Aroma Patterns Characterization of Braised Pork Obtained from a Novel Ingredient by Sensory-Guided Analysis and Gas-Chromatography-Olfactometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials to Food Sample
2.3. Preparation of Self-Made Braised Sauce
2.4. Preparation of Braised Pork
2.5. Sensory Evaluation
2.6. SPME
2.7. SDE
2.8. GC-MS Analysis
2.9. SDE-GC-O Analysis
2.10. Aroma Extraction Dilution Analysis (AEDA)
2.11. Qualitative and Quantitative Analysis of the Volatile Compounds
2.12. Calibration of Standard Curves
2.13. Odor Aroma Value Determination
2.14. Statistical Analysis
3. Results
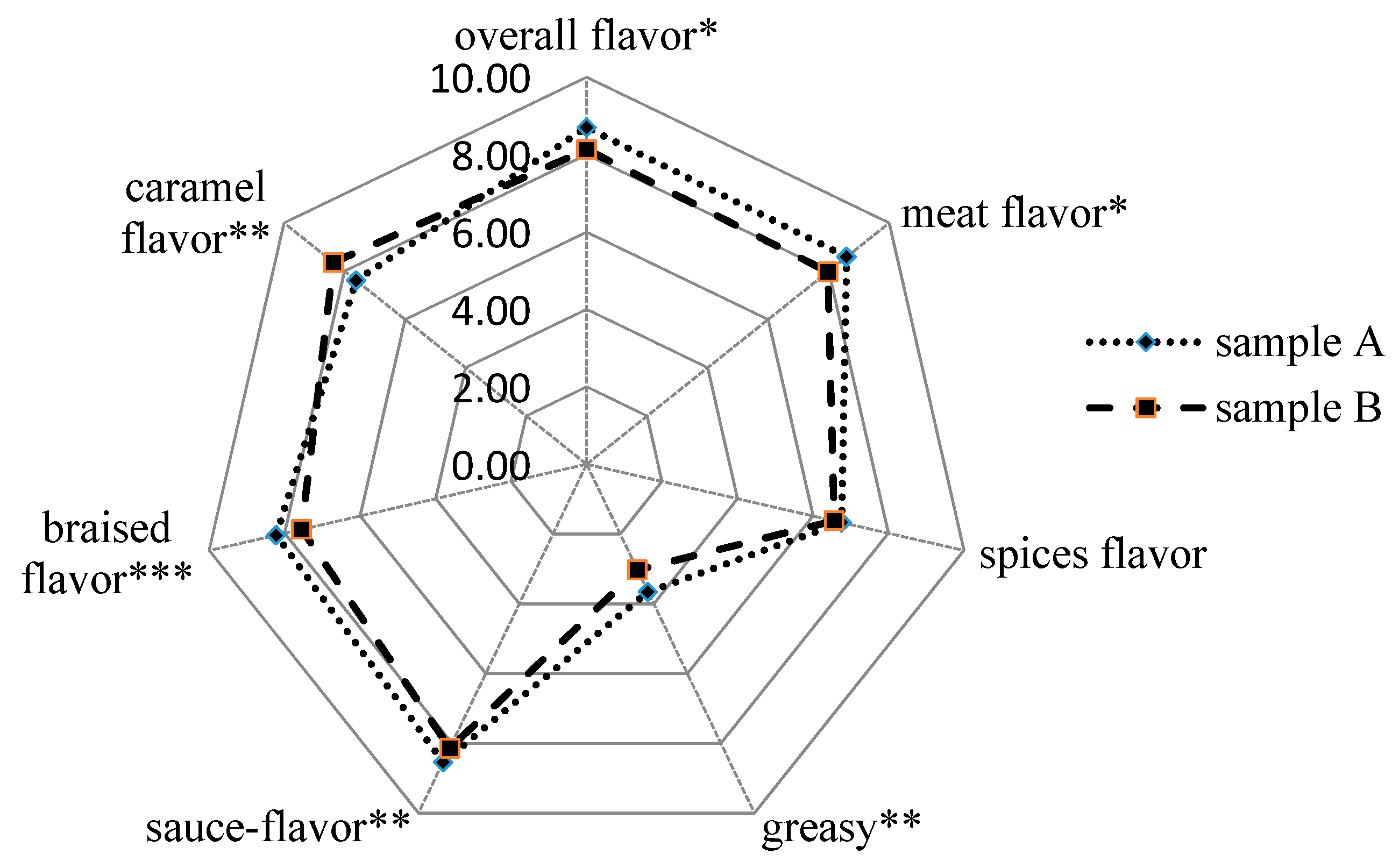
3.1. Sensory Evaluation of the Braised Pork Samples
3.2. Volatile Flavor Compounds Identified from the Braised Pork
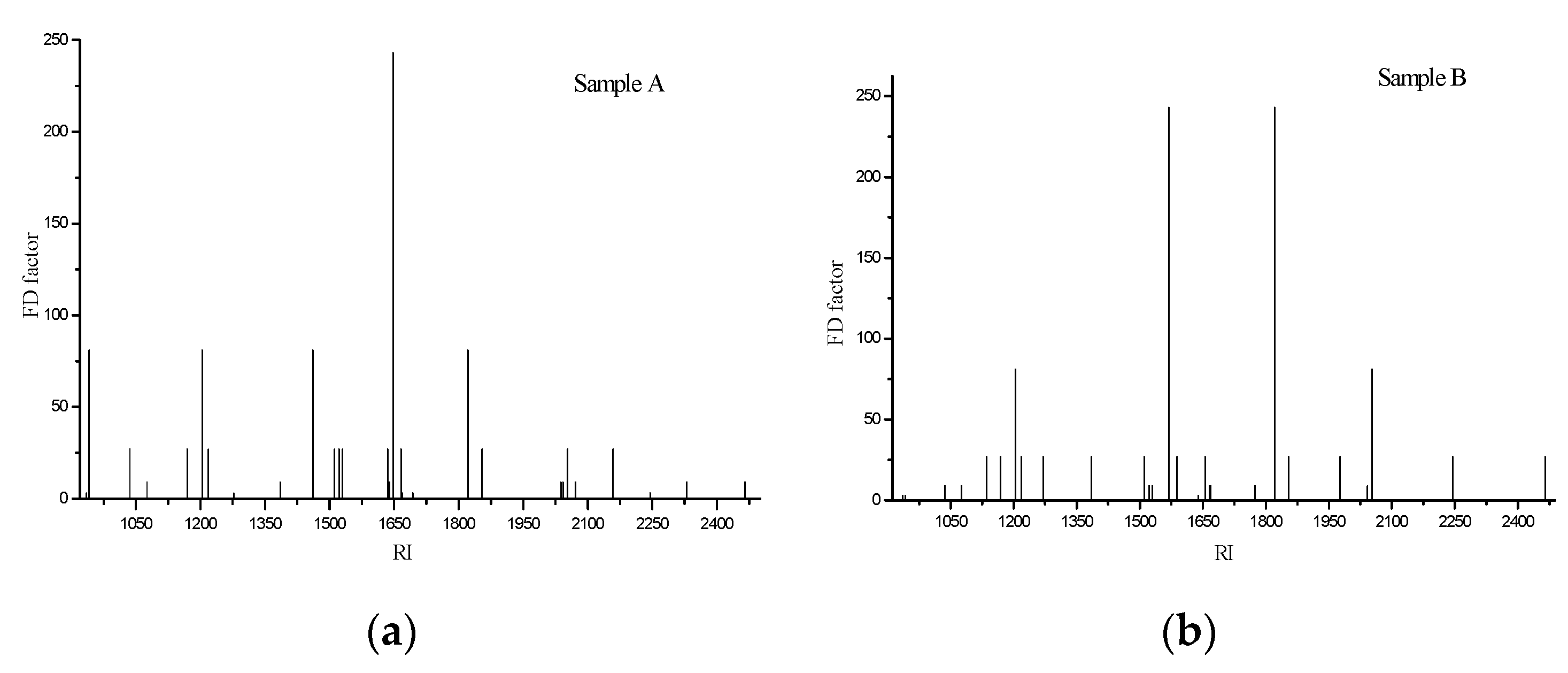
3.3. Identification of the Odor-Active Compounds in Braised Pork
3.4. Quantitation of Important Odorants and Calculation of OAV
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gallagher, E.; O’Brien, C.M.; Agm, S.; Arendt, E.K. Evaluation of sugar replacers in short dough biscuit production. J. Food Eng. 2003, 56, 261–263. [Google Scholar] [CrossRef]
- Esteller, M.S.; Lima, A.C.O.D.; Lannes, S.C.D.S. Color measurement in hamburger buns with fat and sugar replacers. LWT-Food Sci. Technol. 2006, 39, 184–187. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; He, C.; Song, H.; Chen, F. Effect of thermal treatment on the flavor generation from Maillard reaction of xylose and chicken peptide. LWT-Food Sci. Technol. 2015, 64, 316–325. [Google Scholar] [CrossRef]
- Parker, J.K. Thermal Generation or Aroma. In Flavour Development, Analysis and Perception in Food and Beverages; Elmore, J.S., Methven, L., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 151–185. [Google Scholar]
- Perezlocas, C.; Yaylayan, V.A.; Skibsted, L.H.; Risbo, J.; Andersen, M.L. The Maillard reaction and food quality deterioration. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 70–94. [Google Scholar]
- Trevisan, A.J.; De, A.L.D.; Sampaio, G.R.; Soares, R.A.; Markowicz, D.H. Influence of home cooking conditions on Maillard reaction products in beef. Food Chem. 2016, 196, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kesen, S.; Kelebek, H.; Sen, K.; Ulas, M.; Selli, S. GC-MS-olfactometric characterization of the key aroma compounds in Turkish olive oils by application of the aroma extract dilution analysis. Food Res. Int. 2013, 54, 1987–1994. [Google Scholar] [CrossRef]
