Ion Mobility Spectrometry as a Potential Tool for Flavor Control in Chocolate Manufacture
Abstract
1. Introduction
2. Materials and Methods
2.1. Cocoa and Chocolate Samples
2.2. Conching of Chocolate Mass
2.3. Headspace Solid Phase Micro-Extraction GC-MS Analysis
2.4. Ion Mobility Spectrometry
2.5. Statistical Analysis
3. Results and Discussion
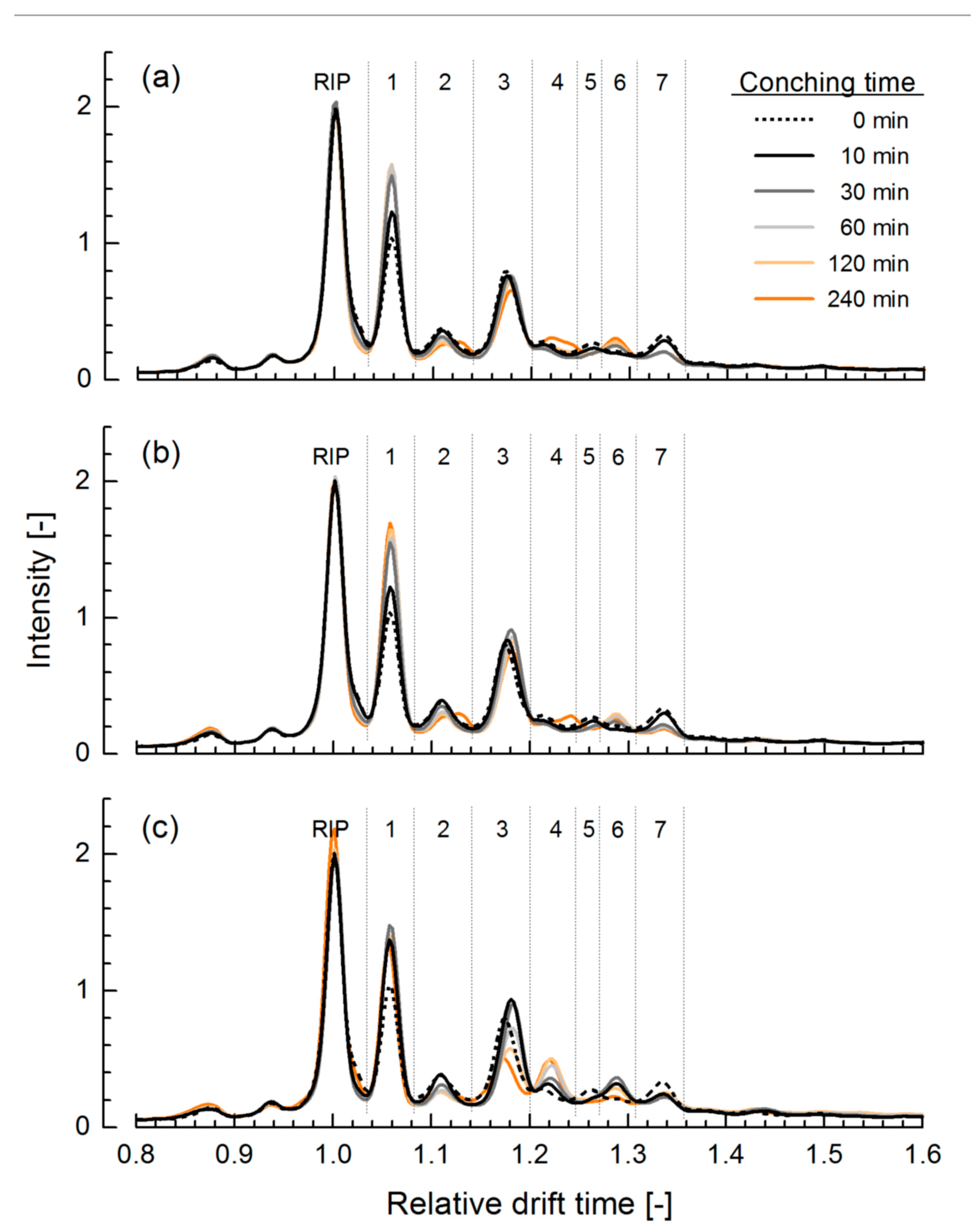
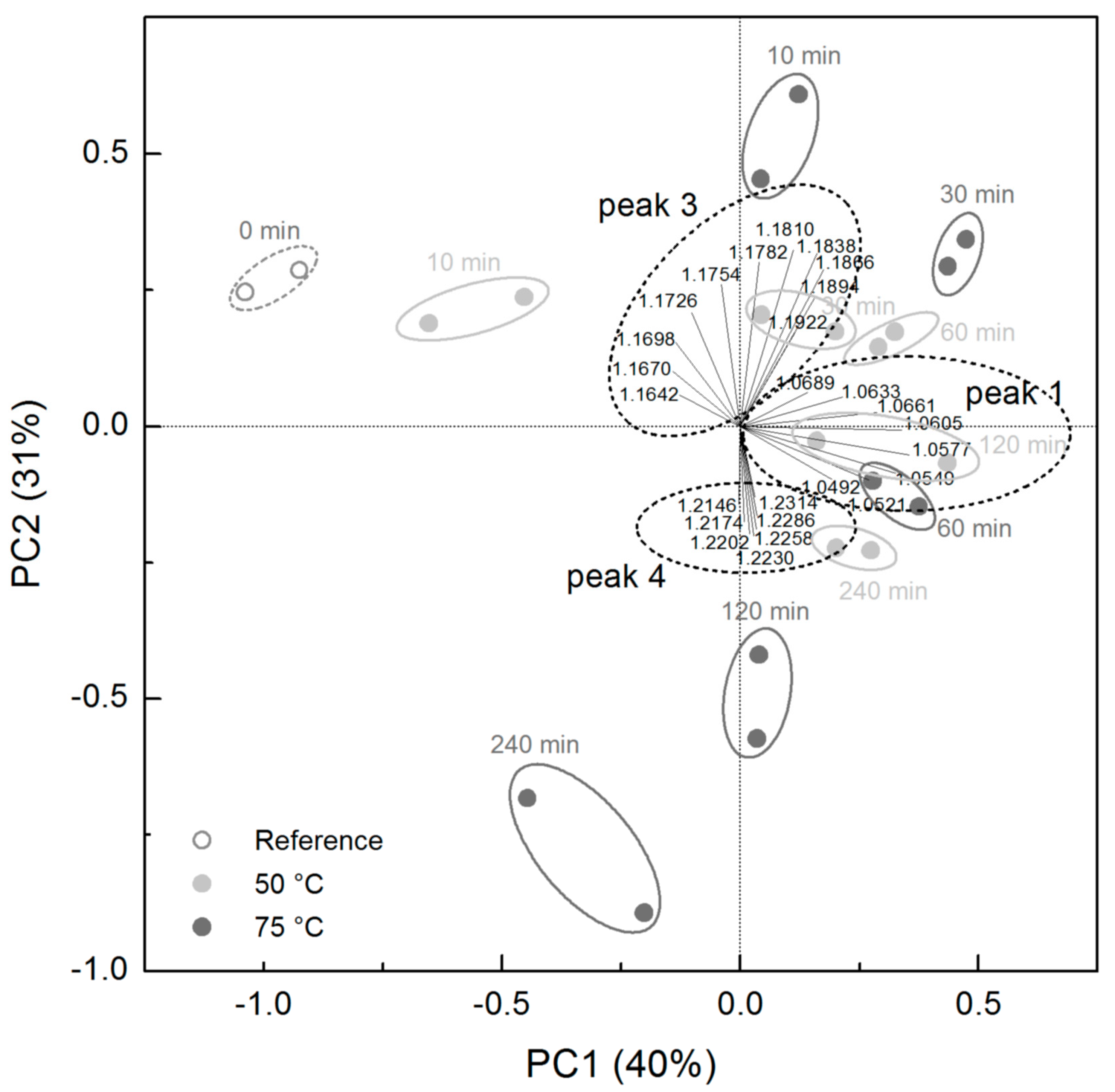
3.1. Ion Mobility Spectra of Chocolate Mass during Conching
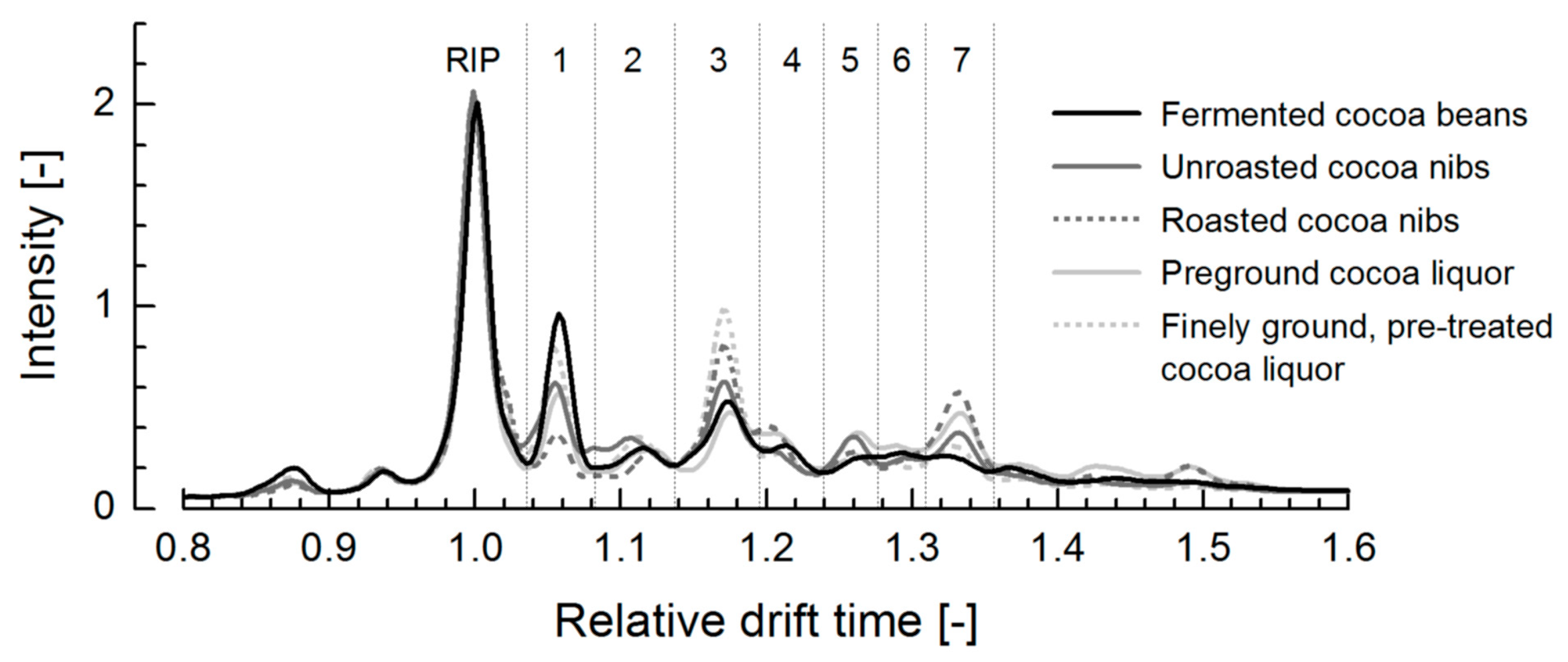
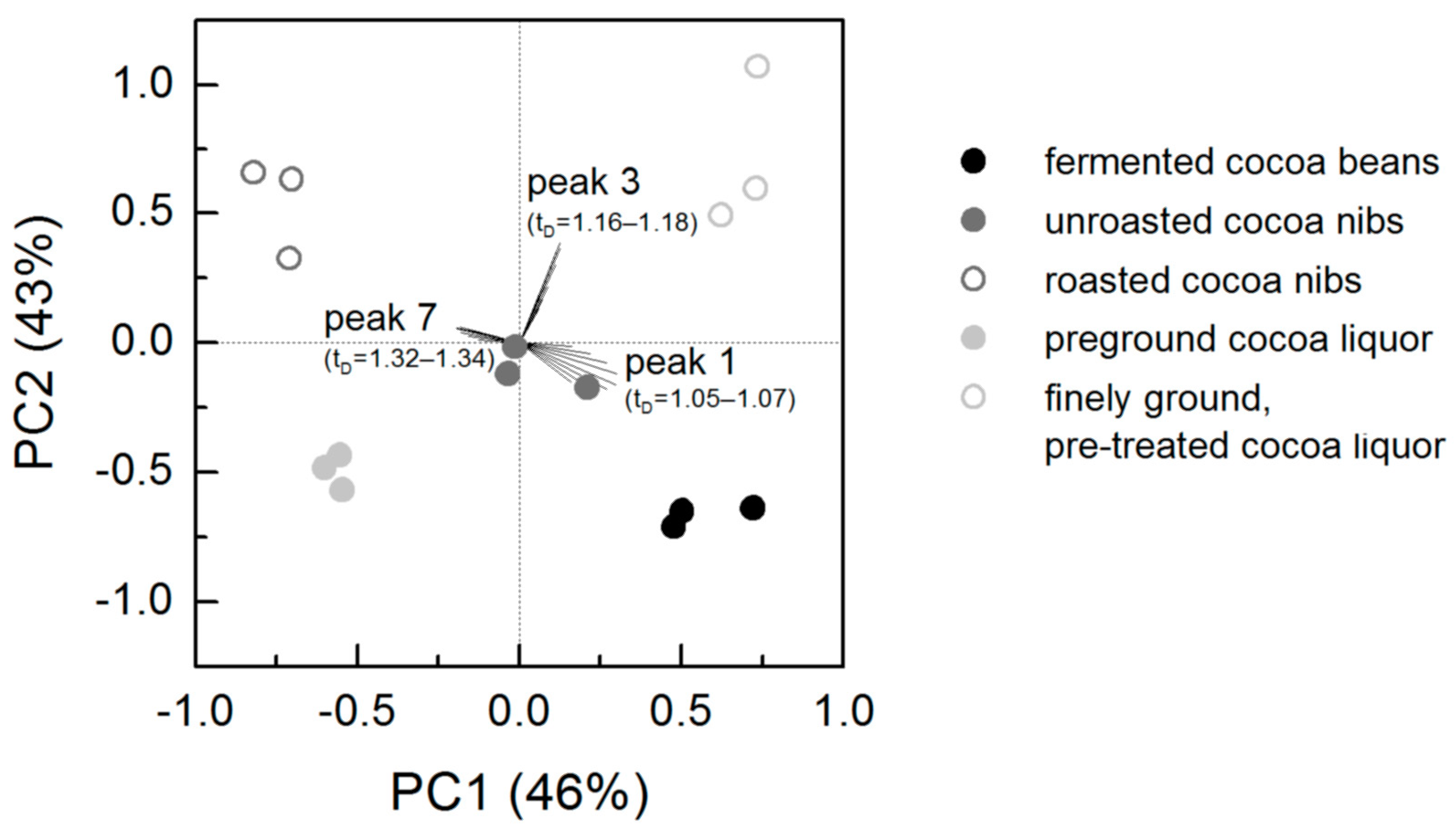
3.2. Ion Mobility Spectra of Bulk Cocoa Samples during Processing
3.3. Correlation of Ion Mobility Spectrometry and HS-SPME-GC-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J. Chocolate and cocoa aroma. In Chocolate in Health and Nutrition; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 89–101. [Google Scholar]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor chemistry of cocoa and cocoa products—An overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Counet, C.; Callemien, D.; Ouwerx, C.; Collin, S. Use of gas chromatography-olfactometry to identify key odorant compounds in dark chocolate. Comparison of samples before and after conching. J. Agric. Food Chem. 2002, 50, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Schnermann, P.; Schieberle, P. Evaluation of key odorants in milk chocolate and cocoa mass by aroma extract dilution analyses. J. Agric. Food Chem. 1997, 45, 867–872. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Changes in key aroma compounds of Criollo cocoa beans during roasting. J. Agric. Food Chem. 2008, 56, 10244–10251. [Google Scholar] [CrossRef] [PubMed]
- Jinap, S.; Rosli, W.I.W.; Russly, A.R.; Nordin, L.M. Effect of roasting time and temperature on volatile component profiles during nib roasting of cocoa beans (Theobroma cacao). J. Sci. Food Agric. 1998, 77, 441–448. [Google Scholar] [CrossRef]
- Jinap, S.; Siti, M.H.; Norsiati, M.G. Formation of methyl pyrazine during cocoa bean fermentation. Pertanika J. Trop. Agric. Sci. 1994, 17, 27–32. [Google Scholar]
- Hofmann, T.; Schieberle, P. Formation of aroma-active Strecker-aldehydes by a direct oxidative degradation of Amadori compounds. J. Agric. Food Chem. 2000, 48, 4301–4305. [Google Scholar] [CrossRef] [PubMed]
- Ziegleder, G. Flavour development in cocoa and chocolate. In Industrial Chocolate Manufacture and Use; Beckett, S.T., Ed.; Blackwell Publishing: Oxford, UK, 2009; pp. 169–191. [Google Scholar]
- Tran, P.D.; Durme, J.V.; de Walle, D.V.; de Winne, A.; Delbaere, C.; de Clercq, N.; Phan, T.T.Q.; Nguyen, C.-H.P.; Tran, D.N.; Dewettinck, K. Quality attributes of dark chocolate produced from Vietnamese cocoa liquors. J. Food Qual. 2016, 39, 311–322. [Google Scholar] [CrossRef]
