Development and Characterization of Gelled Double Emulsions Based on Chia (Salvia hispanica L.) Mucilage Mixed with Different Biopolymers and Loaded with Green Tea Extract (Camellia sinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtention of Green Tea Extract (GTE)
2.2.1. Characterization of GTE
2.3. Extraction of Chia Mucilage
2.4. Preparation of Gelled Double Emulsions
2.4.1. Characterization of Gelled Double Emulsions
2.5. Statistical Analysis
3. Results and Discussion
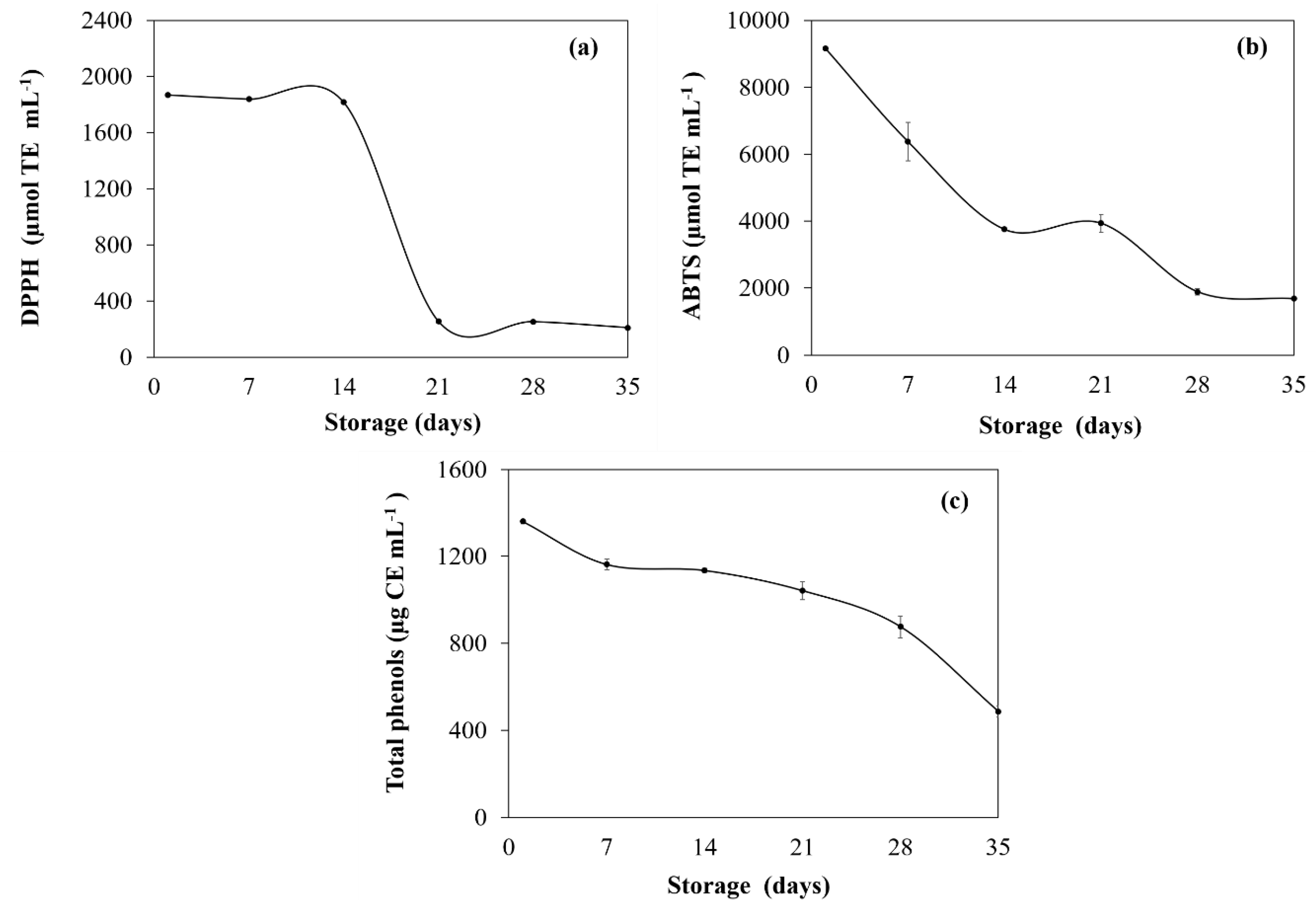
3.1. Obtention of Green Tea Extract (GTE)
Antioxidant Activity and Total Phenolic Content of GTE
3.2. Extraction of Chia Mucilage (CM)
3.3. Characterization and Stability of Gelled Double Emulsions
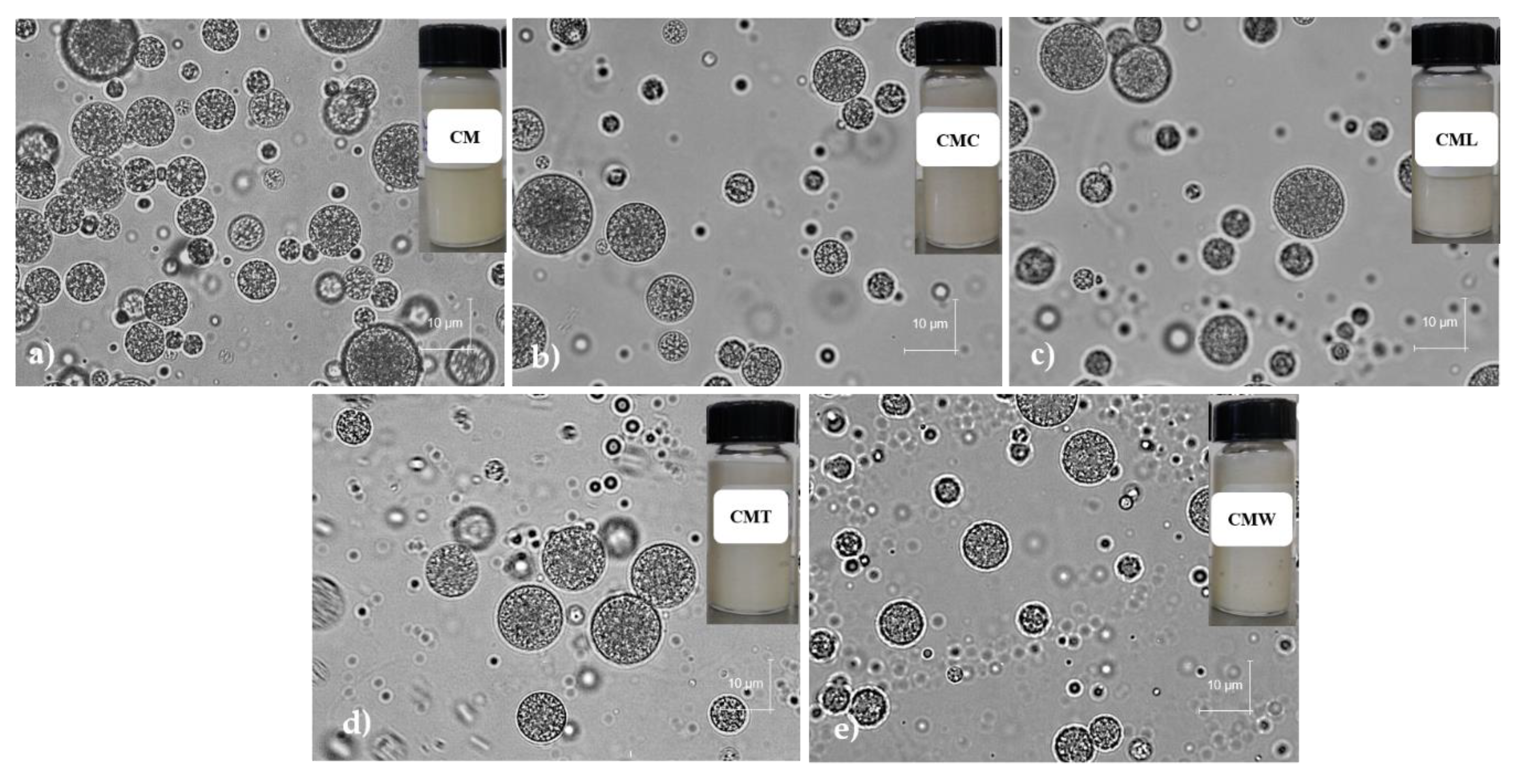
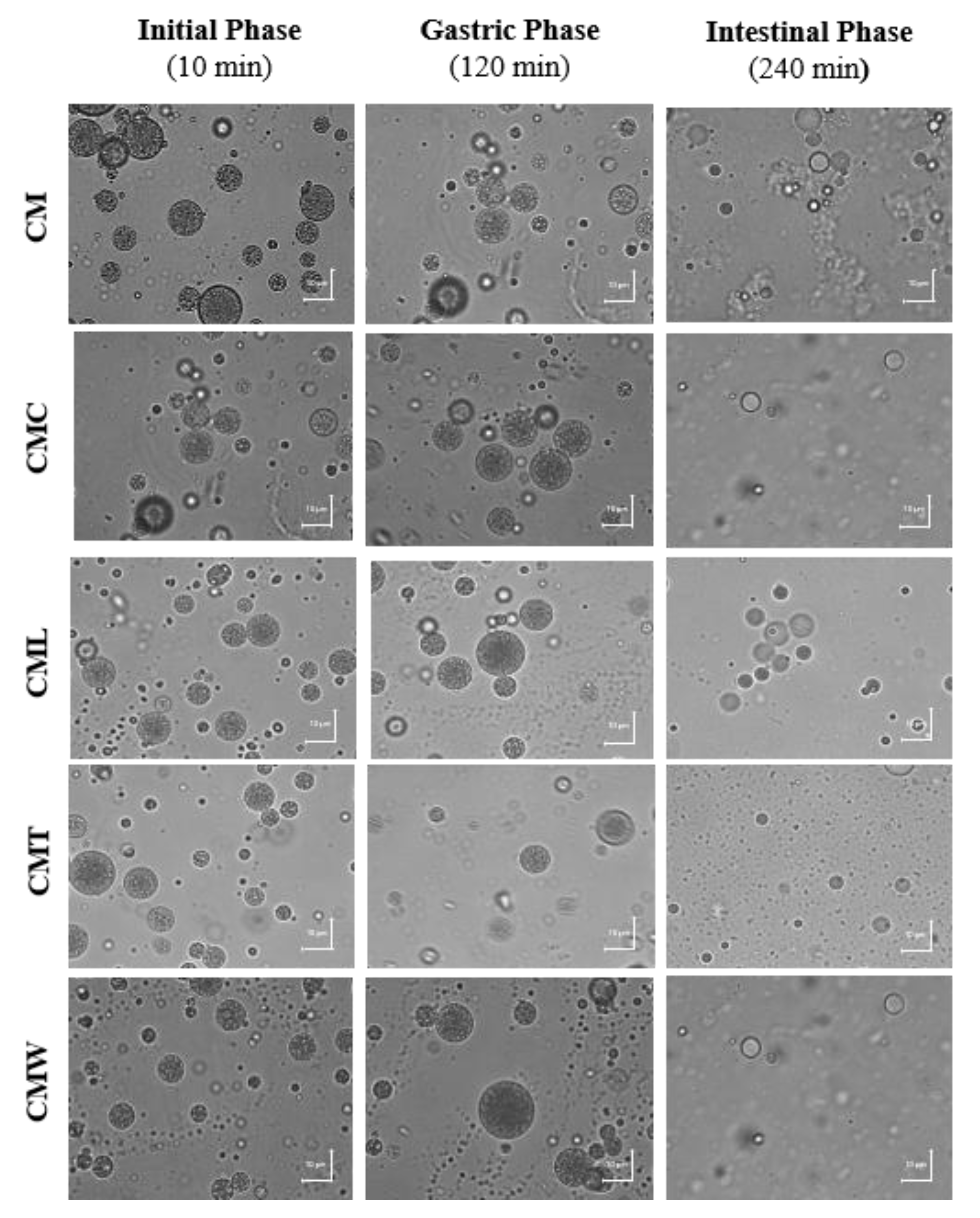
3.3.1. Microscopic Analysis
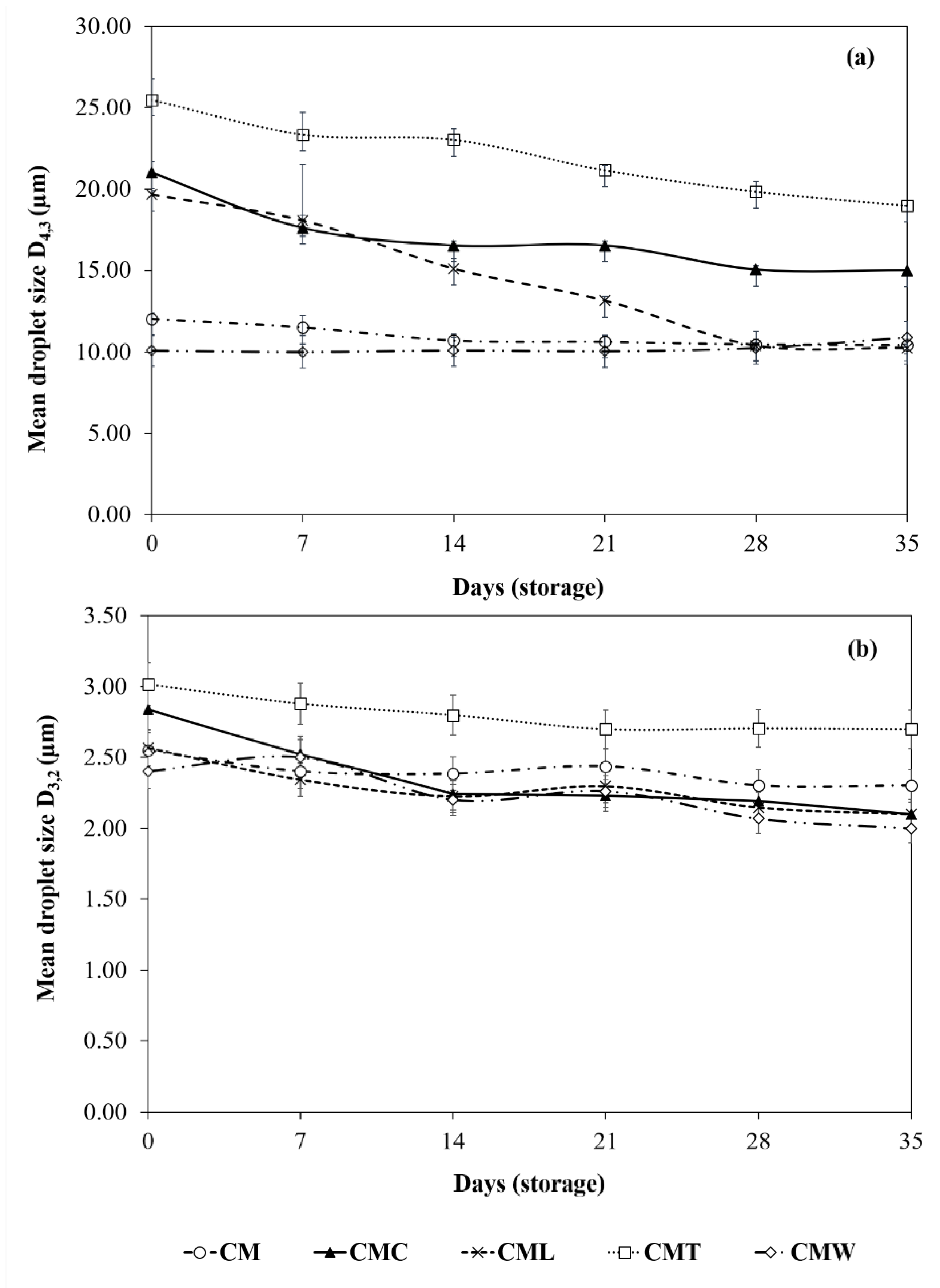
3.3.2. Droplet Size Measurements
3.3.3. Creaming Stability Measurements
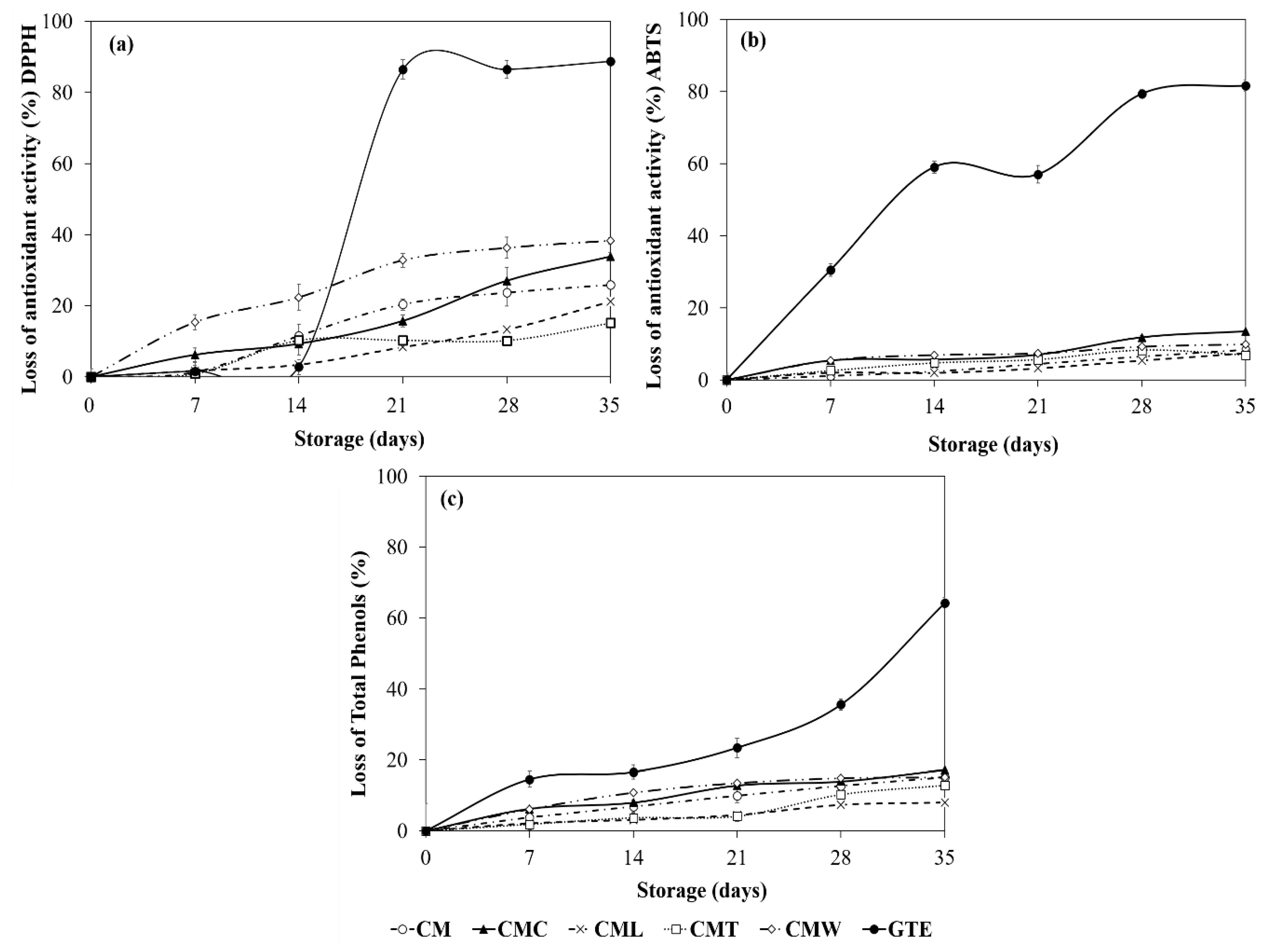
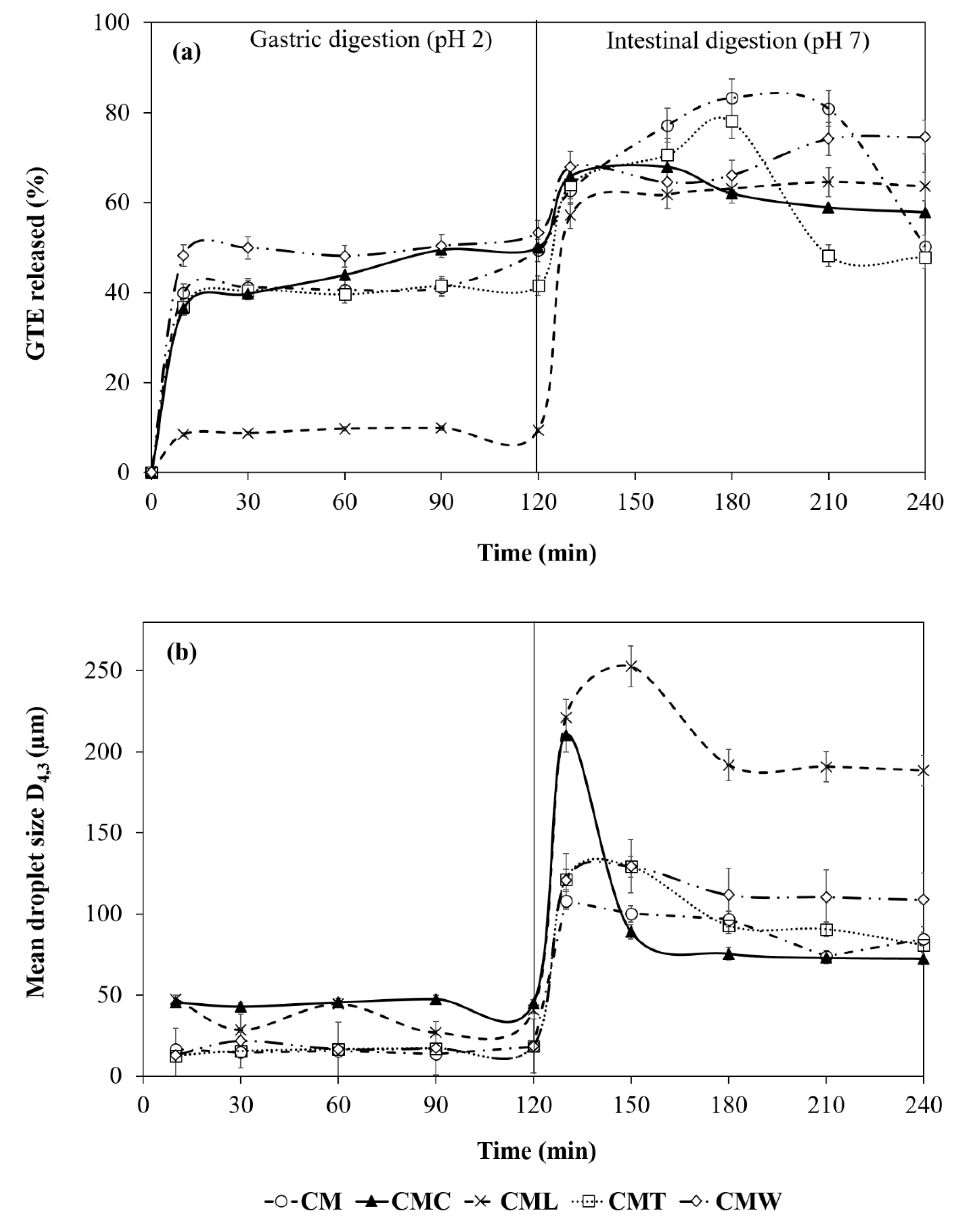
3.3.4. Protective Effect of Gelled Double Emulsion on GTE
3.3.5. Digestibility (In Vitro) of Gelled Double Emulsions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Birch, S.C.; Bonwick, G.A. Ensuring the future of functional foods. Int. J. Food Sci. Technol. 2018, 54, 1467–1485. [Google Scholar] [CrossRef]
- Castro, F.V.R.; Andrade, M.A.; Sanches Silva, A.; Vaz, M.F.; Vilarinho, F. The Contribution of a whey protein film incorporated with green tea extract to minimize the lipid oxidation of salmon (Salmo salar L.). Foods 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Satoh, E.; Tohyama, N.; Nishimura, M. Comparison if the antioxidant activity of roasted tea with green, oolong, and black teas. Int. J. Food Sci. Nutr. 2009, 56, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, X.B.; Tian, D.Q.; Fang, X.P.; Yu, Y.M.; Zhu, H.Q.; Ge, Y.Y.; Ma, G.Y.; Wang, W.Y.; Xiao, W.F.; et al. Antioxidant properties and color parameters of herbal teas in China. Ind. Crop. Prod. 2016, 87, 189–209. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2011, 50, 469–479. [Google Scholar] [CrossRef]