- Chin, S.T.; Eyres, G.T.; Marriott, P.J. Cumulative solid phase microextraction sampling for gas chromatography-olfactometry of Shiraz wine. J. Chromatogr. A 2012, 1255, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Eyres, G.; Marriott, P.J.; Dufour, J.P. The combination of gas chromatography–olfactometry and multidimensional gas chromatography for the characterisation of essential oils. J. Chromatogr. A 2007, 1150, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Casilli, A.; Decorzant, E.; Jaquier, A.; Delort, E. Multidimensional gas chromatography hyphenated to massspectrometry and olfactometry for the volatile analysis of citrushybrid peel extract. J. Chromatogr. A 2014, 1373, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, M.; Scalzo, R.L.; Testoni, A.; Rizzolo, A. Evaluation of Fruit Aroma Quality: Comparison between Gas Chromatography–Olfactometry (GC–O) and Odour Activity Value (OAV) Aroma Patterns of Strawberries. Food Anal. Methods 2008, 1, 270–282. [Google Scholar] [CrossRef]
- Zellner, B.D.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography–olfactometry in food flavour analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jia, C.; Hayat, K.; Yu, J.; Deng, S. Controlled formation of flavor compounds by preparation and application of Maillard reaction intermediate (MRI) derived from xylose and phenylalanine. RSC Adv. 2017, 7, 45442–45451. [Google Scholar] [CrossRef]
- Song, S.; Tang, Q.; Fan, L.; Xu, X.; Song, Z. Identification of pork flavour precursors from enzyme-treated lard using Maillard model system assessed by GC–MS and partial least squares regression. Meat Sci. 2017, 124, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Dermiki, M.; Phanphensophon, N.; Mottram, D.S.; Methven, L. Contributions of non-volatile and volatile compounds to the umami taste and overall flavour of shiitake mushroom extracts and their application as flavour enhancers in cooked minced meat. Food Chem. 2008, 141, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Flavornet. Available online: http://www.flavornet.org/ (accessed on 12 December 2018).
