Effects of Transglutaminase on the Protein Network and In Vitro Starch Digestibility of Asian Wheat Noodles
Abstract
1. Introduction
2. Materials and Methods
2.1. Noodles Preparation
2.2. Confocal Scanning Laser Microscopy (CSLM)
2.3. Protein Solubility
2.4. Differential Scanning Calorimetry (DSC)
2.5. Water Absorption
2.6. Texture Analysis
2.7. In Vitro Digestion
2.8. Statistical and Mathematical Analysis
3. Results
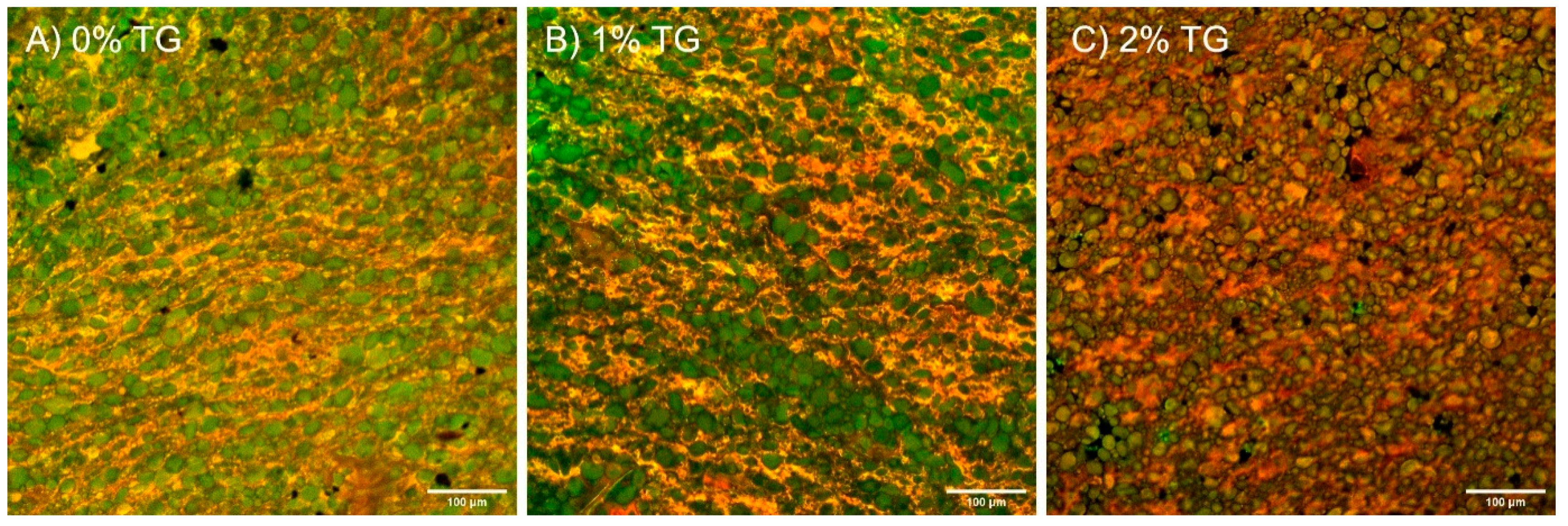
3.1. Microstructure (CSLM)
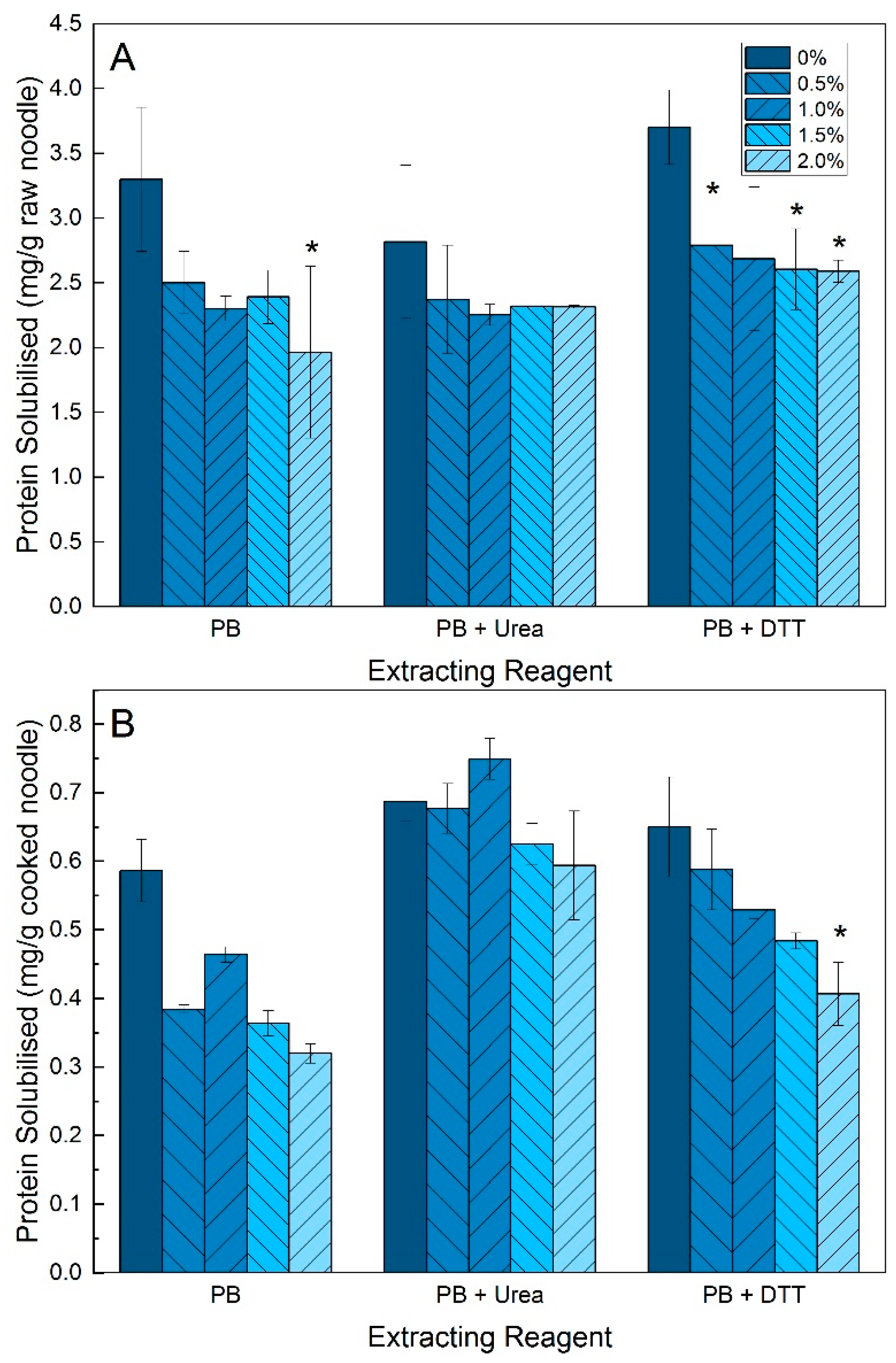
3.2. Protein Solubility
3.3. Thermal Properties (DSC)
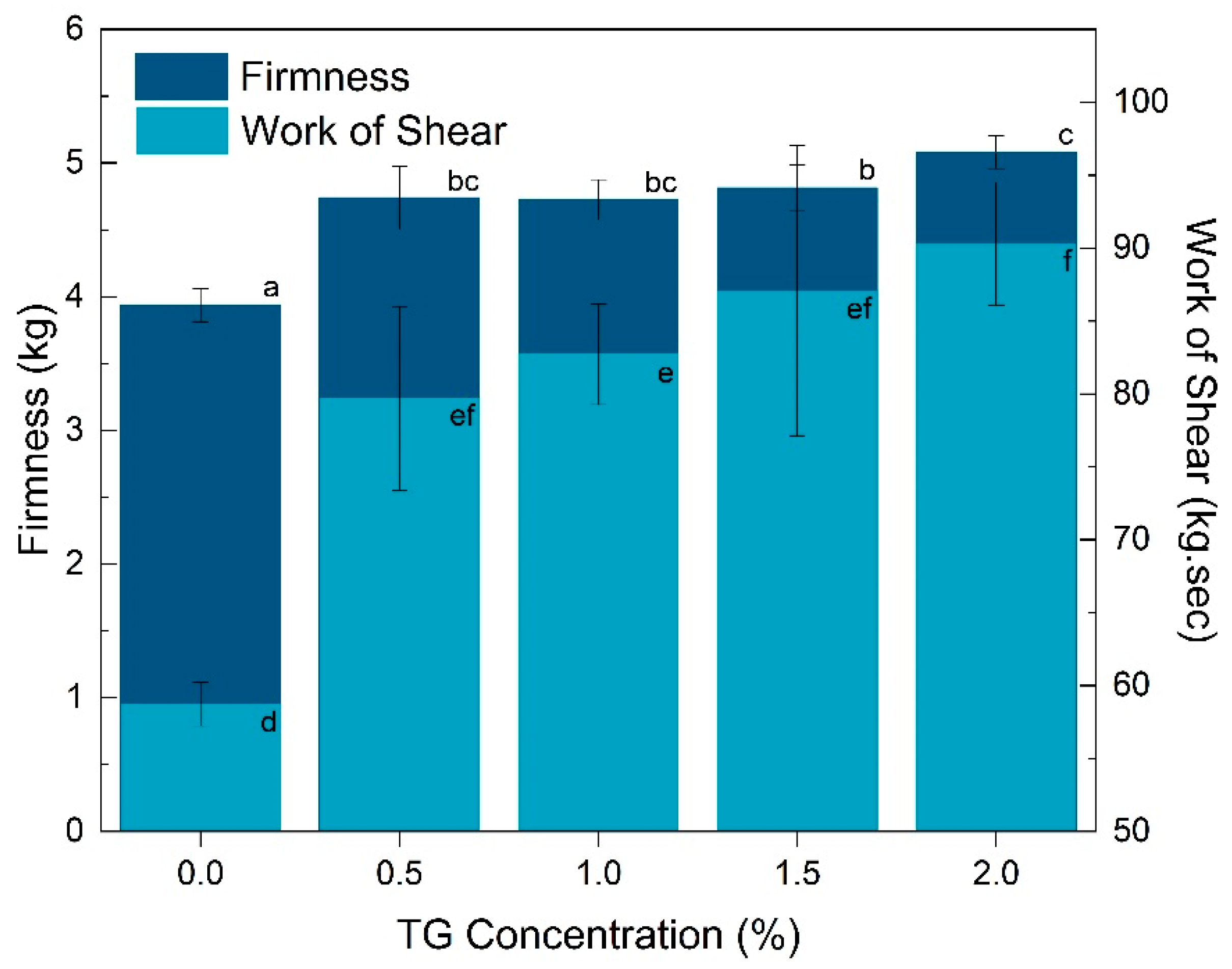
3.4. Textural Properties
3.5. Water Absorption
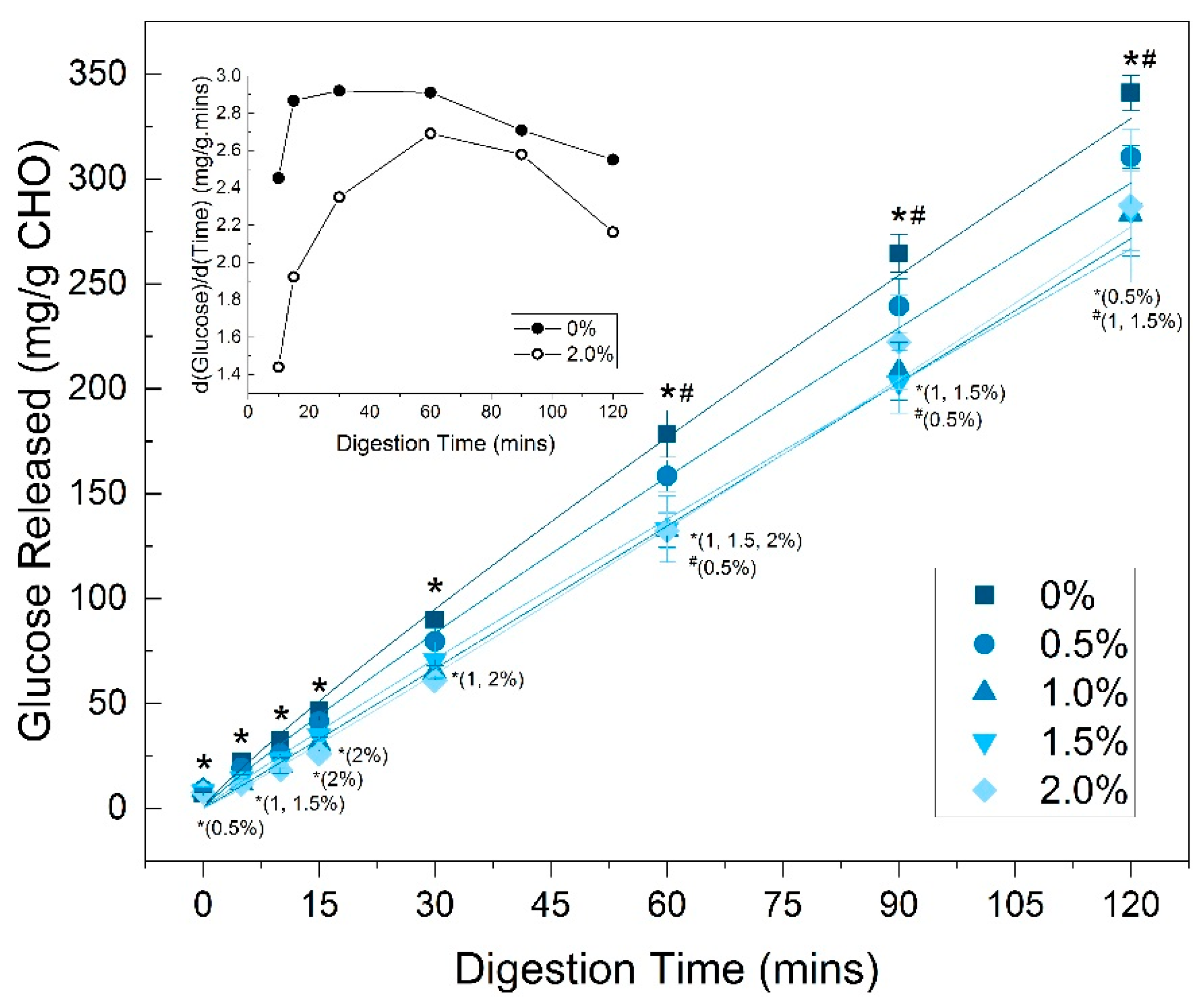
3.6. In Vitro Digestion (Glucose Release)
4. Discussion and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zuniga, Y.L.; Rebello, S.A.; Oi, P.L.; Zheng, H.; Lee, J.; Tai, E.S.; Van Dam, R.M. Rice and noodle consumption is associated with insulin resistance and hyperglycaemia in an Asian population. Br. J. Nutr. 2014, 111, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.-P.; Colette, C. Activation of Oxidative Stress by Acute Glucose Fluctuations Compared with Sustained Chronic Hyperglycemia in Patients With Type 2 Diabetes. JAMA 2006, 295, 1681. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Choi, H.; Lee, S.; Park, J.-M.; Kim, B.R.; Paik, H.-Y.; Song, Y. Establishing a Table of Glycemic Index Values for Common Korean Foods and an Evaluation of the Dietary Glycemic Index among the Korean Adult Population. Korean J. Nutr. 2012, 45, 80. [Google Scholar] [CrossRef]