- Namal, S.S.P.J. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Phongnarisorn, B.; Orfila, C.; Holmes, M.; Marshall, L.J. Enrichment of biscuits with Matcha green tea powder: Its impact on consumer acceptability and acute metabolic response. Foods 2018, 7, 17. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Lamothe, S.; Azimy, N.; Bazinet, L.; Couillard, C.; Britten, M. Interactions of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment. Food Funct. 2014, 5, 2621–2631. [Google Scholar] [CrossRef]
- Bellés, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. Sulfite-free lamb meat: Antimicrobial and antioxidant properties of green tea and carvacrol. J. Sci. Food Agric. 2018, 99, 464–472. [Google Scholar] [CrossRef]
- Ortíz, J.; Ferruzzi, M.G.; Taylor, L.S.; Maurer, L.J. Interaction of Environmental Moisture with Powdered Green Tea Formulations: Effect on Catechin Chemical Stability. J. Agric. Food Chem. 2008, 56, 4068–4077. [Google Scholar]
- Cutrim, C.; Dutra, I.; Sloboda, M.A. Microencapsulation of green tea polyphenols by ionic gelation and spray chilling methods. J. Food Sci. Technol. 2019, 56, 3561–3570. [Google Scholar] [CrossRef] [PubMed]
- Zokti, J.A.; Baharim, B.S.; Mohammed, A.S.; Abas, F. Green tea leaves extract: Microencapsulation, physicochemical and storage stability study. Molecules 2016, 21, 940. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, D.; Ezhilarasi, P.N.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green trea polyphenols and its effect on incorporated bread quality. LWT Food Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Kurrey, N.K.; Anandharamakrishnan, C. Nanoencapsulation of green tea catechins by electrospraying technique and its effects on controlled release and in-vitro permeability. J. Food Eng. 2017, 199, 82–92. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014, 156, 176–183. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C.; Alfonso, M.J.; Caballero, M.A. Antioxidant packaging with encapsulated green tea for fresh minced meat. Food Sci. Emerg. Technol. 2017, 41, 307–313. [Google Scholar] [CrossRef]
