Production and Physicochemical Properties of Starch Isolated from Djulis (Chenopodium formosanum)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition, Starch Yield, and Recovery
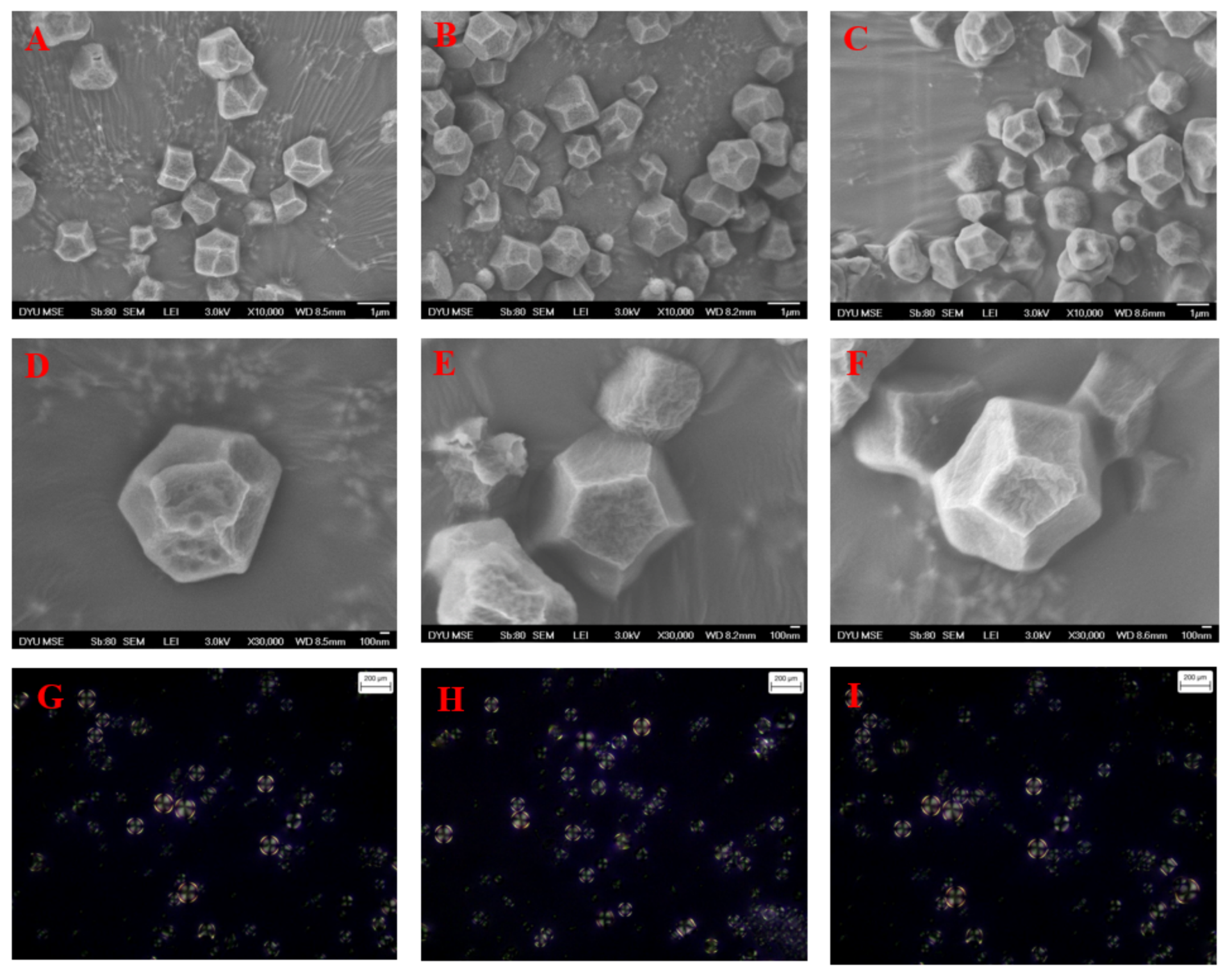
2.2. Morphological Characteristics
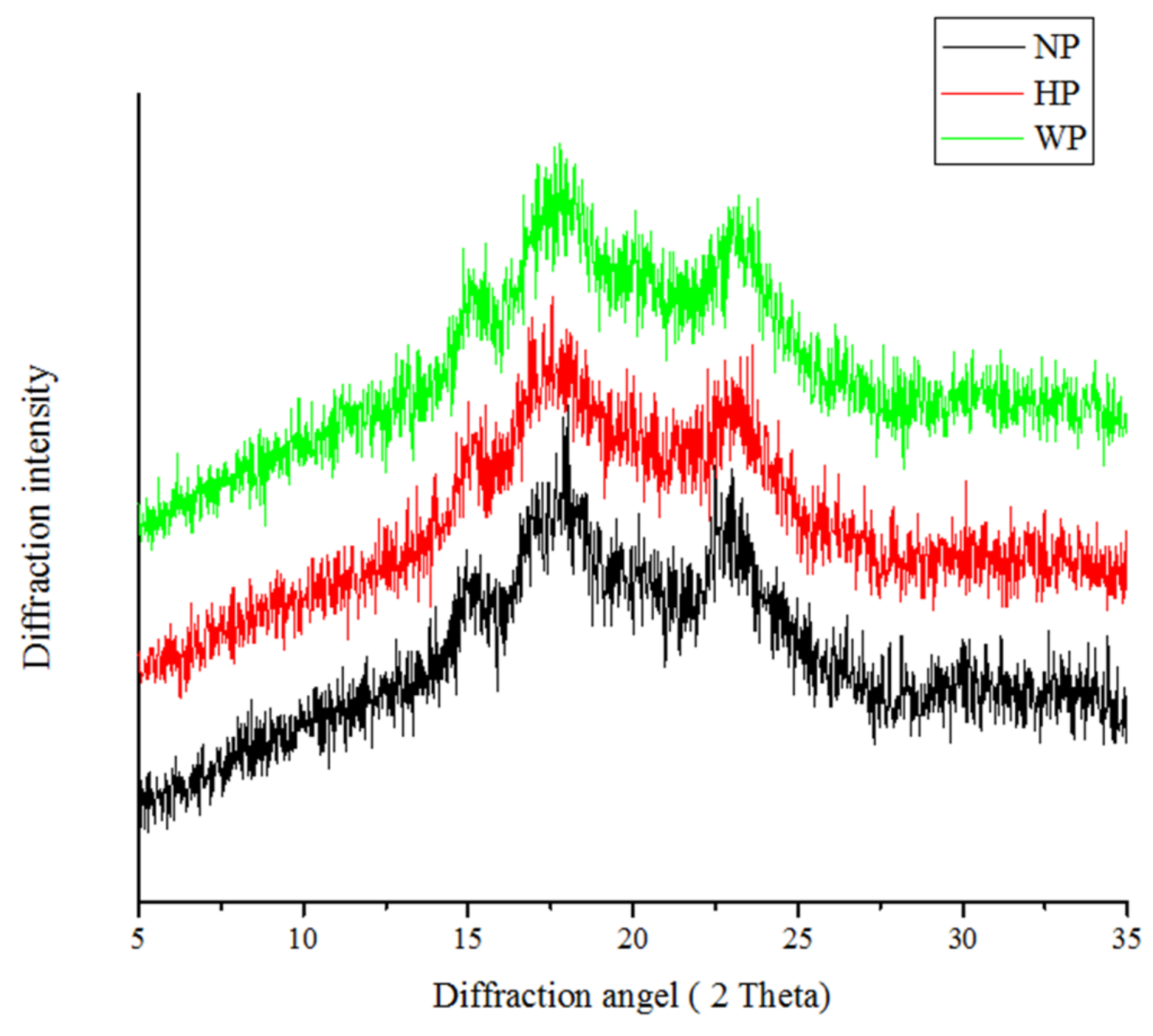
2.3. Crystalline Properties
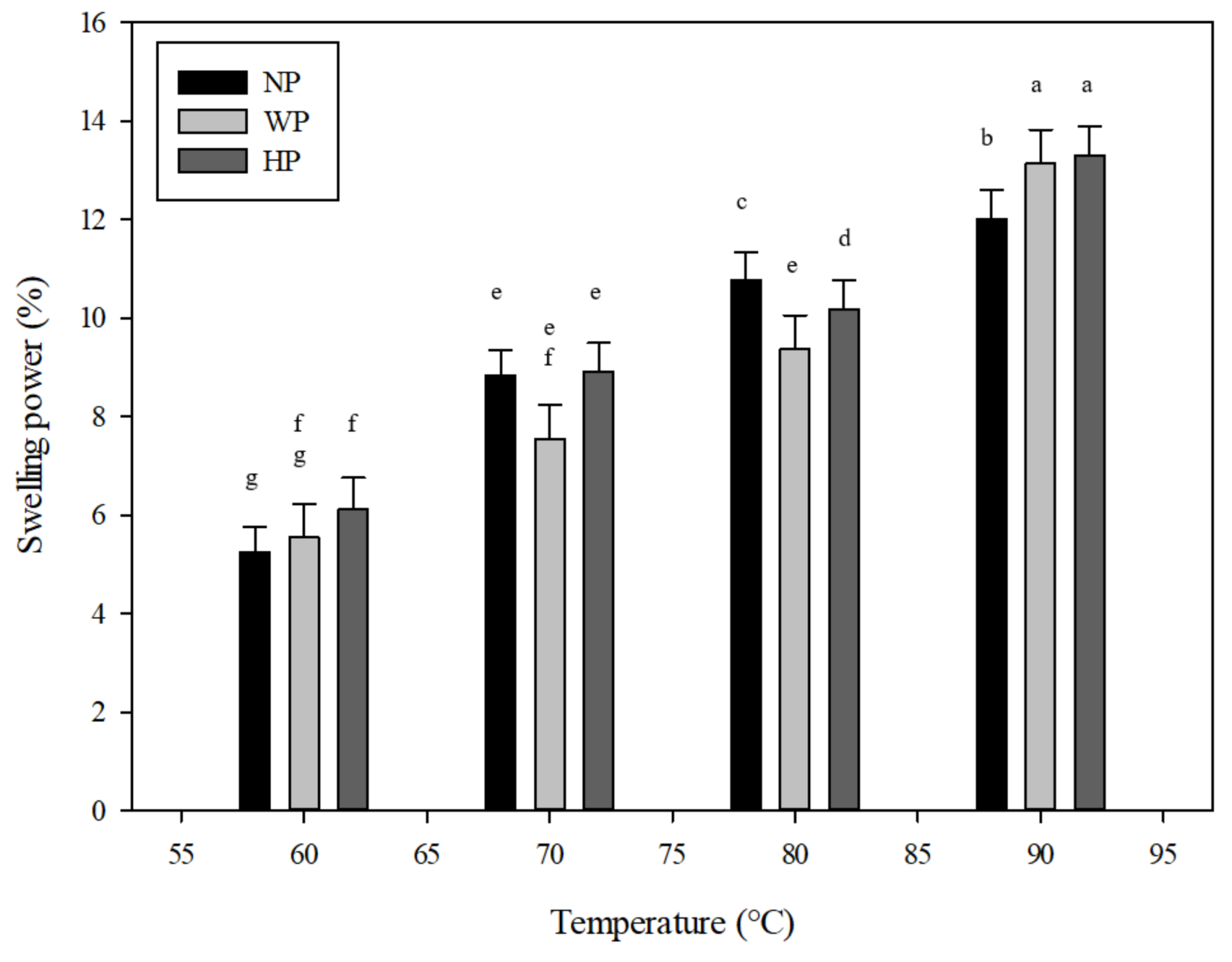
2.4. Swelling Power
2.5. Gelatinization Thermal Properties
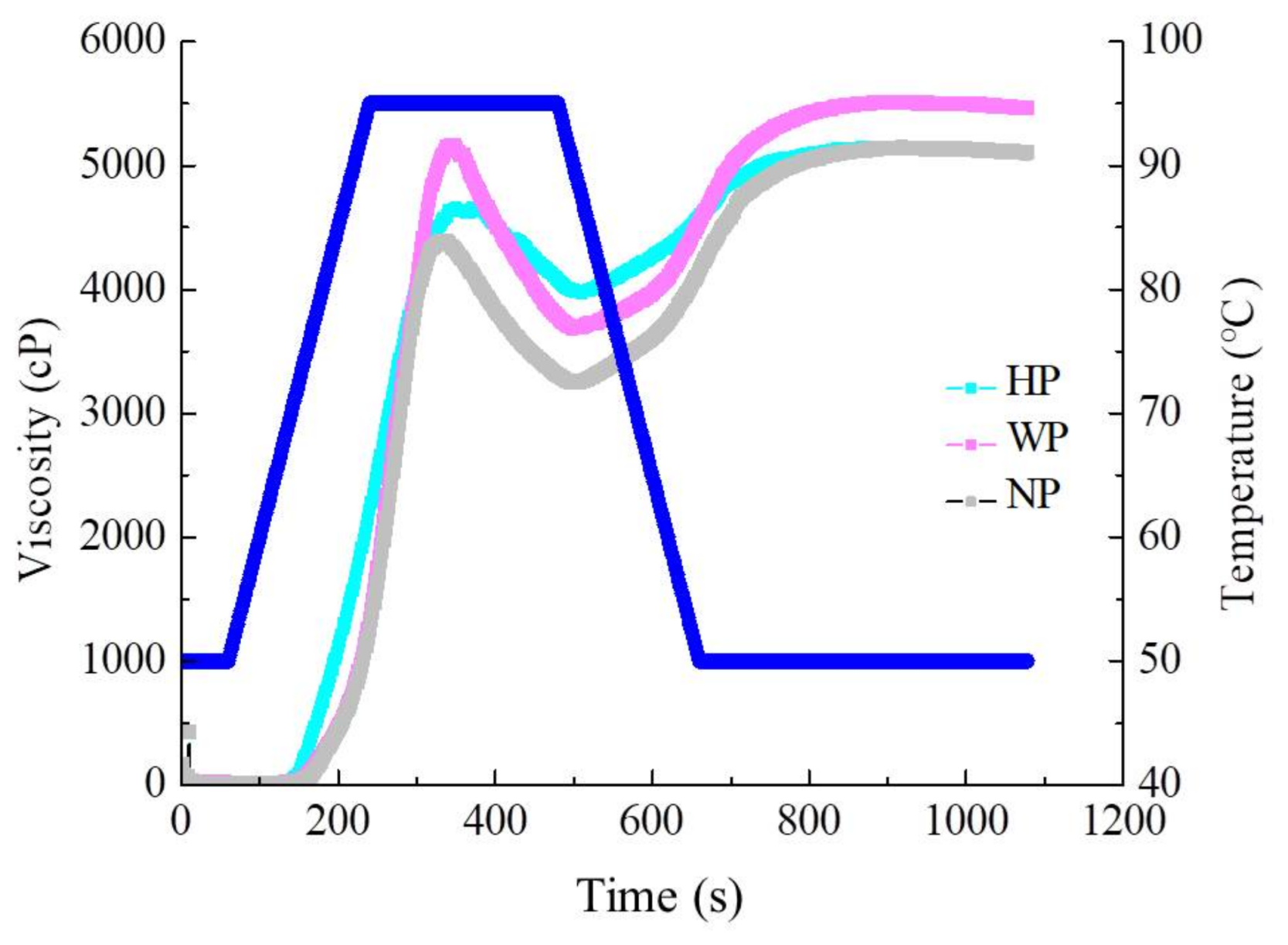
2.6. Pasting Properties
3. Conclusions
4. Experimental
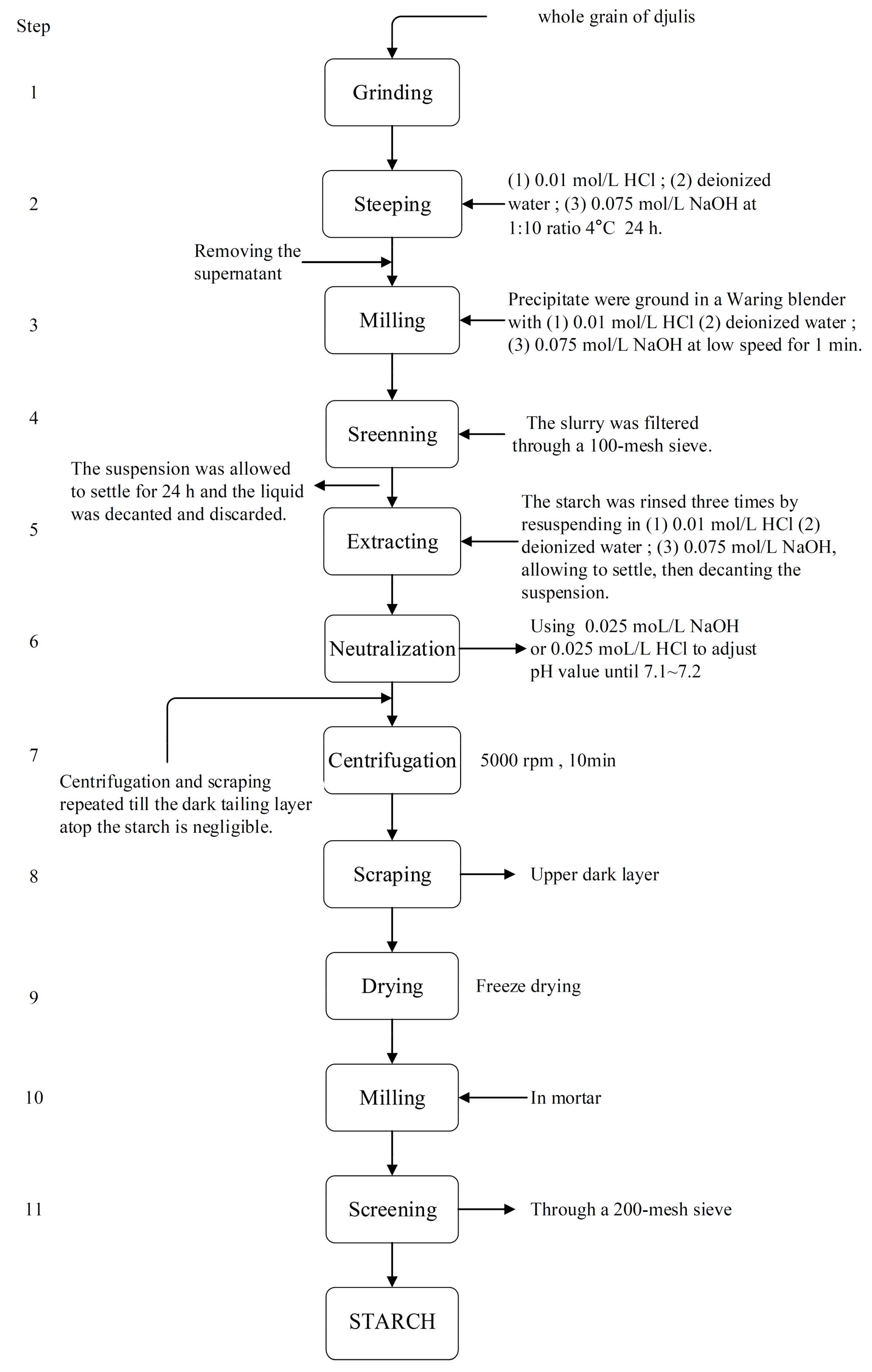
4.1. Starch Isolation Procedure
4.2. The Chemical Composition of Starch
4.3. Starch and Amylose Content
4.4. Production Yield and Recovery
4.5. Granule Size
4.6. Morphological Observation of Starch Granule
4.7. X-Ray Diffraction
4.8. Swelling Power
4.9. Thermal Properties
4.10. Pasting Properties
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa- an Indian perspective. Ind. Crop Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Lu, W.C.; Hsieh, C.W.; Li, T.C.; Huang, D.W. Rheological properties of dough and quality of salted noodles supplemented with djulis (Chenopodium formosanum Koidz.) Flour. J. Agric. Sci. 2015, 7. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chu, Y.L.; Sridhar, K.; Tsai, P.J. Analysis and determination of phytosterols and triterpenes in different inbred lines of Djulis (Chenopodium formosanum Koidz.) hull: A potential source of novel bioactive ingredients. Food Chem. 2019, 297, 124948. [Google Scholar] [CrossRef]