- Xiao, Z.; Wu, M.; Niu, Y.; Chen, F.; Zhang, X.; Zhu, J.; Song, S. Contribution of Chicken Base Addition to Aroma Characteristics of Maillard Reaction Products Based on Gas Chromatography-Mass Spectrometry, Electronic Nose and Statistical Analysis. Food Sci. Biotechnol. 2015, 24, 411–419. [Google Scholar] [CrossRef]
- Tao, N.P.; Wu, R.; Zhou, P.G.; Gu, S.Q.; Wu, W. Characterization of odor-active compounds in cooked meat of farmed obscure puffer (Takifugu obscurus) using gas chromatographyemass spectrometry-olfactometry. J. Food Drug Anal. 2014, 22, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Barra, A.; Baldovini, N.; Loiseau, A.M.; Albino, L.; Lesecq, C. Chemical analysis of French beans (Phaseolus vulgaris L.) by headspace solid phase microextraction (HS-SPME) and simultaneous distillation/extraction (SDE). Food Chem. 2006, 101, 1279–1284. [Google Scholar] [CrossRef]
- Meadus, W.J.; Turner, T.D.; Dugan, M.E.; Aalhus, J.L.; Duff, P. Fortification of pork loins with docosahexaenoic acid (DHA) and its effect on flavor. J. Anim. Sci. Biotechnol. 2013, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Sunesen, L.O.; Dorigoni, V.; Zanardi, E.; Stahnke, L. Volatile compounds released during ripening in Italian dried sausage. Meat Sci. 2001, 58, 93–97. [Google Scholar] [CrossRef]
- Yu, A.N.; Zhang, A.D. Aroma compounds generated from thermal reaction of L-ascorbic acid with L-cysteine. Food Chem. 2010, 121, 1060–1065. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors-A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Benet, I.; Guàrdia, M.D.; Ibañez, C.; Solà, J.; Arnau, J. Analysis of SPME or SBSE extracted volatile compounds from cooked cured pork ham differing in intramuscular fat profiles. LWT-Food Sci. Technol. 2015, 60, 393–399. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, F.; Chen, H.; Huang, M.; Xie, J. Analysis of volatiles in Dezhou Braised Chicken by comprehensive two-dimensional gas chromatography/high resolution-time of flight mass spectrometry. LWT-Food Sci. Technol. 2015, 60, 1235–1242. [Google Scholar] [CrossRef]
- Cerny, C. Origin of carbons in sulfur-containing aroma compounds from the Maillard reaction of xylose, cysteine and thiamine. LWT-Food Sci. Technol. 2007, 40, 1309–1315. [Google Scholar] [CrossRef]
- Yu, A.N.; Tan, Z.W.; Wang, F.S. Mechanism of formation of sulphur aroma compounds from L-ascorbic acid and L-cysteine during the Maillard reaction. Food Chem. 2012, 132, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Timo´n, M.L.; Carrapiso, A.I.; Jurado, A.; Lagemaat, J.V. A study of the aroma of fried bacon and fried pork loin. J. Sci. Food Agric. 2004, 84, 825–831. [Google Scholar] [CrossRef]
- Xie, J.; Sun, B.; Zheng, F.; Wang, S. Volatile flavor constituents in roasted pork of Mini-pig. Food Chem. 2008, 109, 506–514. [Google Scholar] [CrossRef]
- Paleari, M.A.; Moretti, V.M.; Bersani, C.; Beretta, G.; Mentasti, T. Characterisation of a lard cured with spices and aromatic herbs. Meat Sci. 2004, 67, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Ayseli, M.T.; Filik, G.; Selli, S. Evaluation of volatile compounds in chicken breast meat using simultaneous distillation and extraction with odour activity value. J. Food Nutr. Res. 2014, 53, 137–142. [Google Scholar]