- Del Rosario Brunetto, M.; Cayama, Y.D.; Gutiérrez, L.; Roa, S.C.; Méndez, Y.C.; Gallignani, M.; Zambrano, A.; Gómez, Á.; Ramos, G. Headspace gas chromatography–mass spectrometry determination of alkylpyrazines in cocoa liquor samples. Food Chem. 2009, 112, 253–257. [Google Scholar] [CrossRef]
- Ducki, S.; Miralles-Garcia, J.; Zumbé, A.; Tornero, A.; Storey, D.M. Evaluation of solid-phase micro-extraction coupled to gas chromatography–mass spectrometry for the headspace analysis of volatile compounds in cocoa products. Talanta 2008, 74, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Perego, P.; Fabiano, B.; Cavicchioli, M.; Del Borghi, M. Cocoa quality and processing: A study by solid-phase microextraction and gas chromatography analysis of methylpyrazines. Food Bioprod. Process. 2004, 82, 291–297. [Google Scholar] [CrossRef]
- Eiceman, G.A. Ion-mobility spectrometry as a fast monitor of chemical composition. TrAC Trends Anal. Chem. 2002, 21, 259–275. [Google Scholar] [CrossRef]
- Karpas, Z. Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res. Int. 2013, 54, 1146–1151. [Google Scholar] [CrossRef]
- Vautz, W.; Zimmermann, D.; Hartmann, M.; Baumbach, J.I.; Nolte, J.; Jung, J. Ion mobility spectrometry for food quality and safety. Food Addit. Contam. 2006, 23, 1064–1073. [Google Scholar] [CrossRef]
- Karpas, Z.; Tilman, B.; Gdalevsky, R.; Lorber, A. Determination of volatile biogenic amines in muscle food products by ion mobility spectrometry. Anal. Chim. Acta 2002, 463, 155–163. [Google Scholar] [CrossRef]
