Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies alba Mill.) Needles
Abstract
1. Introduction
2. Materials and Methods
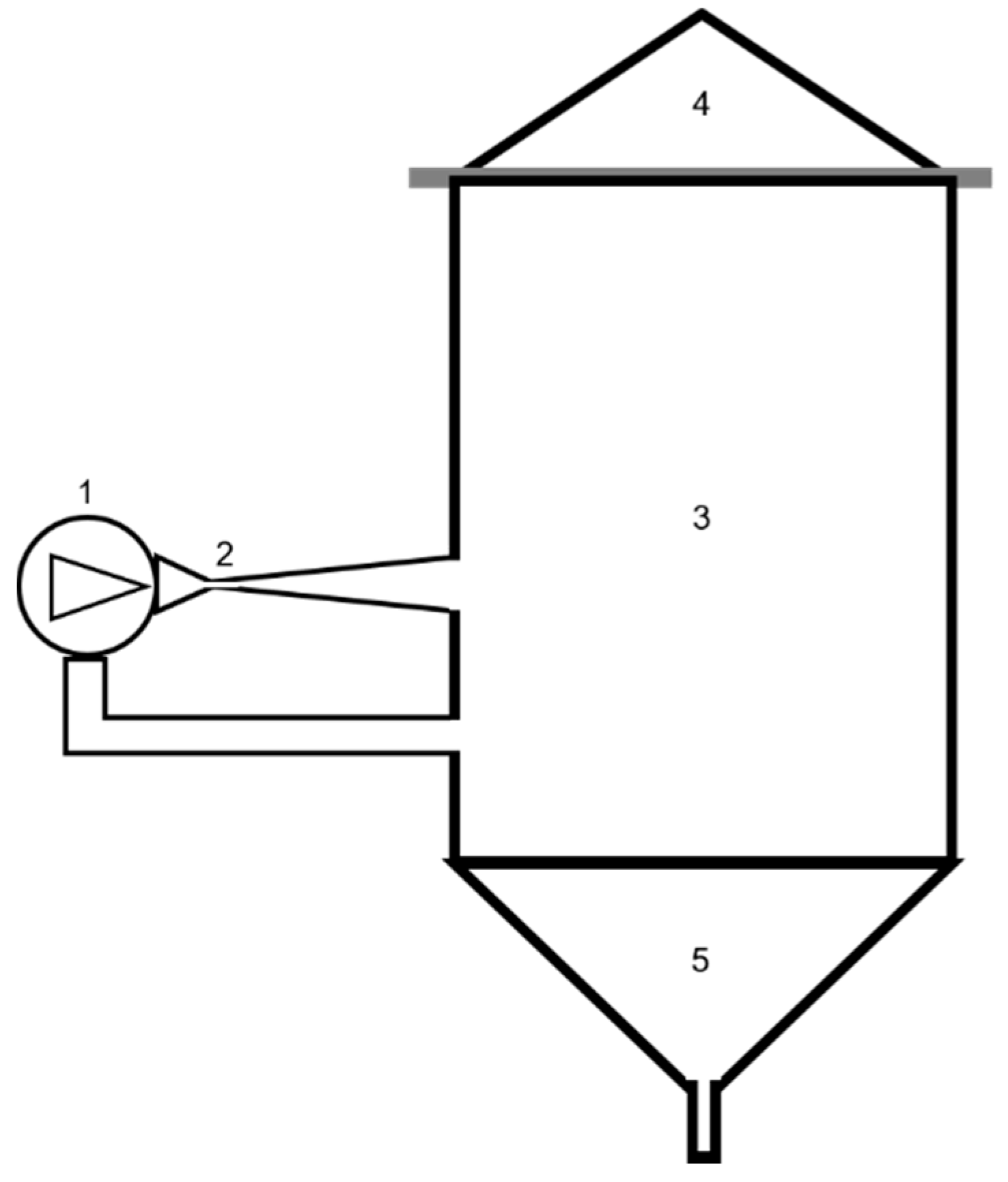
2.1. HC Device and Method
2.2. Silver Fir Needles Samples and Tests
2.3. Analytical Procedures
2.3.1. Total Phenolic Content
2.3.2. Total Flavonoid Content
2.3.3. DPPH Radical Scavenging Assay
2.3.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
3. Results
3.1. Main Operational Parameters
3.2. Total Phenolic and Flavonoids Content
3.3. Antioxidant Activity
3.4. Stability
4. Discussion
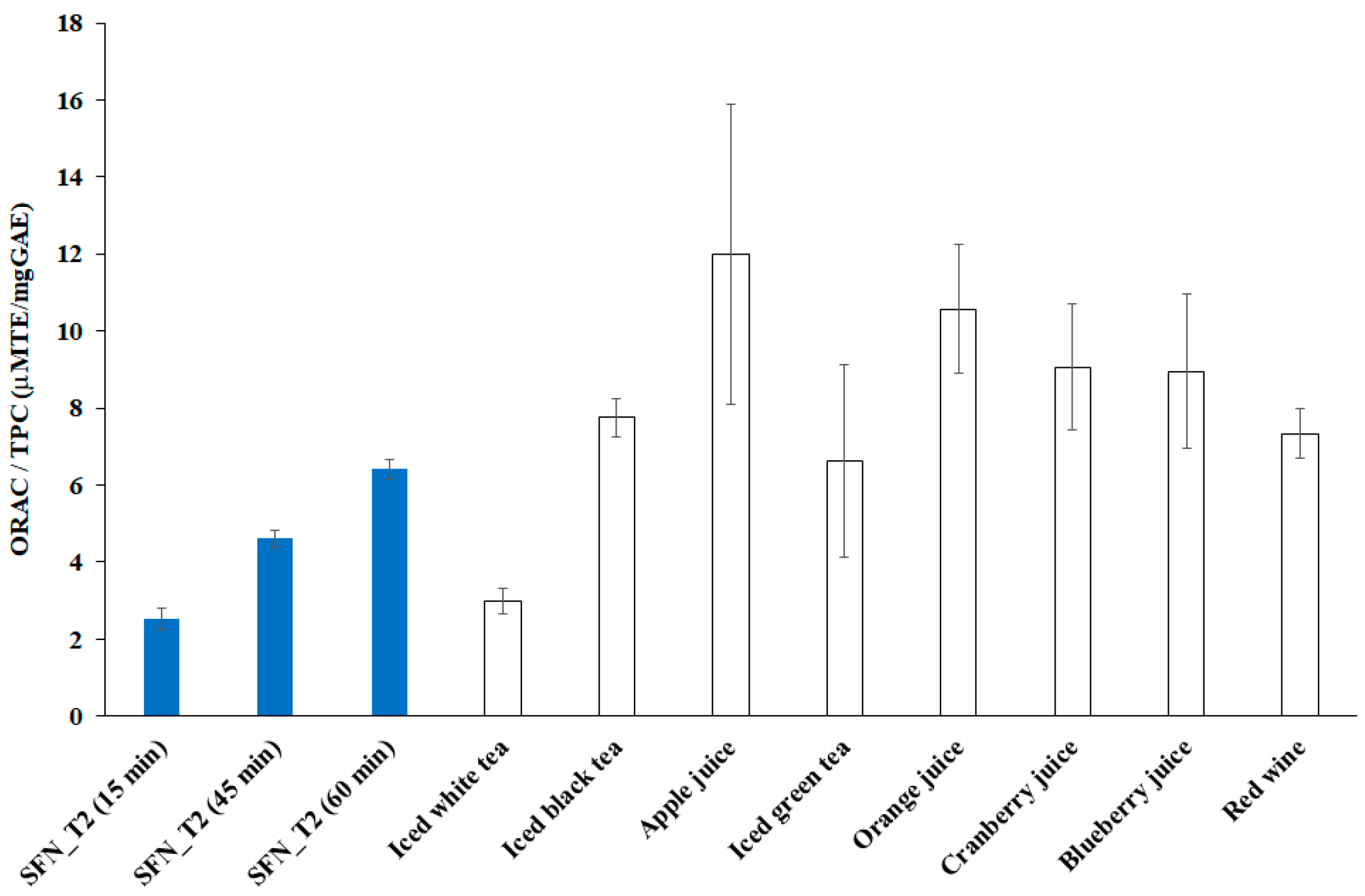
- The ORAC to TPC levels found in this study increased with cavitation time, with the SFN_T2 sample collected after 60 min exhibiting both the highest ORAC level and the highest ORAC to TPC level, which could suggest the HC ability to extract more and more functional polyphenols, likely bound in the raw material, during the process.
- The highest ORAC to TPC level found in this study was comparable with most of the respective levels found for the considered commercial beverages, and it is likely to increase further with longer and/or optimized cavitation process.
- The ORAC levels achieved in this study are likely to increase also after increasing the concentration of the raw material added to water, which was very low in this study; however, the dose-dependency over the ORAC antioxidant activity needs specific investigation.
- (1)
- Innovation by selection of varieties and use of renewable plant resources: Abies alba Mill. is a plant species at risk in Italian northern Apennines, relict of past large populations [51]; moreover, fir needles are abundant and renewable by-products of forest management, and can be used in small proportion to achieve remarkable oxidant activity in aqueous solution.
- (2)
- Use of alternative solvents and principally water or agro-solvents: water was the only solvent used in the discussed extraction method.
- (3)
- Reduce energy consumption by energy recovery and using innovative technologies: as little as 0.04 kWh of electricity per liter of aqueous solution were consumed during 60 min of process time in both tests discussed in this study, with no other energy source used during operation; electricity consumed for centrifuge separation was not accounted for, but it was assumed negligible.