- Pascoal, A.M.; Di-Medeiros, M.C.B.; Batista, K.A.; Leles, M.I.G.; Lião, L.M.; Fernandes, K.F. Extraction and chemical characterization of starch from S. lycocarpum fruits. Carbohydr. Polym. 2013, 98, 1304–1310. [Google Scholar] [CrossRef]
- Beninca, C.; Colman, T.A.D.; Lacerda, L.G.; Filho, M.A.S.C.; Bannach, G.; Schnitzler, E. The thermal, rheological and structural properties of cassva starch granules modified with hydrochloric acid at different temperatures. Thermochim. Acta 2013, 552, 65–69. [Google Scholar] [CrossRef]
- Paula, R.C.; Cristiana, N.M.; Luisa, B.M. The effect of starch isolation method on physical and functional properties of Portuguese nut starches. II. Q. rotundifolia Lam. and Q. suber Lam. acorns starches. Food Hydrocoll. 2013, 30, 448–455. [Google Scholar]
- Li, P.; Zhang, H.; Chen, J.; Shi, Y.; Cai, J.; Yang, J.; Wu, Y. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int. J. Cancer 2014, 135, 1444–1453. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Cai, J.; Han, W.; Huai, H.; Chen, Y.; Wei, C. Comparison of starches isolated from three different Trapa species. Food Hydrocoll. 2014, 37, 174–181. [Google Scholar] [CrossRef]
- Burrell, M.M. Starch: The need for improved quality or quantity- an overview. J. Exp. Bot. 2003, 54, 451–456. [Google Scholar] [CrossRef]