| Compounds | ID X | RI Y | RI Z | Peak Area (%) | |
|---|---|---|---|---|---|
| A | B | ||||
| Hydrocarbons | |||||
| α-Pinene | M | 1036 | 1032 m | 0.29 ± 0.07 | 0.40 ± 0.09 |
| Camphene | M | 1078 | 1075 m | 0.90 ± 0.02 | 1.04 ± 0.05 |
| β-Pinene | M | 1135 | 1116 m | 0.04 ± 0.01 | 0.06 ± 0.02 |
| δ-3-Carene | M | 1169 | 1148 m | 0.04 ± 0.01 | nd |
| β-Myrcene | M | 1171 | 1145 m | 0.12 ± 0.03 | 0.09 ± 0.04 |
| α-Phellandrene | M | 1176 | 1166 m | nd | 0.05 ± 0.02 |
| Limonene | M | 1204 | 1201 m | 0.32 ± 0.11 | 0.22 ± 0.08 |
| Bornylene | S | 1217 | nd | 0.05 ± 0.01 | |
| β-Phellandrene | M | 1214 | 1209 m | 0.37 ± 0.12 | 0.38 ± 0.06 |
| Styrene | M | 1272 | 1241 m | 0.07 ± 0.01 | 0.23 ± 0.11 |
| o-Cymene | S | 1280 | 0.04 ± 0.01 | 0.06 ± 0.02 | |
| Total | 2.19 | 2.59 | |||
| Aldehydes | |||||
| 2-Methylpropanal | M | 834 | 821 m | 0.09 ± 0.01 | 0.07 ± 0.01 |
| 3-Methylbutanal | M | 935 | 910 m | nd | 0.80 ± 0.21 |
| 2-Methylbutanal | M | 933 | 912 m | 0.48 ± 0.13 | nd |
| Pentanal | M | 942 | 935 m | 0.13 ± 0.01 | 0.17 ± 0.02 |
| Hexanal | M | 1076 | 1084 m | 0.25 ± 0.02 | 0.63 ± 0.14 |
| Heptanal | M | 1174 | 1174 m | 0.08 ± 0.03 | 0.08 ± 0.02 |
| Octanal | M | 1303 | 1280 m | 0.04 ± 0.01 | 0.04 ± 0.01 |
| (E)- 2-Heptenal | M | 1344 | 1324 g | 0.11 ± 0.04 | 0.05 ± 0.01 |
| Nonanal | M | 1385 | 1385 m | 0.12 ± 0.02 | 0.15 ± 0.03 |
| Benzaldehyde | M | 1522 | 1495 m | 0.49 ± 0.16 | 0.57 ± 0.13 |
| Phenylacetaldehyde | M | 1639 | 1625 m | 0.06 ±0.02 | 0.08 ± 0.01 |
| (E,E)-2,4-Decadienal | M | 1654 | 1632 m | 0.04 ± 0.01 | 0.02 ± 0.01 |
| Geranial | M | 1745 | 1715 m | 0.03 ± 0.01 | 0.03 ± 0.01 |
| 4-Methoxybenzaldehyde | S | 2042 | 0.04 ± 0.01 | 0.03 ± 0.01 | |
| (Z)-Cinnamaldehyde | S | 2053 | 0.68 ± 0.13 | 0.28 ± 0.04 | |
| Total | 2.64 | 3.00 | |||
| Alcohols | |||||
| Ethanol | M | 959 | 929 m | 0.52 ± 0.13 | 0.80 ± 0.21 |
| Cyclopentanol | S | 1004 | nd | 0.14 ± 0.03 | |
| Allyl alcohol | S | 1141 | nd | 0.06 ± 0.01 | |
| 1-Butanol | M | 1169 | 1145 m | 0.06 ± 0.01 | nd |
| 3-Methyl-1-butanol | M | 1228 | 1205 m | nd | 0.07 ± 0.01 |
| 1-Pentanol | M | 1234 | 1255 m | 0.08 ± 0.02 | 0.10 ± 0.04 |
| 1-Hexanol | M | 1373 | 1360 m | nd | 0.03 ± 0.01 |
| 1-Terpinen-4-ol | M | 1589 | 1591 m | 0.02 ± 0.01 | 0.03 ± 0.01 |
| 2-(2-Ethoxyethoxy)ethanol | S | 1650 | 0.02 ± 0.01 | 0.08 ± 0.02 | |
| α-Terpineol | M | 1681 | 1688 m | 0.02 ± 0.01 | 0.03 ± 0.01 |
| 4-Hydroxy-4-methyl-2-pentanone | S | 2350 | nd | 0.07 ± 0.03 | |
| Total | 0.72 | 1.40 | |||
| Ketones | |||||
| 2-Pentanone | M | 1002 | 983 m | 0.12 ± 0.03 | nd |
| Acetoin | M | 1270 | 1287 m | 0.10 ± 0.02 | 0.07 ± 0.02 |
| 6-Methyl-5-hepten-2-one | M | 1357 | 1336 m | 0.06 ± 0.02 | nd |
| Total | 0.28 | 0.07 | |||
| Acids | |||||
| Acetic acid | M | 1445 | 1450 m | 0.30 ± 0.12 | 0.20 ± 0.09 |
| Propanoic acid | M | 1551 | 1523 m | 0.04 ± 0.02 | nd |
| Butanoic acid | M | 1610 | 1619 m | 0.04 ± 0.01 | 0.06 ± 0.02 |
| Hexanoic acid | M | 1892 | 1888 g | 0.03 ± 0.01 | 0.04 ± 0.01 |
| Decanoic acid | M | 2333 | 2361 m | 0.15 ± 0.07 | nd |
| Total | 0.57 | 0.30 | |||
| Esters and lactones | |||||
| Methyl butanoate | S | 1011 | nd | 0.07 ± 0.02 | |
| γ-Butyrolactone | M | 1630 | 1647 m | 0.03 ± 0.01 | nd |
