Determination of Acrylamide in Biscuits by High-Resolution Orbitrap Mass Spectrometry: A Novel Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Standard Solutions
2.3. Biscuit Samples
2.4. Sample Preparation
2.5. LC-ESI-Orbitrap
2.6. Analysis of Colour
2.7. Moisture Content Determination
2.8. Statistical Analysis
3. Results and Discussion
3.1. Method Performance
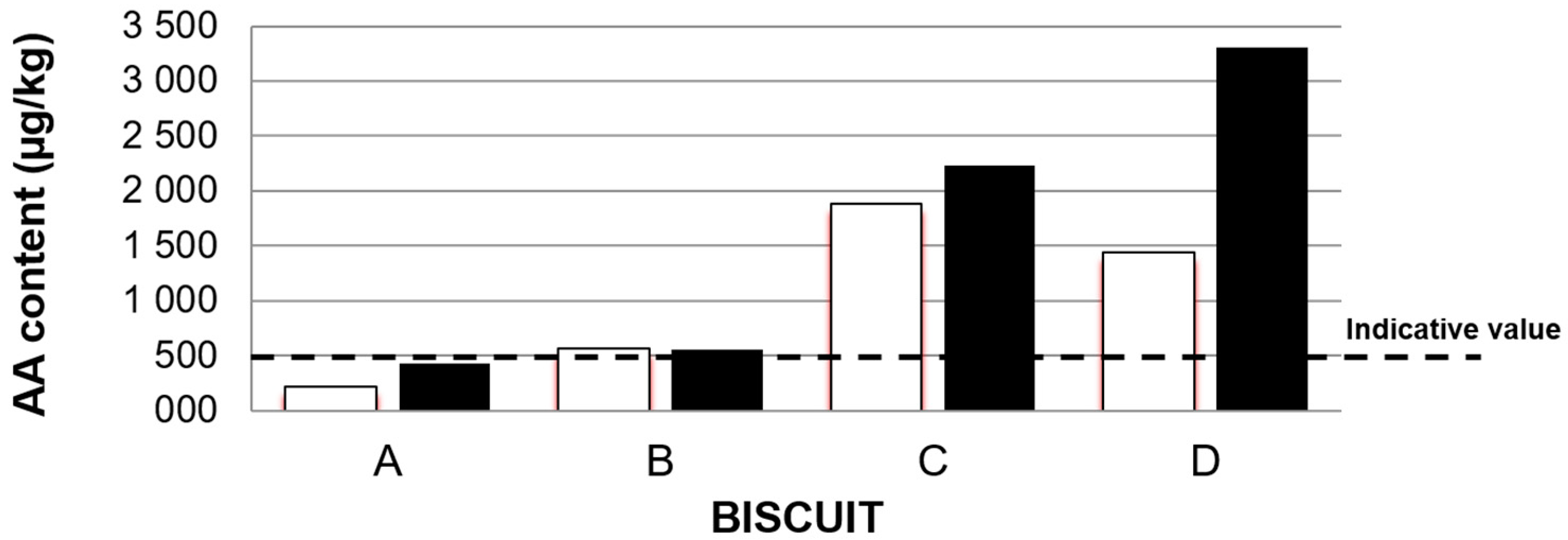
3.2. Acrylamide Content in Biscuits
3.3. Correlation between Acrylamide Content and Biscuit Colour
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Tornqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Bergmark, E. Hemoglobin Adducts of Acrylamide and Acrylonitrile in Laboratory Workers, Smokers and Nonsmokers. Chem. Res. Toxicol. 1997, 10, 78–84. [Google Scholar] [CrossRef]
- Sorgel, F.; Weissenbacher, R.; Kinzig-Schippers, M.; Hofmann, A.; Illauer, M.; Skott, A.; Landersdorfer, C. Acrylamide: Increased concentrations in homemade food and first evidence of its variable absorption from food, variable metabolism and placental and breast milk transfer in humans. Chemotherapy 2002, 48, 267–274. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- WHO. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; W. Press: Beijing, China, 1994. [Google Scholar]
- European Commission. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1998, 41, 32–54. [Google Scholar]
- European Union. Commission Recommendation of 8 November 2013 on investigations into the levels of acrylamide in food. Off. J. Eur. Union 2013, 56, 17. [Google Scholar]
- Yaylayan, V.A.; Wnorowski, A.; Perez Locas, C. Why Asparagine Needs Carbohydrates To Generate Acrylamide. J. Agric. Food Chem. 2003, 51, 1753–1757. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Acrylamide in Foods; Food and Drug Administration: Rockville, MD, USA, 2016. [Google Scholar]
- Halford, N.G.; Curtis, T.Y. Reducing the Acrylamide-Forming Potential of Wheat, Rye and Potato: A Review. In Browned Flavors: Analysis, Formation, and Physiology; American Chemical Society: Washington, DC, USA, 2016; Volume 1237, pp. 35–53. [Google Scholar]
- Postles, J.; Powers, S.J.; Elmore, J.S.; Mottram, D.S.; Halford, N.G. Effects of variety and nutrient availability on the acrylamide-forming potential of rye grain. J. Cereal Sci. 2013, 57, 463–470. [Google Scholar] [CrossRef]
- Przygodzka, M.; Piskula, M.K.; Kukurová, K.; Ciesarová, Z.; Bednarikova, A.; Zieliński, H. Factors influencing acrylamide formation in rye, wheat and spelt breads. J. Cereal Sci. 2015, 65, 96–102. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, B.; Ran, R.; Liu, Y.; Chen, H.; Kai, G.; Shi, J. Risk assessment, formation, and mitigation of dietary acrylamide: Current status and future prospects. Food Chem. Toxicol. 2014, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pittet, A.; Perisset, A.; Oberson, J.M. Trace level determination of acrylamide in cereal-based foods by gas chromatography-mass spectrometry. J. Chromatogr. 2004, 1035, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Kohata, K.; Yamaguchi, Y.; Hayashi, N.; Sawai, Y.; Chuda, Y.; Ono, H.; Yada, H.; Yoshida, M. Analysis of acrylamide in green tea by gas chromatography-mass spectrometry. J. Agric. Food Chem. 2006, 54, 7370–7377. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.M.D.; Fernandes, J.O. MSPD Method to Determine Acrylamide in Food. Food Anal. Methods 2008, 2, 197. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Boyacı Gündüz, C.P. An eco-friendly, quick and cost-effective method for the quantification of acrylamide in cereal-based baby foods. J. Sci. Food Agric. 2014, 94, 2534–2540. [Google Scholar] [CrossRef]