- Manek, R.V.; Kunle, O.O.; Emeje, M.O.; Builders, P.; Rao, G.V.R.; Lopez, G.P.; Kolling, W.M. Physical, thermal and sorption profile of starch obtained from Tacca leontopetaloides. Starch Stärke. 2005, 57, 55–61. [Google Scholar] [CrossRef]
- Man, J.M.; Cai, J.W.; Cai, C.H.; Huai, H.Y.; Wei, C.X. Physicochemical properties of rhizome starch from a traditional Chinese medicinal plant of Anemone altaica. Carbohydr. Polym. 2012, 89, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.R.; Nunes, M.C.; Beirão-da-Costa, M.L. The effect of starch isolation method on physical and functional properties of Portuguese nuts starches. I. chestnuts (Castanea sativa Mill. Var. Martainha and Longal) fruits. Food Hydrocoll. 2012, 27, 256–263. [Google Scholar] [CrossRef]
- Villarreal, M.E.; Ribotta, P.D.; Iturriaga, L.B. Comparing methods for extracting amaranthus starch and the properties of the isolated starches. LWT Food Sci. Technol. 2013, 51, 441–447. [Google Scholar] [CrossRef]
- Zhong, F.; Li, Y.; Ibáñez, A.M.; Oh, M.H.; McKenzie, K.S.; Shoemaker, C. The effect of rice variety and starch isolation method on the pasting and rheological properties of rice starch pastes. Food Hydrocoll. 2009, 23, 406–414. [Google Scholar] [CrossRef]