| Styrallyl acetate | S | 1737 | 0.03 ± 0.01 | 0.05 ± 0.01 | |
| Benzyl isovalerate | S | 1938 | nd | 0.03 ± 0.01 | |
| Total | 0.06 | 0.14 | |||
| Phenols | |||||
| Phenol | S | 2072 | 0.02 ± 0.01 | 0.03 ± 0.01 | |
| Total | 0.02 | 0.03 | |||
| Ethers | |||||
| Estragole | M | 1656 | 1655 m | nd | 0.04 ± 0.01 |
| Anethole | S | 1821 | 1.31 ± 0.05 | 1.32 ± 0.03 | |
| Total | 1.31 | 1.36 | |||
| Sulphur compounds | |||||
| Methanethiol | M | 711 | 696 m | 2.87 ± 0.11 | 3.12 ± 1.01 |
| Allyl mercaptan | S | 916 | 0.34 ± 0.06 | 1.31 ± 0.08 | |
| Allyl methyl sulfide | S | 981 | 0.07 ± 0.01 | 0.10 ± 0.02 | |
| 2-Methylthiophene | M | 1110 | 1084 m | nd | 0.06 ± 0.01 |
| Diallyl sulfide | S | 1165 | nd | 0.11 ± 0.07 | |
| Thiophene | M | 1178 | 1150 m | 0.02 ± 0.01 | nd |
| Thiazole | M | 1271 | 1286 g | 0.02 ± 0.01 | nd |
| Allyl methyl disulfide | S | 1298 | nd | 0.07 ± 0.02 | |
| 2-Methyl-3-furanthiol | M | 1310 | 1339 g | 0.03 ± 0.01 | nd |
| Furfuryl mercaptan | M | 1431 | 1432 m | 0.05 ± 0.01 | nd |
| Methional | M | 1484 | 1458 m | 0.16 ± 0.02 | nd |
| 2-Acetylthiazole | M | 1686 | 1692 g | 0.08 ± 0.03 | 0.10 ± 0.03 |
| Total | 3.65 | 4.87 | |||
| Nitrogenous compounds | |||||
| Pyrazine | M | 1235 | 1254 g | 0.05 ± 0.01 | nd |
| Methanamide | S | 1605 | 0.02 ± 0.01 | nd | |
| 2-Amino-6-methylbenzoic acid | S | 1781 | nd | 0.20 ± 0.09 | |
| 2-Acetylpyrrole | M | 2037 | 2027 g | 0.02 ± 0.01 | nd |
| Dimethylamine | S | 2232 | 0.01 ± 0.00 | 0.11 ± 0.04 | |
| Total | 0.11 | 0.31 | |||
| Oxygen-containing heterocycles | |||||
| 2-Ethylfuran | M | 979 | 986 g | nd | 0.05 ± 0.01 |
| 2-Pentylfuran | M | 1243 | 1240 m | 0.10 ± 0.03 | 0.12 ± 0.05 |
| 2-Methyltetrahydrofuran-3-one | M | 1278 | 1299 g | 0.12 ± 0.04 | nd |
| Furfural | M | 1461 | 1455 m | 0.22 ± 0.11 | 0.04 ± 0.01 |
| 5-Methyl-2-furfural | M | 1570 | 1560 m | 0.06 ± 0.01 | nd |
| Furfuryl alcohol | S | 1647 | 0.03 ± 0.00 | nd | |
| 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | S | 2359 | 0.24 ± 0.10 | nd | |
| 5-Hydroxymethyl-2-furfural | S | 2512 | 0.13 ± 0.03 | nd | |
| Total | 0.91 | 0.20 | |||
| Compounds | ID X | RI Y | RI Z | Peak Area (%) | |
|---|---|---|---|---|---|
| A | B | ||||
| Hydrocarbons | |||||
| α-Pinene | M | 1036 | 1032 m | 0.03 ± 0.01 | 0.03 ± 0.01 |
| β-Pinene | M | 1135 | 1116 m | nd | 0.17 ± 0.04 |
| δ-3-Carene | M | 1169 | 1148 m | 0.03 ± 0.01 | 0.17 ± 0.05 |
| β-Myrcene | M | 1171 | 1145 m | 0.07 ± 0.01 | nd |
| Limonene | M | 1204 | 1201 m | 0.15 ± 0.04 | 0.30 ± 0.12 |
| o-Cymene | S | 1280 | 0.01 ± 0.00 | nd | |
| Total | 0.29 | 0.66 | |||
| Aldehydes | |||||
| 3-Methylbutanal | M | 935 | 910 m | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Pentanal | M | 942 | 935 m | 0.64 ± 0.24 | nd |
| Hexanal | M | 1076 | 1084 m | 0.31 ± 0.11 | 0.05 ± 0.02 |
| Heptanal | M | 1174 | 1174 m | 0.23 ± 0.04 | nd |
| Nonanal | M | 1385 | 1385 m | 0.12 ± 0.03 | 0.14 ± 0.01 |
| Benzaldehyde | M | 1522 | 1495 m | 0.06 ± 0.03 | 1.18 ± 0.05 |
| (E)-2-Decenal | S | 1635 | 0.01 ± 0.00 | 0.01 ± 0.01 | |
| Phenyl acetaldehyde | M | 1639 | 1625 m | 0.19 ± 0.01 | 0.37 ± 0.06 |
| 2,4-Decadienal | M | 1654 | 1632 m | nd | 0.01 ± 0.01 |
| (E)-Cinnamaldehyde | M | 1666 | 1631 m | 0.23 ± 0.08 | 0.11 ± 0.04 |