- Kim, Y.J.; Houng, S.-J.; Kim, J.H.; Kim, Y.; Ji, H.G.; Lee, S. Nanoemulsified green tea extract shows improved hypocholesterolemic effects in C57BL/6 mice. J. Nutr. Biochem. 2012, 23, 186–191. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Noipha, S. Active film from chitosan incorporating green tea extract for shelf life extensión of pork sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Von Staszewski, M.; Ruiz, H.; Vitor, M.P.; Pilosof, A.M.R. Green tea polyphenols-β-lactoglobulin nanocomplexes: Interfacial behavior emulsification and oxidation stability of fish oil. Food Hydrocoll. 2014, 35, 505–511. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N. Stability of a Cosmetic Multiple Emulsion Loaded with Green Tea Extract. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N.; Manickman, S. Interfacial film stabilized W/O/W nano multiple emulsions loaded with green tea and lotus extracts: Systematic characterization of physicochemical properties and shelf-storage stability. J. Nanobiotechnol. 2014, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Vladisavlijevic, G.T.; Mun, S.; McClements, D.J. Preparation and characterization of water/oil and water/oil/water emulsions containing biopolymer-gelled water droplets. J. Agric. Food Chem. 2007, 55, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Dumlu, P.; Vermeir, L.; Lewille, B.; Lesaffer, A. Rheological characterization of gel-in-oil-in-gel type structured emulsions. Food Hydrocoll. 2015, 46, 84–92. [Google Scholar] [CrossRef]
- Altunas, O.Y.; Sumnu, G.; Sahin, S. Preparation and characterization of W/O/W type double emulsion contaning PGPR-Lecithin mixture as lipophilic surfactant. J. Disp. Sci. Technol. 2016, 38, 486–493. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mahdi, J.; Seid Assadpour, E.; Faridi Esfanjani, A. Nano-encapsulation of olive leaf phenolic compounds through WPC-pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2015, 82, 816–822. [Google Scholar] [CrossRef]
- Akhtar, M.; Murray, B.S.; Afeisume, E.I.; Khew, S.H. Encapsulation of flavonoid in multiple emulsion using spinning disc reactor technology. Food Hydrovoll. 2013, 34, 62–67. [Google Scholar] [CrossRef]