- (4)
- Production of co-products instead of waste to include the bio- and agro-refining industry: once deprived of soluble (and solubilized) compounds, the residual fraction of the original mass of fir needle, which had to be separated from the aqueous solution, could be destined to composting, anaerobic digestion, or even to reuse as feedstock for biochar [52].
- (5)
- Reduce unit operations and favor safe, robust and controlled processes: the discussed extraction method comprised only two operations after fir needles harvesting, i.e., HC processing, and mechanical separation; the equipment was simple, safe, robust, and easily controllable; the HC process needed to achieve high levels of the antioxidant activity was very fast (60 min or less).
- (6)
- Aim for a non-denatured and biodegradable extract without contaminants: absent any additives, water and fir needles were the only ingredients; although indirectly inferred, as discussed in Section 3.4, the HC process did not denature the antioxidant compounds of silver fir needles.
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Essential Oils | Range of Concentration |
|---|---|
| β-Pinene 1 | 0.51–32.80% |
| Bornyl acetate 1 | 4.40–30.31% |
| delta-3-Carene 1 | 13.85% 3 |
| Camphene 1 | 6.90–19.91% |
| Limonene 1 | 6.10–13.90% |
| α-Pinene 1 | 2.87–17.30% |
| Tricyclene 1 | 0.80–12.90% |
| β-Caryophyllene 2 | 1.30–6.70% |
| α-Humulene 2 | 0.20–3.80% |
| β-Phellandrene | 0.00–4.90% |
| Santene 2 | 1.20–2.00% |
| Myrcene 1 | 0.80–1.00% |
| Terpinolene 1 | 0.30–1.10% |
| Sabinene 1 | 0.10% 1 |
Appendix B
References
- Hulme, N. Libellus de Natura, Causa, Curationeque Scorbuti. To Which is Annexed a Proposal for Preventing the Scurvy in the British Navy. Available online: https://books.google.com.hk/books?hl=zh-TW&lr=&id=V0ZcAAAAcAAJ&oi=fnd&pg=PA3&dq=Libellus+de+Natura,+Causa,+Curationeque+Scorbuti.+To+Which+is+Annexed+a+Proposal+for+Preventing+the+Scurvy+in+the+British+Navy&ots=-ZMCqtHPGT&sig=iTO1EEITqyp-JDQU0VidxBgSKlc&redir_esc=y#v=onepage&q=Libellus%20de%20Natura%2C%20Causa%2C%20Curationeque%20Scorbuti.%20To%20Which%20is%20Annexed%20a%20Proposal%20for%20Preventing%20the%20Scurvy%20in%20the%20British%20Navy&f=false (accessed on 10 December 2018).
- Lind, J. A Treatise of the Scurvy; Sands, Murray, and Cochran: Edinburgh, UK, 1753. [Google Scholar]
- Smith, A.H. Beer and Scurvy. Some Notes From History. Lancet 1918, 192, 813–815. [Google Scholar] [CrossRef]
- Charters, E. Disease, War, and the Imperial State: The Welfare of the British Armed Forces During the Seven Years’ War; University of Chicago Press: Chicago, IL, USA, 2014; ISBN 978-0226180007. [Google Scholar]
- Charters, E.M. Disease, Wilderness Warfare, and imperial relations: The battle for Quebec, 1759-1760. War Hist. 2009, 16, 1–24. [Google Scholar] [CrossRef]
- Tešević, V.; Milosavljević, S.; Vajs, V.; Dordević, I.; Soković, M.; Lavadinović, V.; Novaković, M. Chemical composition and antifungal activity of the essential oil of Douglas fir (Pseudosuga menziesii Mirb. Franco) from Serbia. J. Serbian Chem. Soc. 2009, 74, 1035–1040. [Google Scholar] [CrossRef]
- Stubbs, B.J. Captain Cook’s beer: The antiscorbutic use of malt and beer in late 18th century sea voyages. Asia Pac. J. Clin. Nutr. 2003, 12, 129–137. [Google Scholar] [PubMed]
- Kodicek, E.H.; Young, F.G. Captain Cook and Scurvy. Notes Rec. R. Soc. Lond. 1969, 24, 43–63. [Google Scholar] [CrossRef]
- Laing, J. Medicine and surgeryin the Artic Circle. Edinb Med. J. 1901, 9, 449–451. [Google Scholar]