| Neral | M | 1669 | 1667 m | 0.10 ± 0.08 | 0.04 ± 0.01 |
| Dodecanal | M | 1693 | 1722 m | 0.13 ± 0.06 | nd |
| Benzenepropanal | S | 1775 | 0.02 ± 0.01 | 0.46 ± 0.12 | |
| (E,E)-2,4-Decadienal | S | 1854 | 0.02 ± 0.01 | nd | |
| 2-Methoxybenzaldehyde | S | 1977 | nd | 0.28 ± 0.05 | |
| 4-Methoxybenzaldehyde | S | 2042 | 0.05 ± 0.01 | 0.34 ± 0.11 | |
| (Z)-Cinnamaldehyde | S | 2053 | 0.74 ± 0.16 | 1.09 ± 0.41 | |
| (Z)-13-Octadecenal | S | 2383 | nd | 0.39 ± 0.11 | |
| 2-Methoxycinnamaldehyde | S | 2401 | nd | 0.34 ± 0.09 | |
| Total | 2.85 | 4.80 | |||
| Alcohols | |||||
| Allyl alcohol | S | 1105 | 0.35 ± 0.02 | nd | |
| 1,8-Cineole | M | 1218 | 1213 m | 0.08 ± 0.01 | 0.13 ± 0.02 |
| 1-Pentanol | M | 1234 | 1255 m | 0.08 ± 0.01 | nd |
| Linalool | M | 1530 | 1537 m | 0.07 ± 0.02 | 0.44 ± 0.11 |
| 1-Terpinen-4-ol | M | 1589 | 1591 m | nd | 0.08 ± 0.02 |
| α-Terpineol | M | 1681 | 1688 m | 0.22 ± 0.10 | 0.08 ± 0.02 |
| β-Fenchyl alcohol | S | 1701 | nd | 0.05 ± 0.01 | |
| Cinnamyl alcohol | M | 2330 | 2300 m | 0.15 ± 0.06 | nd |
| (E)-2-Tetradecen-1-ol | S | 2356 | nd | 0.26 ± 0.08 | |
| Total | 0.96 | 1.04 | |||
| Ketones | |||||
| 2,3-Pentanedione | S | 1057 | 0.10 ± 0.04 | nd | |
| 2-Heptanone | M | 1172 | 1170 m | 0.01 ± 0.00 | nd |
| 2-Octanone | M | 1267 | 1244 m | 0.04 ± 0.01 | nd |
| Acetoin | M | 1270 | 1287 m | nd | 0.21 ± 0.07 |
| Acetol | M | 1286 | 1287 m | nd | 0.09 ± 0.02 |
| 2-Tridecanone | S | 1804 | 0.01 ± 0.01 | nd | |
| 2-Pentadecanone | S | 2020 | 0.06 ± 0.01 | 0.35 ± 0.11 | |
| 1-(4-Methoxyphenyl)-2-propanone | S | 2170 | 0.01 ± 0.00 | nd | |
| 2-Heptadecanone | S | 2208 | 0.01 ± 0.01 | nd | |
| Total | 0.24 | 0.65 | |||
| Acids | |||||
| Butanoic acid | M | 1610 | 1619 m | 0.01 ± 0.01 | nd |
| Heptanoic acid | S | 1935 | 0.01 ± 0.01 | nd | |
| Tetradecanoic acid | M | 2094 | 2094 g | 0.05 ± 0.02 | nd |
| Nonanoic acid | M | 2193 | 2202 m | 0.01 ± 0.01 | nd |
| Decanoic acid | M | 2333 | 2361 m | 0.03 ± 0.01 | nd |
| Oleic acid | M | 2374 | 2430 m | 0.05 ± 0.02 | nd |
| Hexadecanoic acid | S | 2468 | 0.16 ± 0.07 | 0.46 ± 0.11 | |
| Total | 0.32 | 0.46 | |||
| Lactones and esters | |||||
| γ-Butyrolactone | M | 1630 | 1647 m | 0.11 ± 0.03 | nd |
| γ-Nonalactone | M | 2014 | 2042 m | 0.06 ± 0.01 | nd |
| γ-Undecalactone | M | 2145 | 2270 m | 0.27 ± 0.06 | 1.58 ± 0.31 |
| Ethyl cinnamate | M | 2158 | 2139 m | 0.01 ± 0.01 | nd |
| Total | 0.45 | 1.58 | |||
| Phenols | |||||
| Phenol | S | 2072 | 0.78 ± 0.11 | 1.05 ± 0.12 | |
| Total | 0.78 | 1.05 | |||
| Ethers | |||||
| Estragole | M | 1656 | 1655 m | 0.02 ± 0.01 | 0.98 ± 0.09 |
| Anethole | S | 1821 | 1.78 ± 0.08 | 1.16 ± 0.21 | |
| Total | 1.80 | 2.15 | |||
| Nitrogenous compounds | |||||
| 2-Acetylpyrrole | M | 2037 | 2025 g | 0.02 ± 0.01 | nd |
| Total | 0.02 | ||||
| Oxygen-containing heterocycles | |||||
| Furfuryl alcohol | M | 1157 | 1199 m | 0.07 ± 0.02 | nd |
| Furfural | M | 1461 | 1455 m | 0.06 ± 0.02 | nd |
| 2-Acetylfuran | M | 1511 | 1490 m | 0.01 ± 0.01 | 0.01 ± 0.01 |
| 5-Methyl-2-furfural | M | 1570 | 1560 m | nd | 0.05 ± 0.02 |
| 5-Methyl-2-furfuryl alcohol | S | 1700 | 0.03 ± 0.01 | nd | |
| 3,4-Dihydro- 2H-1-benzopyran | S | 2275 | nd | 0.33 ± 0.13 | |