- Foster-Powell, K.; A Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Abecassis, J.; Hoebler, C.; Baldwin, P.; Buléon, A.; Berot, S.; Barry, J.-L. Influence of Technological Modifications of the Protein Network from Pasta on in vitro Starch Degradation. J. Cereal Sci. 1999, 30, 133–145. [Google Scholar] [CrossRef]
- Granfeldt, Y.; Björck, I. Glycemic response to starch in pasta: A study of mechanisms of limited enzyme availability. J. Cereal Sci. 1991, 14, 47–61. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Thorne, M.J.; Wolever, T.M.; Jenkins, A.L.; Rao, A.V.; Thompson, L.U. The effect of starch-protein interaction in wheat on the glycemic response and rate of in vitro digestion. Am. J. Clin. Nutr. 1987, 45, 946–951. [Google Scholar] [CrossRef]
- Laleg, K.; Barron, C.; Santé-Lhoutellier, V.; Walrand, S.; Micard, V. Protein enriched pasta: Structure and digestibility of its protein network. Food Funct. 2016, 7, 1196–1207. [Google Scholar] [CrossRef]
- Zou, W.; Sissons, M.; Gidley, M.J.; Gilbert, R.G.; Warren, F.J. Combined techniques for characterising pasta structure reveals how the gluten network slows enzymic digestion rate. Food Chem. 2015, 188, 559–568. [Google Scholar] [CrossRef]
- López-Barón, N.; Gu, Y.; Vasanthan, T.; Hoover, R. Plant proteins mitigate in vitro wheat starch digestibility. Food Hydrocoll. 2017, 69, 19–27. [Google Scholar] [CrossRef]
- Ryan, K.; Brewer, M. In situ examination of starch granule-soy protein and wheat protein interactions. Food Chem. 2007, 104, 619–629. [Google Scholar] [CrossRef]
- Petitot, M.; Brossard, C.; Barron, C.; Larré, C.; Morel, M.-H.; Micard, V. Modification of pasta structure induced by high drying temperatures. Effects on the in vitro digestibility of protein and starch fractions and the potential allergenicity of protein hydrolysates. Food Chem. 2009, 116, 401–412. [Google Scholar] [CrossRef]