- Friedemann, T.E.; Kraybill, H.F.; Consolazio, C.F. The uses of recommended dietary allowances in military nutrition. Am. J. Public Health 1959. [Google Scholar] [CrossRef]
- Belletti, P.; Ferrazzini, D.; Ducci, F.; De Rogatis, A.; Mucciarelli, M. Genetic diversity of Italian populations of Abies alba. Dendrobiology 2017, 77, 147–159. [Google Scholar] [CrossRef]
- Tavčar Benković, E.; Žigon, D.; Mihailović, V.; Petelinc, T.; Jamnik, P.; Kreft, S. Identification, in vitro and in vivo Antioxidant Activity, and Gastrointestinal Stability of Lignans from Silver Fir (Abies alba) Wood Extract. J. Wood Chem. Technol. 2017, 37, 467–477. [Google Scholar] [CrossRef]
- Sahin, H.; Yalcin, O. Chemical Composition and Utilization of Conifer Needles-A Review. J. Appl. Life Sci. Int. 2017, 14, 1–11. [Google Scholar] [CrossRef]
- Yang, X.-W.; Li, S.-M.; Shen, Y.-H.; Zhang, W.-D. Phytochemical and Biological Studies ofAbies Species. Chem. Biodivers. 2008, 5, 56–81. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Ranade, V.V. Modelling of hydrodynamic cavitation with orifice: Influence of different orifice designs. Chem. Eng. Res. Des. 2018, 136, 698–711. [Google Scholar] [CrossRef]
- Carpenter, J.; Badve, M.; Rajoriya, S.; George, S.; Saharan, V.K.; Pandit, A.B. Hydrodynamic cavitation: An emerging technology for the intensification of various chemical and physical processes in a chemical process industry. Rev. Chem. Eng. 2017, 33, 433–468. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Di Stefano, V.; Delisi, R.; Avellone, G.; Meneguzzo, F.; Pagliaro, M. Beer produced via hydrodynamic cavitation retains higher amounts of xanthohumol and other hops prenylflavonoids. LWT Food Sci. Technol. 2018, 91, 160–167. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Beer-brewing powered by controlled hydrodynamic cavitation: Theory and real-scale experiments. J. Clean. Prod. 2017, 142, 1457–1470. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Gluten reduction in beer by hydrodynamic cavitation assisted brewing of barley malts. LWT Food Sci. Technol. 2017, 82, 342–353. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Innovative beer-brewing of typical, old and healthy wheat varieties to boost their spreading. J. Clean. Prod. 2018, 171, 297–311. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Energy efficient inactivation of Saccharomyces cerevisiae via controlled hydrodynamic cavitation. Energy Sci. Eng. 2015, 3, 221–238. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. Cavitation Generation and Usage Without Ultrasound: Hydrodynamic Cavitation. In Theoretical and Experimental Sonochemistry Involving Inorganic Systems; Pankaj, D.S., Ashokkumar, M., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 69–106. ISBN 978-90-481-3886-9. [Google Scholar]
- Yasui, K.; Tuziuti, T.; Sivakumar, M.; Iida, Y. Sonoluminescence. Appl. Spectrosc. Rev. 2004, 39, 399–436. [Google Scholar] [CrossRef]
- Pawar, S.K.; Mahulkar, A.V.; Pandit, A.B.; Roy, K.; Moholkar, V.S. Sonochemical effect induced by hydrodynamic cavitation: Comparison of venturi/orifice flow geometries. AIChE J. 2017, 63, 4705–4716. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Wastewater remediation via controlled hydrocavitation. Environ. Rev. 2017, 25, 175–183. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen Peroxide: A Key Chemical for Today’s Sustainable Development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Yusaf, T.; Al-Juboori, R.A. Alternative methods of microorganism disruption for agricultural applications. Appl. Energy 2014, 114, 909–923. [Google Scholar] [CrossRef]