- Delevic, V.; Zejnilovic, R.; Jancic Stojanovic, B.; Djordjevic, B.; Tokic, Z.; Zrnic-Ciric, M.; Stankovic, I. Quantification of acrylamide in foods selected by using gas chromatography tandem mass spectrometry. Hem. Ind. 2015, 70, 209–215. [Google Scholar] [CrossRef]
- Sobhi, H.R.; Ghambarian, M.; Behbahani, M.; Esrafili, A. Application of modified hollow fiber liquid phase microextraction in conjunction with chromatography-electron capture detection for quantification of acrylamide in waste water samples at ultra-trace levels. J. Chromatogr. 2017, 1487, 30–35. [Google Scholar] [CrossRef]
- Yamazaki, K.; Isagawa, S.; Kibune, N.; Urushiyama, T. A method for the determination of acrylamide in a broad variety of processed foods by GC-MS using xanthydrol derivatization. Food Addit. Contam. 2011, 29, 705–715. [Google Scholar] [CrossRef]
- Molina-Garcia, L.; Santos, C.S.P.; Melo, A.; Fernandes, J.O.; Cunha, S.C.; Casal, S. Acrylamide in Chips and French Fries: A Novel and Simple Method Using Xanthydrol for Its GC-MS Determination. Food Anal. Methods 2015, 8, 1436–1445. [Google Scholar] [CrossRef]
- Luo, L.; Ren, Y.; Liu, J.; Wen, X. Investigation of a rapid and sensitive non-aqueous reaction system for the determination of acrylamide in processed foods by gas chromatography-mass spectrometry. Anal. Methods 2016, 8, 5970–5977. [Google Scholar] [CrossRef]
- Norouzi, P.; Larijani, B.; Bidhendi, M.E.; Eshraghi, M.; Ebrahimi, M. A Sensitive Biosensor for Acrylamide Detection based on Polyaniline and Au Nanoparticles using FFT Admittance Voltammetry. Anal. Bioanal. Electrochem. 2018, 10, 18–32. [Google Scholar]
- Zokaei, M.; Abedi, A.S.; Kamankesh, M.; Shojaee-Aliababadi, S.; Mohammadi, A. Ultrasonic-assisted extraction and dispersive liquid-liquid microextraction combined with gas chromatography-mass spectrometry as an efficient and sensitive method for determining of acrylamide in potato chips samples. Food Chem. 2017, 234, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lagalante, A.F.; Felter, M.A. Silylation of acrylamide for analysis by solid-phase microextraction/gas chromatography/ion-trap mass spectrometry. J. Agric. Food Chem. 2004, 52, 3744–3748. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Sadowska-Rociek, A.; Cieślik, E. Development of a sample preparation method for acrylamide determination in cocoa via silylation. Anal. Methods 2016, 8, 5874–5880. [Google Scholar] [CrossRef]
- Bermudo, E.; Moyano, E.; Puignou, L.; Galceran, M.T. Liquid chromatography coupled to tandem mass spectrometry for the analysis of acrylamide in typical Spanish products. Talanta 2008, 76, 389–394. [Google Scholar] [CrossRef]
- Claus, A.; Weisz, G.M.; Kammerer, D.R.; Carle, R.; Schieber, A. A method for the determination of acrylamide in bakery products using ion trap LC-ESI-MS/MS. Mol. Nutr. Food Res. 2005, 49, 918–925. [Google Scholar] [CrossRef]