- Capitani, M.I.; Nolasco, S.M.; Tomás, M.C. Stability of oil-in-water (O/W) emulsions with chia (Salvia hispánica L.) mucilage. Food Hydrocoll. 2016, 61, 546–573. [Google Scholar] [CrossRef]
- De Campo, C.; Pereira dos Santos, P.; Hass Costa, T.M.; Paese, K.; Stanisçuaski, S.; de Oliveira Rios, A. Hickmann Flôres, S. Nanoencapsulation of chia seed oil with chia mucilage (Salvia hispánica L.) as wall material: Characterization and stability evaluation. Food Chem. 2017, 234, 1–9. [Google Scholar] [CrossRef]
- Xingú, L.A.; González, H.A.; De la Cruz, T.E.; Sangerman-Jarquín, D.M.; Orozco, D.R.G.; Rubí, A.M. Chía (Salvia hispanica L.) situación actual y tendencias futuras. Rev. Mex. Cienc. Agríc. 2017, 8, 619–1631. [Google Scholar]
- Ferrari, F.M.H.; Wahanik, A.L.; Rodrigues, G.C.; Pedrosa, S.C.M.T.; Kil, C.Y.; Steel, J.C. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar]
- Avila-de la Rosa, G.; Alvarez-Ramirez, J.; Vernon-Carter, E.J.; Carrillo-Nava, H.; Pérez-Alonso, C. Viscoelasticity of chia (Salvia hispánica L.) seed mucilage dispersion in the vicinity of an oil-water interface. Food Hydrocoll. 2015, 49, 200–207. [Google Scholar] [CrossRef]
- Druzyńska, B.; Stępniewska, A.; Wolosiak, R. The influence of time and type of solvent on efficiency of the extraction of polyphenols from green tea and antioxidant properties obtained extracts. Acta Sci. Pol. Technol. Aliment. 2007, 6, 27–36. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Bryne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Ahikari, B. Microencapsulation of chia seed oil using chia seed protein isolate-chia seed gum complex coacervates. Int. J. Biol. Macromol. 2016, 91, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Huezo, M.E.; Pedroza-Islas, R.; Prado-Barragán, L.A.; Beristain, C.I.; Vernon-Carter, E.J. Microencapsulation by spray drying of multiple emulsions containing carotenoids. J. Food Sci. 2004, 69, 351–359. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Rodríguez, B.A.; Lagaron, J.M.; López-Rubio, A. Improved antioxidant capacity of quercetin and ferulic acid during in-vitro digestion through encapsulation within food-grade electrospun fibers. J. Funct. Foods 2015, 12, 332–341. [Google Scholar] [CrossRef]