- Yan, Y.; Thorpe, R.B. Flow regime transitions due to cavitation in the flow through an orifice. Int. J. Multiph. Flow 1990, 16, 1023–1045. [Google Scholar] [CrossRef]
- Šarc, A.; Stepišnik-Perdih, T.; Petkovšek, M.; Dular, M. The issue of cavitation number value in studies of water treatment by hydrodynamic cavitation. Ultrason. Sonochem. 2017, 34, 51–59. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef]
- Gogate, P.R. Cavitation: An auxiliary technique in wastewater treatment schemes. Adv. Environ. Res. 2002, 6, 335–358. [Google Scholar] [CrossRef]
- Bartolini, G.; Grifoni, D.; Magno, R.; Torrigiani, T.; Gozzini, B. Changes in temporal distribution of precipitation in a Mediterranean area (Tuscany, Italy) 1955-2013. Int. J. Climatol. 2018, 38, 1366–1374. [Google Scholar] [CrossRef]
- Costantini, E.; Fantappie, M.; L’Abate, G. Climate and Pedoclimate of Italy. In The Soils of Italy; Costantini, E., Dazzi, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; p. 354. ISBN 9400756429. [Google Scholar]
- Albanese, L.; Baronti, S.; Liguori, F.; Meneguzzo, F.; Barbaro, P.; Vaccari, F.P. Hydrodynamic cavitation as an energy efficient process to increase biochar surface area and porosity: A case study. J. Clean. Prod. 2019, 210, 159–169. [Google Scholar] [CrossRef]
- Torti, S.D.; Dearing, M.D.; Kursar, T.A. Extraction of phenolic compounds from fresh leaves: A comparison of methods. J. Chem. Ecol. 1995, 21, 117–125. [Google Scholar] [CrossRef]
- Talari, S.; Shyamsundarachary, R.; Srinivas, P.; Swamy, N.R. Quantification of total phenolic and total flavonoid contents in extracts of Oroxylum indicum L.Kurz. Asian J. Pharm. Clin. Res. 2012, 5, 177–179. [Google Scholar]
- Pekal, A.; Pyrzynska, K. Effect of pH and metal ions on DPPH radical scavenging activity of tea. Int. J. Food Sci. Nutr. 2015, 66, 58–62. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-Based Antioxidants and the In Vitro Methods Used for Their Assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar]
- Yang, S.-A.; Jeon, S.-K.; Lee, E.-J.; Im, N.-K.; Jhee, K.-H.; Lee, S.-P.; Lee, I.-S. Radical Scavenging Activity of the Essential Oil of Silver Fir (Abies alba). J. Clin. Biochem. Nutr. 2009, 44, 253–259. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH—Assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, P.; Cañigueral, S. Biological and Nonbiological Antioxidant Activity of Some Essential Oils. J. Agric. Food Chem. 2016, 64, 4716–4724. [Google Scholar] [CrossRef]
- Merah, S.; Dahmane, D.; Krimat, S.; Metidji, H.; Nouasri, A.; Lamari, L.; Dob, T. Chemical analysis of phenolic compounds and determination of anti-oxidant, antimicrobial and cytotoxic activities of organic extracts of Pinus coulteri. Bangladesh J. Pharmacol. 2018, 13, 120. [Google Scholar] [CrossRef]
- Park, Y.S.; Jeon, M.H.; Hwang, H.J.; Park, M.R.; Lee, S.H.; Kim, S.G.; Kim, M. Antioxidant activity and analysis of proanthocyanidins from pine (Pinus densiflora) needles. Nutr. Res. Pract. 2011, 5, 281–287. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Piovani, P.; Leonardi, S.; Piotti, A.; Menozzi, P. Conservation genetics of small relic populations of silver fir (Abies alba Mill.) in the northern Apennines. Plant Biosyst. 2010, 144, 683–691. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2015, 237, 80. [Google Scholar] [CrossRef]