- Tsutsumiuchi, K.; Hibino, M.; Kambe, M.; Oishi, K.; Okada, M.; Miwa, J.; Taniguchi, H. Application of ion-trap LC/MS/MS for determination of acrylamide in processed foods. Shokuhin Eiseigaku Zasshi 2004, 45, 95–99. [Google Scholar] [CrossRef][Green Version]
- Cajka, T.; Hajšlová, J. Gas Chromatography-Time-of-Flight Mass Spectrometry in Food Analysis. LC GC Eur. 2007, 20, 25. [Google Scholar]
- Huang, Y.S.; Hsieh, T.J.; Lu, C.Y. Simple analytical strategy for MALDI-TOF-MS and nanoUPLC-MS/MS: Quantitating curcumin in food condiments and dietary supplements and screening of acrylamide-induced ROS protein indicators reduced by curcumin. Food Chem. 2015, 174, 571–576. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Wu, G.; Zhang, H.; Gu, L.; Wang, L.; Qian, H.; Qi, X. Mitigation effects of proanthocyanidins with different structures on acrylamide formation in chemical and fried potato crisp models. Food Chem. 2018, 250, 98–104. [Google Scholar] [CrossRef]
- Omar, M.M.; Elbashir, A.A.; Schmitz, O.J. Determination of acrylamide in Sudanese food by high performance liquid chromatography coupled with LTQ Orbitrap mass spectrometry. Food Chem. 2015, 176, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Troise, A.D.; Fiore, A.; Fogliano, V. Quantitation of Acrylamide in Foods by High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2014, 62, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Pugajeva, I.; Jaunbergs, J.; Bartkevics, V. Development of a sensitive method for the determination of acrylamide in coffee using high-performance liquid chromatography coupled to a hybrid quadrupole Orbitrap mass spectrometer. Food Addit. Contam. 2015, 32, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.C. Fundamental Statistics for the Behavioural Sciences, 4th ed.; Duxbury Resource Center: Pacific Grove, CA, USA, 1999. [Google Scholar]
- European Union. European Union. European Directorate for the Quality of Medicines. In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2007. [Google Scholar]
- ICH, Q.R. Validation of Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 8–13 November 2005. [Google Scholar]
- European Commission. Commission Regulation (EU) 2017/2158 Establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, 304, 24–44. [Google Scholar]
- Bratinova, S.; Karasek, L. Determination of the acrylamide content in potato chips. Report on proficiency test organised by the EURL-PAH. In JRC Technical Reports; European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Kaufmann, A. Analytical performance of the various acquisition modes in Orbitrap MS and MS/MS. J. Mass Spectrom 2018, 53, 725–738. [Google Scholar] [CrossRef]
- Kumar, P.; Rubies, A.; Centrich, F.; Granados, M.; Cortes-Francisco, N.; Caixach, J.; Companyo, R. Targeted analysis with benchtop quadrupole-orbitrap hybrid mass spectrometer: Application to determination of synthetic hormones in animal urine. Anal. Chim. Acta 2013, 780, 65–73. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. The effect of asparaginase on acrylamide formation in French fries. Food Chem. 2008, 109, 386–392. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti, S.; Granby, K.; Risum, J. Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. LWT-Food Sci. Technol. 2011, 44, 1473–1476. [Google Scholar] [CrossRef]
- Mustatea, G.; Popa, M.; Mioara, N. Influence of Flour Extraction Degree on Acrylamide Formation in Biscuits. Rom. Biotechnol. Lett. 2016, 21, 11328–11336. [Google Scholar]