- Unno, T.; Osakabe, N. Green tea extract and black tea extract differentially influence cecal levels of short-chain fatty acids in rats. Food Sci. Nutr. 2018, 6, 728–735. [Google Scholar] [CrossRef]
- Wang, C.W.; Liang, C. Reductive lindane degradation with tea extracts in aqueous phase. Chem. Eng. J. 2018, 338, 157–165. [Google Scholar] [CrossRef]
- Afify, A.E.-M.; Shalaby, E.A.; El-Beltagi, H.S. Antioxidant activity of aqueous extracts of different caffeine products. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 117–123. [Google Scholar] [CrossRef][Green Version]
- Gramza, A.; Pawlak-Lemańska, K.; Korczak, J.; Wąsowicz, E.; Rudzinka, M. Tea extracts as Free Radical Scavengers. Pol. J. Environ. Stud. 2005, 6, 861–867. [Google Scholar]
- Kodama, D.H.; Gonçalves, A.E.; de Souza Schmidt, L.; Franco, M.; Genovese, M.I. Flavonoids, total phenolic and antioxidant capacity: Comparison between commercial Green tea preparations. Ciênc. Technol. Aliment. 2009, 30, 1077–1082. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kozukue, N. Stability of green tea catechins in commercial tea leaves during storage for 6 months. J. Food Sci. 2009, 74, 47–51. [Google Scholar] [CrossRef]
- Campos, B.; Ruivo, T.D.; Scapim, M.; Madrona, G.; Bergamasco, R. Optimization of the mucilage extraction process from chia application in ice cream as a stabilizer and emulsifier. LWT Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia sedes: Microstructure, mucilage extraction and hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- Frasch-Melnik, S.; Spyropoulos, F.; Norton Ian, T. W1/O/W2 double emulsions stabilized by fat crystals – Formulation, stability and salt release. J. Colloid. Interf. Sci. 2010, 350, 178–185. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.-J.; Kim, H.W.; Park, S.O.; Lee, J.; Ko, S. Curcumin and catechin co-loaded water-in-oil-in-water emulsion and its beverage application. J. Funct. Foods 2015, 15, 35–43. [Google Scholar] [CrossRef]
- Von Staszewski, M.; Pilosof, A.M.R.; Jagus, R.J. Antioxidant and antimicrobial performance of different Argentinean green tea varieties as affected by whey proteins. Food Chem. 2011, 125, 186–192. [Google Scholar] [CrossRef]