- Sun, X.; Kang, C.H.; Park, J.J.; Kim, H.S.; Om, A.S.; Yoon, J.Y. An experimental study on the thermal performance of a novel hydrodynamic cavitation reactor. Exp. Therm. Fluid Sci. 2018, 99, 200–210. [Google Scholar] [CrossRef]
- Garuti, M.; Langone, M.; Fabbri, C.; Piccinini, S. Monitoring of full-scale hydrodynamic cavitation pretreatment in agricultural biogas plant. Bioresour. Technol. 2018, 247, 599–609. [Google Scholar] [CrossRef]
- Cravotto, G.; Mariatti, F.; Gunjevic, V.; Secondo, M.; Villa, M.; Parolin, J.; Cavaglià, G. Pilot Scale Cavitational Reactors and Other Enabling Technologies to Design the Industrial Recovery of Polyphenols from Agro-Food By-Products, a Technical and Economical Overview. Foods 2018, 7, 130. [Google Scholar] [CrossRef]
- Martynenko, A.; Astatkie, T.; Satanina, V. Novel hydrothermodynamic food processing technology. J. Food Eng. 2015, 152, 8–16. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K.; Meletharayil, G.H. Application of hydrodynamic cavitation to improve antioxidant activity in sorghum flour and apple pomace. Food Bioprod. Process. 2016, 100, 335–343. [Google Scholar] [CrossRef]
- Li, F.; Chen, G.; Zhang, B.; Fu, X. Current applications and new opportunities for the thermal and non-thermal processing technologies to generate berry product or extracts with high nutraceutical contents. Food Res. Int. 2017, 100, 19–30. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging Applications of a Key Biotechnology Industrial Product. Chem. Cent. J. 2017, 11, 1–9. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Inés Rodríguez Hernández, A.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels, Bioprod. Biorefining 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.; Flagella, Z.; Pastore, D.; Soccio, M.; Laus, M.N.; Flagella, Z.; Pastore, D. Assessment of Antioxidant Capacity and Putative Healthy Effects of Natural Plant Products Using Soybean Lipoxygenase-Based Methods. An Overview. Molecules 2018, 23, 3244. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Sidibé, L.; Maksimovic, Z.A.; Petrovic, S.D.; Gorunovic, M.S. Essential oil of Abies alba mill., Pinaceae, from the pilot production in Montenegro. J. Essent. Oil Res. 2001, 13, 288–289. [Google Scholar] [CrossRef]
- Zeneli, G.; Tsitsimpikou, C.; Petrakis, P.V.; Naxakis, G.; Habili, D.; Roussis, V. Foliar and cortex oleoresin variability of Silver fir (Abies alba Mill.) in Albania. Zeitschrift fur Naturforsch. Sect. C 2001, 56, 531–539. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Vukovic, N.; Puchalski, C.; Roychoudhury, S.; Kunová, S.; Klūga, A.; Tokár, M.; Kluz, M.; Ivanišová, E. The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp. isolated from fish. Saudi Pharm. J. 2017, 25, 1108–1116. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Hossain, M.; Rai, D.K. Waste/By-Product Utilisations. In Innovative Technologies in Beverage Processing; Aguiló-Aguayoi, I., Plaza, L., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 297–309. [Google Scholar]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef]
- Singh, G.; Upadhyay, R.K.; Narayanan, C.S.; Padmkumari, K.P.; Rao, G.P. Chemical and fungitoxic investigations on the essential oil of Citrus sinensis (L.) Pers. J. Plant Dis. Prot. 1993, 100, 69–74. [Google Scholar]
- Hollingsworth, R.G. Limonene, a Citrus Extract, for Control of Mealybugs and Scale Insects. J. Econ. Entomol. 2005, 98, 772–779. [Google Scholar] [CrossRef]
- Keinan, E.; Alt, A.; Amir, G.; Bentur, L.; Bibi, H.; Shoseyov, D. Natural ozone scavenger prevents asthma in sensitized rats. Bioorganic Med. Chem. 2005, 13, 557–562. [Google Scholar] [CrossRef]