- Petitot, M.; Abecassis, J.; Micard, V. Structuring of pasta components during processing: Impact on starch and protein digestibility and allergenicity. Trends Food Sci. Technol. 2009, 20, 521–532. [Google Scholar] [CrossRef]
- Goñi, I.; Valentín-Gamazo, C. Chickpea flour ingredient slows glycemic response to pasta in healthy volunteers. Food Chem. 2003, 81, 511–515. [Google Scholar] [CrossRef]
- Chillo, S.; Ranawana, D.V.; Pratt, M.; Henry, C.J.K. Glycemic response and glycemic index of semolina spaghetti enriched with barley β-glucan. Nutrition 2011, 27, 653–658. [Google Scholar] [CrossRef]
- Wee, M.; Loud, D.; Tan, V.; Forde, C. Physical and sensory characterisation of noodles with added native and denatured pea protein isolate. Food Chem. 2019, 294, 152–159. [Google Scholar] [CrossRef]
- Motoki, M.; Seguro, K. Transglutaminase and its use for food processing. Trends Food Sci. Technol. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Quaglia, G.; Gennaro, L. ENZYMES|Uses in Food Processing. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Baltimore, MD, USA, 2003. [Google Scholar]
- Gharibzahedi, S.M.T.; Yousefi, S.; Chronakis, I.S. Microbial transglutaminase in noodle and pasta processing. Crit. Rev. Food Sci. Nutr. 2017, 59, 313–327. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, F.; Goff, H.D.; Li, Y. Study on starch-protein interactions and their effects on physicochemical and digestible properties of the blends. Food Chem. 2019, 280, 51–58. [Google Scholar] [CrossRef]
- Alamprese, C.; Iametti, S.; Rossi, M.; Bergonzi, D. Role of pasteurisation heat treatments on rheological and protein structural characteristics of fresh egg pasta. Eur. Food Res. Technol. 2005, 221, 759–767. [Google Scholar] [CrossRef]