- McClements, D. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; Taylor & Francis Group: Florida, FL, USA, 2015; pp. 304–306. [Google Scholar]
- Kosegarten-Conde, C.E.; Jiménez, M. Factores que intervienen en la estabilidad de una emulsión doble. Temas Sel. Ing. Aliment. 2012, 6, 1–18. [Google Scholar]
- Schuch, A.; Helfenritter, C.; Funck, M.; Schuchmann, H.P. Observations on the influence of different biopolymers on coalescence of inner water droplets in W/O/W (water-in-oil-in-water) double emulsions. Colloid. Surf. A Phys. Eng. Asp. 2015, 475, 2–8. [Google Scholar] [CrossRef]
- Ye, A.; Hemar, Y.; Singh, H. Influence of polysaccharides on the rate of coalescence in oil-in-water emulsions formed with highly hydrolyzed whey proteins. J. Agric. Food Chem. 2004, 52, 5491–5498. [Google Scholar] [CrossRef]
- Lizarraga, M.S.; Vicini, D.; González, A.; Rubiolo, A.; Santiago, L. Rheological behavior of whey protein concentrate and ĸ-carrageenan aqueous mixtures. Food Hydrocoll. 2006, 20, 740–748. [Google Scholar] [CrossRef]
- Manoi, K.; Rizvi, S.H. Rheological characterizations of texturized whey protein concentrate-based powders produced by reactive supercritical fluid extrusion. Food Res. Int. 2008, 41, 786–796. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of Polyphenol- Protein Interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- López de Lacey, A.M.; Pérez-Satín, E.; López-Caballero, M.E.; Montero, P. Survival and metabolic activity of probiotic bacteria in green tea. LWT Food Sci. Technol. 2014, 55, 314–322. [Google Scholar] [CrossRef]
- Tokle, T.; Lesmes, U.; Andrew Decker, E.; Julian McClements, D. Impact of dietary fiber coatings on behavios of protein-stabilized lipid droplets under simulated gastrointestinal conditions. Food Funct. 2012, 3, 58–66. [Google Scholar] [CrossRef]
- Sreedhara, A.; Vegarud, G.E.; Ekeberg, D.; Devols, T.G.; Rukke, E.O.; Schüller, R.B. Rheological behavior and droplet size distribution of emulsions stabilized by whey proteins and chitosan during ex vivo digestion. Annu. Trans. Nord. Rheol. Soc. 2014, 22, 245–252. [Google Scholar]
