan, M.; Toker, O.S.; Aktar, T.; Goksel, M. Optimization of gum combination in prebiotic instant hot chocolate beverage model system in terms of rheological aspect: Mixture design approach. Food Bioprocess Technol. 2013, 6, 783–794. [Google Scholar] [CrossRef]
- Brookfield. More Solutions to Sticky Problems. 2014. Available online: http://www.brookfield.nl/fileadmin/assets-brookfield/documents/More_Solutions_2014.pdf (accessed on 4 March 2018).
- Cho, H.M.; Yoo, B. Rheological characteristics of cold thickened beverages containing xanthan gum–based food thickeners used for dysphagia diets. J. Acad. Nutr. Diet. 2015, 115, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M. Food Texture and Viscosity: Concept and Measurement; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Yanes, M.; Durán, L.; Costell, E. Rheological and optical properties of commercial chocolate milk beverages. J. Food Eng. 2002, 51, 229–234. [Google Scholar] [CrossRef]
- Dogan, M.; Toker, O.S.; Goksel, M. Rheological behaviour of instant hot chocolate beverage: Part 1. Optimization of the effect of different starches and gums. Food Biophys. 2011, 6, 512–518. [Google Scholar] [CrossRef]
- Torres, M.D.; Moreira, R.; Chenlo, F.; Vázquez, M.J. Water adsorption isotherms of carboxymethyl cellulose, guar, locust bean, tragacanth and xanthan gums. Carbohydr. Polym. 2012, 89, 592–598. [Google Scholar] [CrossRef]
- Dogan, M.; Kayacier, A.; Ic, E. Rheological characteristics of some Food Hydrocoll. processed with gamma irradiation. Food Hydrocoll. 2007, 21, 392–396. [Google Scholar] [CrossRef]
- Toker, O.S.; Dogan, M.; Canıyılmaz, E.; Ersöz, N.B.; Kaya, Y. The effects of different gums and their interactions on the rheological properties of a dairy dessert: A mixture design approach. Food Bioprocess Technol. 2013, 6, 896–908. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, H.I.; Song, K.W.; Lee, J. Sensory and rheological characteristics of thickened liquids differing concentrations of a xanthan gum-based thickener. J. Text. Stud. 2017, 48, 571–585. [Google Scholar] [CrossRef]
- Garcıa-Ochoa, F.; Santos, V.; Casas, J.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Urlacher, B.; Noble, O. Xanthan gum. In Thickening and Gelling Agents for Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 284–311. [Google Scholar]
- Pettitt, D.J.; Wayne, J.E.B.; Nantz, J.J.R.; Shoemaker, C.F. Rheological properties of solutions and emulsions stabilized with xanthan gum and propylene glycol alginate. J. Food Sci. 1995, 60, 528–531. [Google Scholar] [CrossRef]
- Yilmazer, G.; Carrillo, A.R.; Kokini, J.L. Effect of propylene glycol alginate and xanthan gum on stability of o/w emulsions. J. Food Sci. 1991, 56, 513–517. [Google Scholar] [CrossRef]
- Nakauma, M.; Ishihara, S.; Funami, T.; Nishinari, K. Swallowing profiles of food polysaccharide solutions with different flow behaviors. Food Hydrocoll. 2011, 25, 1165–1173. [Google Scholar] [CrossRef]
- Napper, D.H. Polymeric Stabilization of Colloidal Dispersions; Academic Press: Cambridge, MA, USA, 1983; Volume 3. [Google Scholar]
- Singh, H. Aspects of milk-protein-stabilised emulsions. Food Hydrocoll. 2011, 25, 1938–1944. [Google Scholar] [CrossRef]