- Vallons, K.J.; Arendt, E.K. Understanding high pressure-induced changes in wheat flour–water suspensions using starch–gluten mixtures as model systems. Food Res. Int. 2010, 43, 893–901. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.; Egan, N.; Fellows, C. Effect of insoluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012, 130, 299–309. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised staticin vitrodigestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.-J.; Petrie, J.R.; Motoi, L.; Morgenstern, M.P.; Sutton, K.H.; Mishra, S.; Simmons, L.D. Effect of Structural and Physicochemical Characteristics of the Protein Matrix in Pasta on In Vitro Starch Digestibility. Food Biophys. 2008, 3, 229–234. [Google Scholar] [CrossRef]
- Bellido, G.; Hatcher, D. Effects of a cross-linking enzyme on the protein composition, mechanical properties, and microstructure of Chinese-style noodles. Food Chem. 2011, 125, 813–822. [Google Scholar] [CrossRef]
- Laleg, K.; Cassan, D.; Barron, C.; Prabhasankar, P.; Micard, V. Structural, Culinary, Nutritional and Anti-Nutritional Properties of High Protein, Gluten Free, 100% Legume Pasta. PLoS ONE 2016, 11, e0160721. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, F.-H. Protein—Protein Interactions during High-Moisture Extrusion for Fibrous Meat Analogues and Comparison of Protein Solubility Methods Using Different Solvent Systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef]