- Zou, L.; Peng, S.; Liu, W.; Gan, L.; Liu, W.; Liang, R.; Liu, C.; Niu, J.; Cao, Y.; Liu, Z. Improved in vitro digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Res. Int. 2014, 64, 492–499. [Google Scholar] [CrossRef]
- Kaimainen, M.; Marze, S.; Järvenpää, E.; Anton, M.; Huopalahti, R. Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT Food Sci. Technol. 2015, 60, 899–904. [Google Scholar] [CrossRef]
| Primary Emulsion (W1/O) (ф1 = 0.2) | Second Aqueous Phase (W2) (ф2 = 0.2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous Phase | Canola Oil Phase | Aqueous Phase | ||||||||
| Emul-sions | WPC (%) | NaCl (%) | Sodium azide (%) | GTE mg kg−1 | PGPR (%) | CM (%) | C (%) | L (%) | T (%) | W (%) |
| CM | 15 | 0.02 | 0.02 | 500 | 7.5 | 2 | - | - | - | - |
| CMC | 15 | 0.02 | 0.02 | 500 | 7.5 | 1 | 1 | - | - | - |
| CML | 15 | 0.02 | 0.02 | 500 | 7.5 | 1 | - | 1 | - | - |
| CMT | 15 | 0.02 | 0.02 | 500 | 7.5 | 1 | - | - | 1 | - |
| CMW | 15 | 0.02 | 0.02 | 500 | 7.5 | 1 | - | - | - | 1 |
| Gelled Double Emulsions | DPPH·+ (µmol TE mL−1) | ABTS·+ (µmol TE mL−1) | Total Phenols (µg CE mL−1) | |||
|---|---|---|---|---|---|---|
| Day 0 | Day 35 | Day 0 | Day 35 | Day 0 | Day 35 | |
| CM | b 1954.50 ± 11.40 A | b 1449.08 ± 80.29 B | b 17,343.51 ± 20.68 A | b 15,898.77 ± 20.68 B | d 3951.02 ± 82.05 A | e 3353.23 ± 2.86 B |
| CMC | c 2176.75 ± 44.54 A | b 1439.21 ± 5.70 B | c 18,704.68 ± 41.36 A | c 16,197.27 ± 54.71 B | d 4010.47 ± 2.86 A | d 3318.56 ± 2.86 B |
| CML | d 2333.15 ± 7.54 A | c 1839.26 ± 2.85 B | c 19,098.70 ± 20.68 A | e 17,689.77 ± 20.86 B | e 4135.97 ±5.72 A | f 3805.70 ± 8.58 B |
| CMT | c 2143.82 ± 18.08 A | c 1819.50 ± 11.40 B | b 17,844.99 ± 593.28 A | d 16,615.17 ± 230.29 B | b 2656.36 ± 42.09 A | b 2312.88 ± 2.86 B |
| CMW | c 2208.03 ± 69.14 A | b 1361.83 ± 22.81 B | c 18,609.16 ± 62.04 A | c 16,782.33 ± 35.82 B | c 3526.63 ± 5.72 A | c 2993.24 ± 8.58 B |
| GTE | a 1868.57 ± 12.43 A | a 208.82 ± 5.70 B | a 9164.30 ± 18.80 A | a 1685.45 ± 16.28 B | a 1360.49 ± 176.08 A | a 486.14 ± 24.99 B |
| Gelled Double Emulsions | Initial Conditions (10 min) | Gastric Conditions (120 min) | Intestinal Conditions (240 min) |