- Menéndez, M.; Garrido-Delgado, R.; Arce, L.; Valcárcel, M. Direct determination of volatile analytes from solid samples by UV-ion mobility spectrometry. J. Chromatogr. A 2008, 1215, 8–14. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Arce, L.; Guamán, A.V.; Pardo, A.; Marco, S.; Valcárcel, M. Direct coupling of a gas–liquid separator to an ion mobility spectrometer for the classification of different white wines using chemometrics tools. Talanta 2011, 84, 471–479. [Google Scholar] [CrossRef]
- Khalesi, M.; Sheikh-Zeinoddin, M.; Tabrizchi, M. Determination of ochratoxin A in licorice root using inverse ion mobility spectrometry. Talanta 2011, 83, 988–993. [Google Scholar] [CrossRef]
- Sheibani, A.; Tabrizchi, M.; Ghaziaskar, H.S. Determination of aflatoxins B1 and B2 using ion mobility spectrometry. Talanta 2008, 75, 233–238. [Google Scholar] [CrossRef]
- Banach, U.; Tiebe, C.; Hübert, T. Multigas sensors for the quality control of spice mixtures. Food Control 2012, 26, 23–27. [Google Scholar] [CrossRef]
- Eiceman, G.A.; Karpas, Z. Ion Mobility Spectrometry, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Tan, J.; Kerr, W.L. Characterizing cocoa refining by electronic nose using a Kernel distribution model. LWT-Food Sci. Technol. 2019, 104, 1–7. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Arroyo-Manzanares, N.; Arce, C.; Arce, L. HS-GC-IMS and chemometric data treatment for food authenticity assessment: Olive oil mapping and classification through two different devices as an example. Food Control 2019, 98, 82–93. [Google Scholar] [CrossRef]