- Li, Y.; Shoemaker, C.F.; Ma, J.; Luo, C.; Zhong, F. Effects of alcalase/propease N treatments on rice starch isolation and their effects on its properties. Food Chem. 2009, 114, 821–828. [Google Scholar] [CrossRef]
- Correia, P.R.; Beirão-da-Costa, M.L. Starch isolation from chestnut and acorn flours through alkaline and enzymatic methods. Food Bioprod. Process. 2012, 90, 309–316. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Rincón-Londoño, N.; Contreras-Padilla, M.; Millan-Malo, B.M.; Rodriguez-Garcia, M.E. Morphological, structural, thermal, compositional, vibrational, and pasting characterization of white, yellow, and purple Arracacha Lego-like starches and flours (Arracacia xanthorrhiza). Int. J. Biol. Macromol. 2018, 113, 1188–1197. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhu, F. Physicochemical properties of quinoa starch. Carbohydr. Polym. 2016, 137, 328–338. [Google Scholar] [CrossRef]
- Lorenz, K. Quinoa (Chenopodium quinoa) Starch - Physico-chemical properties and functional characteristics. Starch Stärke 1990, 42, 81–86. [Google Scholar] [CrossRef]
- Qian, J.; Kuhn, M. Characterization of Amaranthus cruentus and Chenopodium quinoa starch. Starch Stärke 1999, 51, 116–120. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Torres-Vargas, O.L.; Rodríguez-García, M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chem. 2019, 298, 124982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q. Understanding starches and their role in food. In Food Carbohydrate: Chemistry, Physical Properties, and Applications; Ciu, S., Ed.; CRC Press Taylor & Francis Group: New York, NY, USA, 2005; pp. 309–349. [Google Scholar]