- Mustatea, G.; Popa, M.; Mioara, N.; Israel, F. Asparagine and sweeteners—How they influence acrylamide formation in wheat flour biscuits? J. Biotechnol. 2015, 208, S81. [Google Scholar] [CrossRef]
- Delatour, T.; Périsset, A.; Goldmann, T.; Riediker, S.; Stadler, R.H. Improved Sample Preparation to Determine Acrylamide in Difficult Matrixes Such as Chocolate Powder, Cocoa, and Coffee by Liquid Chromatography Tandem Mass Spectroscopy. J. Agric. Food Chem. 2004, 52, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Van Der Fels-Klerx, H.J. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: NaCl and temperature–time profile effects and kinetics. Food Res. Int. 2014, 57, 210–217. [Google Scholar] [CrossRef]
- Nguyen, H.T.; van der Fels-Klerx, H.J.I.; van Boekel, M. Acrylamide and 5-hydroxymethylfurfural formation during biscuit baking. Part II: Effect of the ratio of reducing sugars and asparagine. Food Chem. 2017, 230, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Jozinović, A.; Šarkanj, B.; Ačkar, Đ.; Panak Balentić, J.; Šubarić, D.; Cvetković, T.; Ranilović, J.; Guberac, S.; Babić, J. Simultaneous Determination of Acrylamide and Hydroxymethylfurfural in Extruded Products by LC-MS/MS Method. Molecules 2019, 24, 1971. [Google Scholar] [CrossRef] [PubMed]
- Constantin, O.E.; Kukurova, K.; Dasko, L.; Stanciuc, N.; Ciesarova, Z.; Croitoru, C.; Rapeanu, G. Modelling Contaminant Formation during Thermal Processing of Sea Buckthorn Puree. Molecules 2019, 24, 1571. [Google Scholar] [CrossRef]

| Assay | Acrylamide (AA) Content (µg kg−1) | Average AA Content (µg kg−1) | SD | RSDr % | LOD (µg kg−1) | LOQ (µg kg−1) |
|---|---|---|---|---|---|---|
| 1 | 254.1 | |||||
| 2 | 277.3 | |||||
| 3 | 343.9 | |||||
| 4 | 309.8 | |||||
| 5 | 269.8 | 297.9 | 33.1 | 11.1 | 3.55 | 11.8 |
| 6 | 345.0 | |||||
| 7 | 290.9 | |||||
| 8 | 292.0 |
| Biscuit | Edges of the Baking Oven (µg kg−1) | Middle of the Baking Oven (µg kg−1) | Average (µg kg−1) |
|---|---|---|---|
| A | 216 | 431 | 324 ± 36 |
| B | 563 | 551 | 557 ± 61 |
| C | 1881 | 2231 | 2056 ± 226 |
| D | 1443 | 3303 | 2373 ± 261 |
| AA Content | L | a | b | Moisture | |
|---|---|---|---|---|---|
| AA content | 1.0 | ||||
| L | 0.541 | 1.0 | |||
| a | 0.310 | 0.352 | 1.0 | ||
| b | 0.276 | 0.864 | 0.663 | 1.0 | |
| Moisture | −0.277 | 0.307 | 0.304 | 0.576 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, C.L.; Carvalho, D.O.; Guido, L.F. Determination of Acrylamide in Biscuits by High-Resolution Orbitrap Mass Spectrometry: A Novel Application. Foods 2019, 8, 597. https://doi.org/10.3390/foods8120597
Fernandes CL, Carvalho DO, Guido LF. Determination of Acrylamide in Biscuits by High-Resolution Orbitrap Mass Spectrometry: A Novel Application. Foods. 2019; 8(12):597. https://doi.org/10.3390/foods8120597
Chicago/Turabian StyleFernandes, Cristiana L., Daniel O. Carvalho, and Luis F. Guido. 2019. "Determination of Acrylamide in Biscuits by High-Resolution Orbitrap Mass Spectrometry: A Novel Application" Foods 8, no. 12: 597. https://doi.org/10.3390/foods8120597
APA StyleFernandes, C. L., Carvalho, D. O., & Guido, L. F. (2019). Determination of Acrylamide in Biscuits by High-Resolution Orbitrap Mass Spectrometry: A Novel Application. Foods, 8(12), 597. https://doi.org/10.3390/foods8120597