| 2-Methyltetrahydrofuran-3-one | S | 2393 | 0.02 ± 0.01 | nd | |
| Coumarin | M | 2465 | 2465 m | 0.03 ± 0.01 | 0.31 ± 0.12 |
| Total | 0.22 | 0.70 | |||
| RI a | Compounds | Odor Description b | Odor Threshold (μg/g) | A | B | ||||
|---|---|---|---|---|---|---|---|---|---|
| CN c | FD d | OAV e | CN | FD | OAV | ||||
| Aldehydes | |||||||||
| 935 | 3-Methylbutanal | burnt-sweet, roast | 0.001 | 0.335 | 3 | 335 | 0.211 | 3 | 211 |
| 942 | Pentanal | almond, pungent | 0.009 | 3.0012 | 81 | 333 | 0.1045 | 3 | 12 |
| 1076 | Hexanal | grass, tallow, fat | 0.0045 | 1.4725 | 9 | 34 | 0.2148 | 9 | 5 |
| 1385 | Nonanal | fat, green | 0.0011 | 0.5744 | 9 | 522 | 0.5637 | 27 | 512 |
| 1461 | Furfural | bread, almond, sweet | 3 | 0.3468 | 81 | <1 | |||
| 1522 | Benzaldehyde | almond, burnt sugar | 0.35 | 0.3224 | 27 | <1 | 4.6550 | 9 | 13 |
| 1635 | (E)-2-Decenal | grass, earthy | 0.0004 | 0.0735 | 27 | 180 | |||
| 1639 | Phenylacetaldehyde | hawthorne, honey, sweet | 0.004 | 0.8829 | 9 | 220 | 1.4606 | 3 | 365 |
| 1666 | (E)-Cinnamaldehyde | cinnamon, paint | −f | 1.0826 | 27 | 0.4173 | 9 | ||
| 1669 | Neral | lemon | 0.053 | 0.4836 | 3 | 9 | 0.1457 | 9 | 3 |
| 1693 | Dodecanal | lily, fat, citrus | 0.002 | 0.6133 | 3 | 307 | |||
| 1775 | 3-Phenylpropanal | grass, fat | 0.12 | 1.8005 | 9 | 9 | |||
| 1854 | (E,E)-2,4-Decadienal | fat, roast | 0.00007 | 0.0913 | 27 | 1304 | 0.0237 | 27 | 339 |
| 1977 | 2-Methoxybenzaldehyde | wax, medicinal | - | 1.1077 | 27 | ||||
| 2042 | 4-Methoxybenzaldehyde | similar hawkthorn | 0.03 | 0.2233 | 9 | 7 | 1.3564 | 9 | 45 |
| 2053 | (Z)-Cinnamaldehyde | cinnamon | 0.16 | 3.4719 | 27 | 22 | 4.2745 | 81 | 27 |
| Hydrocarbons | |||||||||
| 1036 | α-Pinene | green, fresh | 0.006 | 0.1321 | 27 | 22 | 0.2148 | 9 | 36 |
| 1135 | β-Pinene | mild, green | 0.14 | 0.6577 | 27 | 5 | |||
| 1169 | δ-3-Carene | lemon, resin | 0.77 | 0.1547 | 27 | <1 | 0.6634 | 27 | <1 |
| 1204 | Limonene | fruity, orange | 0.01 | 0.7133 | 81 | 71 | 1.1866 | 81 | 119 |
| Alcohols | |||||||||
| 1530 | Linalool | floral, lavender | 0.006 | 0.3115 | 27 | 52 | 1.7199 | 9 | 287 |
| 1589 | 1-Terpinen-4-ol | turpentine, nutmeg, must | 1.29 | 0.3126 | 27 | <1 | |||
| 2330 | Cinnamyl alcohol | oily | 0.077 | 0.7314 | 9 | 9 | |||
| Ketones | |||||||||
| 1270 | Acetoin | butter, cream | 0.8 | 0.8223 | 27 | 1 | |||
| Esters and lactones | |||||||||
| 2245 | γ-Undecalactone | flower, wax | 0.042 | 1.2843 | 3 | 31 | 6.2292 | 27 | 148 |
| 2158 | Ethyl cinnamate | cinnamon, honey | 0.04 | 0.0734 | 27 | 2 | |||
| Phenols | |||||||||
| 2072 | Phenol | phenol | 5.9 | 3.6822 | 9 | <1 | |||
| Ethers | |||||||||
| 1218 | 1,8-Cineole | mint, sweet | 0.012 | 0.4129 | 27 | 34 | 0.5122 | 27 | 43 |
| 1821 | Anethole | anise, slight sweet | 0.16 | 8.3842 | 81 | 52 | 4.5618 | 243 | 29 |
| Nitrogenous compounds | |||||||||
| 2037 | 2-Acetylpyrrole | nut, walnut, burnt | 170 | 0.0934 | 9 | <1 | |||
| Oxygen-containing heterocycles | |||||||||
| 1278 | 2-Methyltetrahydrofuran-3-one | sweet, caramel | - | 0.0937 | 3 | ||||
| 1511 | 2-Acetylfuran | butter, meaty | 0.01 | 0.0429 | 27 | 4 | 0.0419 | 27 | 4 |
| 1647 | Furfuryl alcohol | sauce-flavor | 2 | 0.3126 | 243 | <1 | |||
| 2465 | Coumarin | green, sweet | 0.33 | 0.1622 | 9 | <1 | 1.2419 | 27 | 4 |