- Jan, R.; Saxena, D.C.; Singh, S. Pasting, thermal, morphological, rheological and structural characteristics of Chenopodium (Chenopodium album) starch. LWT Food Sci. Technol. 2016, 66, 267–274. [Google Scholar] [CrossRef]
- Matignon, A.; Tecante, A. Starch retrogradation: From starch components to cereal products. Food Hydrocoll. 2017, 68, 43–52. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Hoover, H.; Donner, E.; Liu, Q.; Jaiswal, S.; Chibbar, R.; Nantanga, K.K.M.; Seetharaman, K. Structure of fava bean, black bean and pinto bean at different levels of granule organization and their physicochemical properties. Food Res. Int. 2011, 44, 2962–2974. [Google Scholar] [CrossRef]
- Singh, G.D.; Bawa, A.S.; Singh, S.; Saxena, D.C. Physicochemical, pasting, thermal and morphological characteristics on Indian water chestnut (Trapa natans) starch. Starch Stärke 2009, 61, 35–42. [Google Scholar] [CrossRef]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. 1. Effects of amylopectin, amylose and lipids. Cereal Chem. 1990, 67, 551–557. [Google Scholar]
- Wang, S.; Copeland, I. New insight into loss of swelling power and pasting profiles of acid-hydrolyzed starch granule. Starch Stärke 2012, 64, 538–544. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Falk, K.C.; Tyler, R.T. Characteristics of starch from eight quinoa lines. Cereal Chem. 2005, 82, 216–222. [Google Scholar] [CrossRef]
- Rincón-Londoño, N.; Millan-Malo, B.; Rodríguez-García, M.E. Analysis of thermal pasting profile in corn starch rich in amylose and amylopectin: Physicochemical transformations, part II. Int. J. Biol. Macromol. 2016, 89, 43–53. [Google Scholar] [CrossRef]
- Ratnayake, W.; Hoover, R.; Warkentin, T. Pea starch: Composition, structure and properties—A review. Starch Stärke 2002, 54, 217–243. [Google Scholar] [CrossRef]
- Puchongkavarin, H.; Varavinit, S.; Bergthaller, W. Comparative study of pilot scale rice starch production by an alkaline and an enzymatic process. Starch Stärke 2005, 57, 134–144. [Google Scholar] [CrossRef]
- Ahamed, N.T.; Singhal, R.S.; Kulkarni, P.R.; Pal, M. Physicochemical and functional properties of Chenopodium quinoa starch. Carbohydr. Polym. 1996, 31, 99–103. [Google Scholar] [CrossRef]
- Wang, C.C.R.; Chiang, P.Y.; Li, P.H.; Huang, C.C. Physicochemical properties of water caltrop (Trapa taiwanensis Nakai) starch during growth period. Carbohydr. Polym. 2008, 71, 310–315. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Aparicio, M.A.; Bello, L.A.; Monroy, J.A. Changes of banana starch by chemical and physical modification. Carbohydr. Polym. 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Zhou, M.X.; Mendham, N.J. Predicting barley malt extract with a rapid viscoanalyser. J. Cereal Sci. 2005, 41, 31–36. [Google Scholar] [CrossRef]