- Barbiroli, A.; Bonomi, F.; Casiraghi, M.C.; Iametti, S.; Pagani, M.A.; Marti, A. Process conditions affect starch structure and its interactions with proteins in rice pasta. Carbohydr. Polym. 2013, 92, 1865–1872. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Rombouts, I.; Nivelle, M.A.; Delcour, J.A. The impact of protein characteristics on the protein network in and properties of fresh and cooked wheat-based noodles. J. Cereal Sci. 2017, 75, 234–242. [Google Scholar] [CrossRef]
- Bruneel, C.; Buggenhout, J.; Lagrain, B.; Brijs, K.; Delcour, J.A. Redox agents and N-ethylmaleimide affect protein polymerization during laboratory scale dry pasta production and cooking. Food Chem. 2016, 196, 646–653. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Rombouts, I.; Nivelle, M.A.; Delcour, J.A. The Role of Wheat and Egg Constituents in the Formation of a Covalent and Non-Covalent Protein Network in Fresh and Cooked Egg Noodles. J. Food Sci. 2017, 82, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Paulik, S.; Jekle, M.; Becker, T. Mechanically and Thermally Induced Degradation and Modification of Cereal Biopolymers during Grinding. Polymer 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glycaemic response of pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Tazrart, K.; Zaidi, F.; Salvador, A.; Haros, C.M. Effect of broad bean (Vicia faba) addition on starch properties and texture of dry and fresh pasta. Food Chem. 2019, 278, 476–481. [Google Scholar] [CrossRef]
- Rombouts, I.; Jansens, K.J.; Lagrain, B.; Delcour, J.A.; Zhu, K.-X. The impact of salt and alkali on gluten polymerization and quality of fresh wheat noodles. J. Cereal Sci. 2014, 60, 507–513. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Heiniö, R.-L.; Cassan, D.; Holopainen-Mantila, U.; Micard, V.; Lantto, R.; Sozer, N. Effect of bioprocessing and fractionation on the structural, textural and sensory properties of gluten-free faba bean pasta. LWT 2016, 67, 27–36. [Google Scholar] [CrossRef]