- Robins, M.M.; Watson, A.D.; Wilde, P.J. Emulsions—Creaming and rheology. Curr. Opin. Colloid Interface Sci. 2002, 7, 419–425. [Google Scholar] [CrossRef]
| Sample Codes | Gum and Emulsifier Proportions (%) | ||
|---|---|---|---|
| Lecithin (X1) | PGA (X2) | XG (X3) | |
| S00 (Control) | 0 | 0 | 0 |
| S01 | 100 | 0 | 0 |
| S02 | 0 | 100 | 0 |
| S03 | 0 | 0 | 100 |
| S04 | 50 | 50 | 0 |
| S05 | 50 | 0 | 50 |
| S06 | 0 | 50 | 50 |
| S07 | 33.3 | 33.4 | 33.3 |
| Sample | pH | °Brix | aw | Viscosity (mPa-s) | VSI | Viscosity Ratio | Color | ||
|---|---|---|---|---|---|---|---|---|---|
| L | a | b | |||||||
| S00 | 6.26 ± 0.01 a | 14.00 ± 0.14 c | 0.99 ± 0.00 a | 8.23 ± 0.04 f | 0.92 ± 0.00 c | 1.91 ± 0.01 f | 65.12 ± 0.08 d | 5.66 ± 0.25 ab | 17.51 ± 0.08 de |
| S01 | 6.18 ± 0.01 b | 15.65 ± 0.07 a | 0.99 ± 0.00 a | 5.23 ± 0.04 h | 0.82 ± 0.00 d | 2.14 ± 0.01 e | 67.06 ± 0.08 b | 5.67 ± 0.02 ab | 17.32 ± 0.06 e |
| S02 | 6.11 ± 0.01 d | 13.90 ± 0.14 c | 0.99 ± 0.00 a | 11.38 ± 0.03 e | 0.98 ± 0.00 b | 1.38 ± 0.00 g | 65.04 ± 0.12 d | 5.88 ± 0.05 a | 17.95 ± 0.03 bc |
| S03 | 6.12 ± 0.01 d | 13.70 ± 0.14 c | 0.98 ± 0.00 b | 65.70 ± 0.01 a | 1.00 ± 0.00 a | 4.10 ± 0.00 a | 66.33 ± 0.08 c | 5.71 ± 0.17 ab | 18.07 ± 0.10 b |
| S04 | 6.13 ± 0.01 cd | 14.80 ± 0.14 b | 0.99 ± 0.00 a | 6.99 ± 0.01 g | 0.98 ± 0.00 b | 1.39 ± 0.00 g | 66.18 ± 0.16 c | 5.59 ± 0.04 ab | 17.80 ± 0.02 bcd |
| S05 | 6.19 ± 0.01 b | 15.80 ± 0.28 a | 0.99 ± 0.00 a | 54.55 ± 0.07 c | 1.00 ± 0.00 a | 3.55 ± 0.00 b | 66.96 ± 0.03 b | 5.42 ± 0.00 ab | 17.71 ± 0.03 cd |
| S06 | 6.16 ± 0.01 bc | 15.40 ± 0.00 ab | 0.99 ± 0.00 a | 55.68 ± 0.04 b | 1.00 ± 0.00 a | 3.29 ± 0.00 c | 67.53 ± 0.06 a | 5.32 ± 0.22 b | 18.52 ± 0.13 a |
| S07 | 6.16 ± 0.01 bc | 15.70 ± 0.14 a | 0.99 ± 0.00 a | 35.89 ± 0.01 d | 1.00 ± 0.00 a | 2.95 ± 0.00 d | 66.98 ± 0.01 b | 5.25 ± 0.08 b | 17.85 ± 0.08 bc |
| Term | Estimate | 95% CI | χ2 | P | VIF |
|---|---|---|---|---|---|
| X1 (Lecithin) | 0.82 | 0.81–0.84 | 6086.72 | <0.0001 | 0.9913 |
| X2 (PGA) | 0.98 | 0.97–1.00 | 8678.29 | <0.0001 | 0.8295 |
| X3 (XG) | 1.00 | 0.99–1.02 | 9017.56 | <0.0001 | 0.8295 |
| X1X2 | 0.30 | 0.23–0.37 | 38.53 | <0.0001 | 20.9249 |
| X1X3 | 0.34 | 0.27–0.41 | 48.86 | <0.0001 | 20.9249 |
| Model Fit Statistics | |||||
| R2 | 0.9950 | ||||
| Adjusted R2 | 0.9849 | ||||
| RMSE | 0.0043 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gama, A.P.; Hung, Y.-C.; Adhikari, K. Optimization of Emulsifier and Stabilizer Concentrations in a Model Peanut-Based Beverage System: A Mixture Design Approach. Foods 2019, 8, 116. https://doi.org/10.3390/foods8040116
Gama AP, Hung Y-C, Adhikari K. Optimization of Emulsifier and Stabilizer Concentrations in a Model Peanut-Based Beverage System: A Mixture Design Approach. Foods. 2019; 8(4):116. https://doi.org/10.3390/foods8040116
Chicago/Turabian StyleGama, Aggrey P., Yen-Con Hung, and Koushik Adhikari. 2019. "Optimization of Emulsifier and Stabilizer Concentrations in a Model Peanut-Based Beverage System: A Mixture Design Approach" Foods 8, no. 4: 116. https://doi.org/10.3390/foods8040116
APA StyleGama, A. P., Hung, Y.-C., & Adhikari, K. (2019). Optimization of Emulsifier and Stabilizer Concentrations in a Model Peanut-Based Beverage System: A Mixture Design Approach. Foods, 8(4), 116. https://doi.org/10.3390/foods8040116