- Maté, J.; Periago, P.M.; Palop, A. When nanoemulsified, d-limonene reduces Listeria monocytogenes heat resistance about one hundred times. Food Control 2016, 59, 824–828. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Orange Oil. In Green Pesticides Handbook: Essential Oils for Pest Control; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 291–303. ISBN 978-1-49-875938-0. [Google Scholar]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Nanoencapsulation of D-limonene within nanocarriers produced by pectin-whey protein complexes. Food Hydrocoll. 2018, 77, 152–162. [Google Scholar] [CrossRef]
- Baǧci, E.; Diǧrak, M. Antimicrobial activity of essential oils of some Abies (fir) species from Turkey. Flavour Fragr. J. 1996, 11, 251–256. [Google Scholar] [CrossRef]
- Karkabounas, S.; Assimakopoulos, D.; Malamas, M.; Skaltsounis, A.L.; Leonce, S.; Zelovitis, J.; Stefanou, D.; Evangelou, A. Antiproliferative and anticarcinogenic effects of an aqueous preparation of Abies alba and Viscum album se abies, on a L-1210 malignant cell line and tumor-bearing Wistar rats. Anticancer Res. 2000, 20, 4391–4395. [Google Scholar]
- Benković, E.T.; Grohar, T.; Žigon, D.; Švajger, U.; Janeš, D.; Kreft, S.; Štrukelj, B. Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Ind. Crops Prod. 2014, 52, 23–28. [Google Scholar] [CrossRef]
- Drevenšek, G.; Lunder, M.; Benković, E.T.; Mikelj, A.; Štrukelj, B.; Kreft, S. Silver fir (Abies alba) trunk extract protects Guinea pig arteries from impaired functional responses and morphology due to an atherogenic diet. Phytomedicine 2015, 22, 856–861. [Google Scholar] [CrossRef]
- Drevenšek, G.; Lunder, M.; Benković, E.T.; Štrukelj, B.; Kreft, S. Cardioprotective effects of silver fir (Abies alba) extract in ischemic-reperfused isolated rat hearts. Food Nutr. Res. 2016, 60. [Google Scholar] [CrossRef]
- Lunder, M.; Roškar, I.; Hošek, J.; Štrukelj, B. Silver Fir (Abies alba) Extracts Inhibit Enzymes Involved in Blood Glucose Management and Protect against Oxidative Stress in High Glucose Environment. Plant Foods Hum. Nutr. 2018. [Google Scholar] [CrossRef]
- Whiteland, H.L.; Chakroborty, A.; Forde-Thomas, J.E.; Crusco, A.; Cookson, A.; Hollinshead, J.; Fenn, C.A.; Bartholomew, B.; Holdsworth, P.A.; Fisher, M.; et al. An Abies procera-derived tetracyclic triterpene containing a steroid-like nucleus core and a lactone side chain attenuates in vitro survival of both Fasciola hepatica and Schistosoma mansoni. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 465–474. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef]
- Pavelková, A.; Bobko, M.; Haščík, P.; Kačániová, M.; Tkáčová, J. Oxidative stability of chicken thigh meat after treatment of Abies alba essential oil. Potravin. Sci. J. Food Ind. 2015, 9, 451–457. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Santini, M.; Landi, L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 2013, 75, 24–27. [Google Scholar] [CrossRef]
- Korolev, K.G.; Lomovskii, O.I.; Rozhanskaya, O.A.; Vasil Ev, V.G. Mechanochemical preparation of water-soluble forms of triterpene acids. Chem. Nat. Compd. 2003, 39, 366–372. [Google Scholar] [CrossRef]

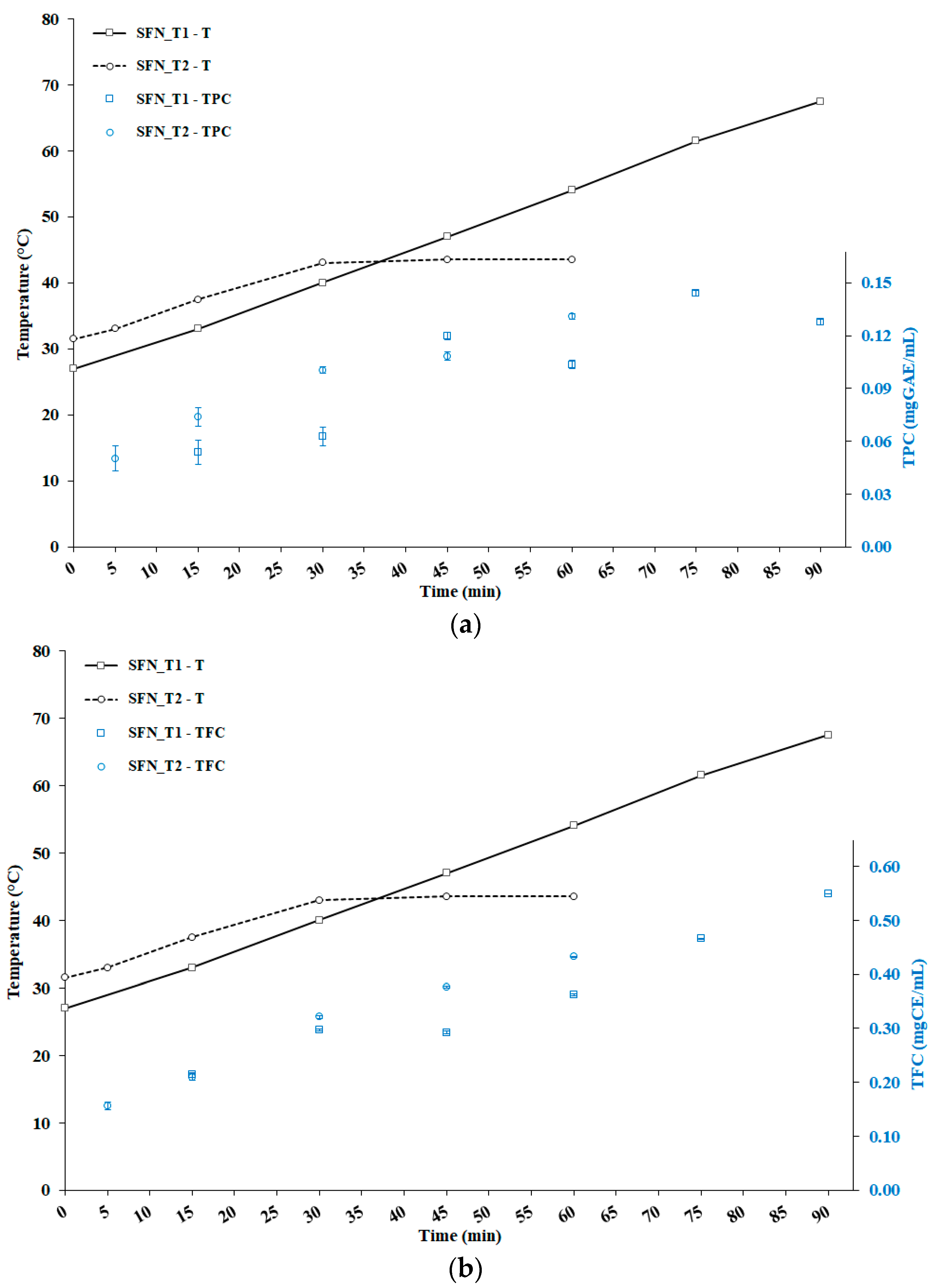
| Time (min) | T (°C) | DPPH (IC50, μg/mL) | ORAC (μMTE/L) |
|---|---|---|---|
| 0 | 27.0 | ||
| 15 | 33.0 | 16.5 ± 1.0 a | 201.7 ± 14.0 b |
| 30 | 40.0 | 14.4 ± 0.7 a | 163.0 ± 11.4 b |
| 45 | 47.0 | 10.1 ± 0.4 | 184.6 ± 12.9 b |