| Process | Moisture (%) | Protein (%) * | Lipid (%) * | Ash (%) * | Amylose (%) | Damage Starch (%) |
|---|---|---|---|---|---|---|
| HP | 4.89 ± 0.21 c | 0.29 ± 0.02 b | 0.82 ± 0.07 b | 0.11 ± 0.02 a | 22.14 ± 0.31 b | 3.93 ± 0.27 a |
| WP | 6.35 ± 0.36 a | 0.37 ± 0.07 a | 0.94 ± 0.04 a | 0.12 ± 0.01 a | 24.15 ± 0.28 a | 2.12 ± 0.19 c |
| NP | 5.48 ± 0.19 b | 0.22 ± 0.03 b | 0.75 ± 0.05 b | 0.14 ± 0.03 a | 22.43 ± 0.26 b | 3.05 ± 0.11 b |
| Process | Yield (%) | Recovery (%) | Starch Granule Size | |
|---|---|---|---|---|
| Range (μm) | Mean (μm) | |||
| HP | 29.74 ± 0.45 a | 78.49 ± 0.76 a | 0.56−1.96 | 1.57 ± 0.09 b |
| WP | 30.19 ± 0.29 a | 79.68 ± 0.58 a | 0.74−3.02 | 1.84 ± 0.13 a |
| NP | 26.41 ± 0.33 b | 69.15 ± 0.26 b | 0.62−2.48 | 1.62 ± 0.21 b |
| Process | T0 (°C) | Tp (°C) | Tc (°C) | ΔT(°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| HP | 59.45 ± 0.47 a | 64.35 ± 0.62 a | 72.19 ± 1.73 a | 12.74 ± 0.44 b | 9.24 ± 1.03 a |
| WP | 60.27 ± 0.54 a | 65.21 ± 0.22 a | 72.95 ± 0.38 a | 12.68 ± 0.31 b | 8.51 ± 0.68 a |
| NP | 60.73 ± 0.89 a | 66.02 ± 1.10 a | 70.74 ± 1.35 a | 10.01 ± 0.25 a | 6.95 ± 0.98 b |
| Process | PV (cP) | TV (cP) | BD (cP) | FV (cP) | SB (cP) | PT (°C) |