- Shiau, S.-Y.; Chang, Y.-H. Instrumental Textural and Rheological Properties of Raw, Dried, and Cooked Noodles with Transglutaminase. Int. J. Food Prop. 2013, 16, 1429–1441. [Google Scholar] [CrossRef]
- Takács, K.; Gelencsér, É.; Kovács, E.T. Effect of transglutaminase on the quality of wheat-based pasta products. Eur. Food Res. Technol. 2008, 226, 603–611. [Google Scholar] [CrossRef]
- Romano, A.; Giosafatto, C.V.L.; Di Pierro, P.; Romano, R.; Masi, P.; Mariniello, L. Impact of transglutaminase treatment on properties and in vitro digestibility of white bean (Phaseolus vulgaris L.) flour. Food Res. Int. 2016, 88, 239–246. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of starch digestion by α-amylase—Structural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef]
- Edwards, C.H.; Warren, F.J.; Milligan, P.J.; Butterworth, P.J.; Ellis, P.R. A novel method for classifying starch digestion by modelling the amylolysis of plant foods using first-order enzyme kinetic principles. Food Funct. 2014, 5, 2751–2758. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Liu, L.; Wang, S.; Copeland, L. Structural Orders of Wheat Starch Do Not Determine theIn VitroEnzymatic Digestibility. J. Agric. Food Chem. 2017, 65, 1697–1706. [Google Scholar] [CrossRef]
- Arranz-Martínez, P.; Srikaeo, K.; González-Sánchez, A.L. Effects of non-starch polysaccharides on physicochemical properties and in vitro starch digestibility of rice starches. Bioact. Carbohydr. Diet. Fibre 2014, 4, 6–15. [Google Scholar] [CrossRef]