| 60 | 54.0 | 44.0 ± 2.1 | 457.9 ± 24.1 |
| 75 | 61.5 | 150.5 ± 8.4 | 585.9 ± 27.1 |
| 90 | 67.5 | 350.8 ± 23.7 | 295.4 ± 18.7 |
| Time (min) | T (°C) | DPPH (IC50, μg/mL) | ORAC (μMTE/L) |
|---|---|---|---|
| 0 | 31.5 | ||
| 5 | 33.0 | 27.4 ± 1.6 | 190.6 ± 7.3 c |
| 15 | 37.5 | 19.5 ± 0.9 a | 186.6 ± 18.1 c |
| 30 | 43.0 | 19.5 ± 0.9 a | 393.8 ± 25.6 |
| 45 | 43.0 | 13.7 ± 0.5 b | 497.9 ± 22.8 |
| 60 | 43.0 | 14.7 ± 0.8 b | 840.8 ± 31.4 |
| SFN_T1 | SFN_T2 | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 48 | Diff. | Day 1 | Day 9 | Diff. | |
| TPC (mgGAE/mL) | 0.103 ± 0.002 | 0.053 ± 0.007 | −48% | 0.131 ± 0.002 | 0.091 ± 0.003 | −31% |
| TFC (mgCE/mL) | 0.363 ± 0.002 | 0.209 ± 0.004 | −42% | 0.432 ± 0.001 | 0.309 ± 0.002 | −28% |
| DPPH (IC50, μg/mL) | 44.0 ± 2.1 | 65.8 ± 3.0 | 50% | 14.7 ± 0.8 a | 14.4 ± 1.0 a | 0% |
| ORAC (μMTE/L) | 457.9 ± 24.1 | 128.3 ± 8.5 | −72% | 840.8 ± 31.4 | 152.3 ± 5.7 | −82% |
| Substance | DPPH (IC50, μg/mL) | Ref. |
|---|---|---|
| Abies alba needles extract | 10.1 ± 0.4 | This study a |
| Ascorbic acid (reference substance) | 5.85 | [45] |
| Ascorbic acid (reference substance) | 7.62 | [12] |
| Ascorbic acid (reference substance) | 20 ± 1.3 | [44] |
| Ascorbic acid (reference substance) | 50 | [45] |
| Resveratrol (reference substance) | 16.62 | [12] |
| Quercetin (reference substance) | 10.5 ± 4.6 | [46] |
| Butylated hydroxytoluene (synthetic antioxidant, reference substance) | 11.58 | [12] |
| Butylated hydroxytoluene (synthetic antioxidant, reference substance) | 21.30 | [45] |
| α-Tocopherol (vitamin E) | 27.1 | [45] |
| Epigalocatechin gallate (a type of catechin) | 7.06 | [12] |
| Abies alba twigs and needles (essential oil) | 27 ± 6.3 | [44] |
| Clove (essential oil) | 13.2 ± 2.9 | [46] |
| Abies alba wood (extract) | 35.46 | [12] |
| Pinus coulteri needles (extract) b | 22.7 ± 0.6 | [47] |
| Pinus densiflora needles (extract) c | 270 | [48] |
| Substance | ORAC (μMTE/L) | TPC (mgGAE/L) | Ref. |
|---|---|---|---|
| Abies alba needles extract | 186.6 ± 18.1 | 74 ± 4 | This study a |
| Abies alba needles extract | 497.9 ± 22.8 | 108 ± 2 | This study b |
| Abies alba needles extract | 840.8 ± 31.4 | 131 ± 2 | This study c |
| Iced white tea | 2700 ± 300 | 900 ± 0 | [49] |
| Iced black tea | 3100 ± 200 | 400 ± 0 | |
| Apple juice | 4800 ± 1000 | 400 ± 100 | |
| Iced green tea | 5300 ± 1900 | 800 ± 100 | |
| Orange juice | 7400 ± 500 | 700 ± 100 | |
| Cranberry juice | 15,400 ± 2100 | 1700 ± 200 | |
| Blueberry juice | 20,600 ± 2900 | 2300 ± 400 | |
| Red wine | 25,700 ± 2100 | 3500 ± 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albanese, L.; Bonetti, A.; D’Acqui, L.P.; Meneguzzo, F.; Zabini, F. Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies alba Mill.) Needles. Foods 2019, 8, 65. https://doi.org/10.3390/foods8020065
Albanese L, Bonetti A, D’Acqui LP, Meneguzzo F, Zabini F. Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies alba Mill.) Needles. Foods. 2019; 8(2):65. https://doi.org/10.3390/foods8020065
Chicago/Turabian StyleAlbanese, Lorenzo, Alessandra Bonetti, Luigi Paolo D’Acqui, Francesco Meneguzzo, and Federica Zabini. 2019. "Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies alba Mill.) Needles" Foods 8, no. 2: 65. https://doi.org/10.3390/foods8020065
APA StyleAlbanese, L., Bonetti, A., D’Acqui, L. P., Meneguzzo, F., & Zabini, F. (2019). Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies alba Mill.) Needles. Foods, 8(2), 65. https://doi.org/10.3390/foods8020065