|---|---|---|---|---|---|---|
| HP | 4674.67 ± 61.45 b | 3974.33 ± 33.71 a | 772.67 ± 167.05 c | 5113.17 ± 16.56 b | 1139.00 ± 50.27 c | 71.70 ± 0.30 a |
| WP | 5200.01 ± 22.61 a | 3683.67 ± 58.71 b | 1516.33 ± 37.07 a | 5462.33 ± 28.50 a | 1778.67 ± 51.39 b | 72.80 ± 0.61 a |
| NP | 4397.67 ± 17.02 c | 3248.00 ± 83.29 c | 1149.67 ± 90.31 b | 5104.73 ± 59.72 b | 1856.33 ± 37.31 a | 69.53 ± 0.29 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.-C.; Chan, Y.-J.; Tseng, F.-Y.; Chiang, P.-Y.; Li, P.-H. Production and Physicochemical Properties of Starch Isolated from Djulis (Chenopodium formosanum). Foods 2019, 8, 551. https://doi.org/10.3390/foods8110551
Lu W-C, Chan Y-J, Tseng F-Y, Chiang P-Y, Li P-H. Production and Physicochemical Properties of Starch Isolated from Djulis (Chenopodium formosanum). Foods. 2019; 8(11):551. https://doi.org/10.3390/foods8110551
Chicago/Turabian StyleLu, Wen-Chien, Yung-Jia Chan, Fang-Yu Tseng, Po-Yuan Chiang, and Po-Hsien Li. 2019. "Production and Physicochemical Properties of Starch Isolated from Djulis (Chenopodium formosanum)" Foods 8, no. 11: 551. https://doi.org/10.3390/foods8110551
APA StyleLu, W.-C., Chan, Y.-J., Tseng, F.-Y., Chiang, P.-Y., & Li, P.-H. (2019). Production and Physicochemical Properties of Starch Isolated from Djulis (Chenopodium formosanum). Foods, 8(11), 551. https://doi.org/10.3390/foods8110551