- Bernklau, I.; Lucas, L.; Jekle, M.; Becker, T.; Lucas, I. Protein network analysis—A new approach for quantifying wheat dough microstructure. Food Res. Int. 2016, 89, 812–819. [Google Scholar] [CrossRef]
| TG Concentration (%) | Onset Temperature, To (°C) | Peak Temperature, Tp (°C) | End Temperature, Te (°C) | Enthalpy of Gelatinisation, ΔH (J/g) |
|---|---|---|---|---|
| 0 | 57.1 ± 0.2 | 65.7 ± 0.9 | 72.4 ± 1.0 | 3.4 ± 0.2 |
| 0.5 | 56.7 ± 0.4 | 65.9 ± 0.2 | 72.8 ± 0.6 | 4.8 ± 0.7 |
| 1.0 | 58.6 ± 3.6 | 66.3 ± 1.6 | 73.6 ± 1.5 | 5.4 ± 1.1 |
| 1.5 | 58.1 ± 0.9 | 65.4 ± 0.1 | 72.7 ± 9.2 | 4.9 ± 0.9 |
| 2.0 | 60.0 ± 2.1 | 66.0 ± 1.1 | 73.5 ± 0.6 | 4.8 ± 0.6 |
| TG Concentration (%) | Water Absorption (%) |
|---|---|
| 0 | 87.3 ± 9.4 |
| 0.5 | 93.8 ± 2.7 |
| 1.0 | 90.0 ± 1.7 |
| 1.5 | 89.7 ± 4.6 |
| 2.0 | 91.1 ± 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wee, M.S.M.; Jeyakumar Henry, C. Effects of Transglutaminase on the Protein Network and In Vitro Starch Digestibility of Asian Wheat Noodles. Foods 2019, 8, 607. https://doi.org/10.3390/foods8120607
Wee MSM, Jeyakumar Henry C. Effects of Transglutaminase on the Protein Network and In Vitro Starch Digestibility of Asian Wheat Noodles. Foods. 2019; 8(12):607. https://doi.org/10.3390/foods8120607
Chicago/Turabian StyleWee, May Sui Mei, and Christiani Jeyakumar Henry. 2019. "Effects of Transglutaminase on the Protein Network and In Vitro Starch Digestibility of Asian Wheat Noodles" Foods 8, no. 12: 607. https://doi.org/10.3390/foods8120607
APA StyleWee, M. S. M., & Jeyakumar Henry, C. (2019). Effects of Transglutaminase on the Protein Network and In Vitro Starch Digestibility of Asian Wheat Noodles. Foods, 8(12), 607. https://doi.org/10.3390/foods8120607