|---|---|---|---|
| CM | b 5134.72 ± 107.15 A | c 6353.32 ± 353.86 B | a 6465.80 ± 479.39 B |
| CMC | b 5265.95 ± 19.36 A | d 7228.21 ± 96.81 B | b 8365.56 ± 413.47 C |
| CML | a 1466.48 ± 36.96 A | a 1631.46 ± 53.39 A | c 11,008.98 ± 294.04 B |
| CMT | b 5059.73 ± 290.84 A | b 5697.15 ± 161.56 A | a 6565.79 ± 836.07 B |
| CMW | c 7315.70 ± 294.28 A | e 8090.60 ± 479.39 B | c 11,315.20 ± 117.78 C |
| GTE | d 8984.24 ± 586.05 A | f 9315.45 ± 411.19 A | d 16,470.80 ± 152.61 B |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Díaz, D.A.; Treviño-Garza, M.Z.; Rodríguez-Romero, B.A.; Gallardo-Rivera, C.T.; Amaya-Guerra, C.A.; Báez-González, J.G. Development and Characterization of Gelled Double Emulsions Based on Chia (Salvia hispanica L.) Mucilage Mixed with Different Biopolymers and Loaded with Green Tea Extract (Camellia sinensis). Foods 2019, 8, 677. https://doi.org/10.3390/foods8120677
Guzmán-Díaz DA, Treviño-Garza MZ, Rodríguez-Romero BA, Gallardo-Rivera CT, Amaya-Guerra CA, Báez-González JG. Development and Characterization of Gelled Double Emulsions Based on Chia (Salvia hispanica L.) Mucilage Mixed with Different Biopolymers and Loaded with Green Tea Extract (Camellia sinensis). Foods. 2019; 8(12):677. https://doi.org/10.3390/foods8120677
Chicago/Turabian StyleGuzmán-Díaz, Diana A., Mayra Z. Treviño-Garza, Beatriz A. Rodríguez-Romero, Claudia T. Gallardo-Rivera, Carlos Abel Amaya-Guerra, and Juan G. Báez-González. 2019. "Development and Characterization of Gelled Double Emulsions Based on Chia (Salvia hispanica L.) Mucilage Mixed with Different Biopolymers and Loaded with Green Tea Extract (Camellia sinensis)" Foods 8, no. 12: 677. https://doi.org/10.3390/foods8120677
APA StyleGuzmán-Díaz, D. A., Treviño-Garza, M. Z., Rodríguez-Romero, B. A., Gallardo-Rivera, C. T., Amaya-Guerra, C. A., & Báez-González, J. G. (2019). Development and Characterization of Gelled Double Emulsions Based on Chia (Salvia hispanica L.) Mucilage Mixed with Different Biopolymers and Loaded with Green Tea Extract (Camellia sinensis). Foods, 8(12), 677. https://doi.org/10.3390/foods8120677