| Unknown | |||||||||
| 1570 | Unknown1 | almond, caramel, toasty | - | 243 | |||||
| 1656 | Unknown2 | licorice, anise | - | 27 | 27 | ||||
| No | Compounds | Quantitative Ions a | R2 | LOD (μg/kg) b | LOQ (μg/kg) c |
|---|---|---|---|---|---|
| 1 | 3-Methyl- butanal | 41,43,44 | 0.987 | 1.8 | 6.0 |
| 2 | Pentanal | 41,44,58 | 0.974 | 4.4 | 14.7 |
| 3 | α-Pinene | 91,92,93 | 0.992 | 2.0 | 6.7 |
| 4 | Hexanal | 44,56,57 | 0.978 | 25.1 | 83.6 |
| 5 | β-Pinene | 69,91,93 | 0.988 | 1.5 | 5.0 |
| 6 | δ-3-Carene | 77,91,93 | 0.995 | 6.4 | 21.3 |
| 7 | Limonene | 67,68,93 | 0.996 | 1.9 | 6.3 |
| 8 | 1,8-Cineole | 43,81,108 | 0.995 | 1.6 | 5.3 |
| 9 | Acetoin | 43,45,88 | 0.996 | 6.2 | 20.6 |
| 10 | 2-Methyltetrahydrofuran-3-one | 43,72,100 | 0.978 | 2.1 | 7.0 |
| 11 | Nonanal | 41,56,57 | 0.986 | 7.0 | 23.3 |
| 12 | Furfural | 39,95,96 | 0.986 | 10.6 | 35.3 |
| 13 | 2-Acetylfuran | 39,95,110 | 0.976 | 1.2 | 4.0 |
| 14 | Benzaldehyde | 77,105,106 | 0.985 | 16.3 | 54.3 |
| 15 | Linalool | 55,71,93 | 0.971 | 4.3 | 14.3 |
| 16 | 1-Terpinen-4-ol | 71,93,111 | 0.983 | 1.5 | 5.0 |
| 17 | (E)-2-Decenal | 41,55,70 | 0.977 | 0.3 | 1.0 |
| 18 | Phenyl acetaldehyde | 69,91,92 | 0.972 | 4.3 | 14.3 |
| 19 | Furfuryl alcohol | 81,97,98 | 0.994 | 6.8 | 22.6 |
| 20 | (E)-Cinnamaldehyde | 103,131,132 | 0.964 | 3.2 | 10.7 |
| 21 | Neral | 41,69,94 | 0.979 | 11.8 | 39.3 |
| 22 | Dodecanal | 41,57,82 | 0.986 | 2.2 | 7.3 |
| 23 | 3-Phenylpropanal | 91,92,134 | 0.974 | 2.4 | 8.0 |
| 24 | Anethole | 132,133,148 | 0.995 | 1.7 | 5.7 |
| 25 | (E,E)-2,4-Decadienal | 41,67,81 | 0.995 | 0.8 | 2.7 |
| 26 | 2-Methoxybenzaldehyde | 77,118,136 | 0.986 | 5.3 | 17.6 |
| 27 | 2-Acetylpyrrole | 66,94,109 | 0.976 | 1.7 | 5.7 |
| 28 | 4-Methoxybenzaldehyde | 77,92,135 | 0.989 | 14.6 | 48.6 |
| 29 | (Z)-Cinnamaldehyde | 103,131,132 | 0.977 | 0.6 | 2.0 |
| 30 | Phenol | 65,66,94 | 0.994 | 5.4 | 18.0 |
| 31 | Ethyl cinnamate | 103,131,176 | 0.974 | 1.6 | 5.3 |
| 32 | γ-Undecalactone | 55,85,128 | 0.983 | 2.1 | 7.0 |
| 33 | Cinnamyl alcohol | 91,92,134 | 0.986 | 0.6 | 2.0 |
| 34 | Coumarin | 89,118,146 | 0.976 | 2.1 | 7.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Fan, L.; Xu, X.; Xu, R.; Jia, Q.; Feng, T. Aroma Patterns Characterization of Braised Pork Obtained from a Novel Ingredient by Sensory-Guided Analysis and Gas-Chromatography-Olfactometry. Foods 2019, 8, 87. https://doi.org/10.3390/foods8030087
Song S, Fan L, Xu X, Xu R, Jia Q, Feng T. Aroma Patterns Characterization of Braised Pork Obtained from a Novel Ingredient by Sensory-Guided Analysis and Gas-Chromatography-Olfactometry. Foods. 2019; 8(3):87. https://doi.org/10.3390/foods8030087
Chicago/Turabian StyleSong, Shiqing, Li Fan, Xiaodong Xu, Rui Xu, Qian Jia, and Tao Feng. 2019. "Aroma Patterns Characterization of Braised Pork Obtained from a Novel Ingredient by Sensory-Guided Analysis and Gas-Chromatography-Olfactometry" Foods 8, no. 3: 87. https://doi.org/10.3390/foods8030087
APA StyleSong, S., Fan, L., Xu, X., Xu, R., Jia, Q., & Feng, T. (2019). Aroma Patterns Characterization of Braised Pork Obtained from a Novel Ingredient by Sensory-Guided Analysis and Gas-Chromatography-Olfactometry. Foods, 8(3), 87. https://doi.org/10.3390/foods8030087