- Rottiers, H.; Tzompa Sosa, D.A.; Van de Vyver, L.; Hinneh, M.; Everaert, H.; De Wever, J.; Messens, K.; Dewettinck, K. Discrimination of cocoa liquors based on their odor fingerprint: A fast GC electronic nose suitability study. Food Anal. Methods 2019, 12, 475–488. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Effect of fermentation method, roasting and conching conditions on the aroma volatiles of dark chocolate. J. Food Process. Preserv. 2012, 36, 446–456. [Google Scholar] [CrossRef]
- Tzschoppe, M.; Haase, H.; Höhnisch, M.; Jaros, D.; Rohm, H. Using ion mobility spectrometry for screening the autoxidation of peanuts. Food Control 2016, 64, 17–21. [Google Scholar] [CrossRef]
- Albak, F.; Tekin, A.R. Variation of total aroma and polyphenol content of dark chocolate during three phase of conching. J. Food Sci. Technol. 2016, 53, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the raw seed to chocolate: Volatile profile of Blanco de Criollo in different phases of the processing chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Jurado-Compos, N.; Garrido-Delgado, R.; Martínez-Haya, B.; Eiceman, G.A.; Arce, L. Stability of proton-bound clusters of alkyl alcohols, aldehydes and ketones in Ion Mobility Spectrometry. Talanta 2018, 185, 299–308. [Google Scholar] [CrossRef]
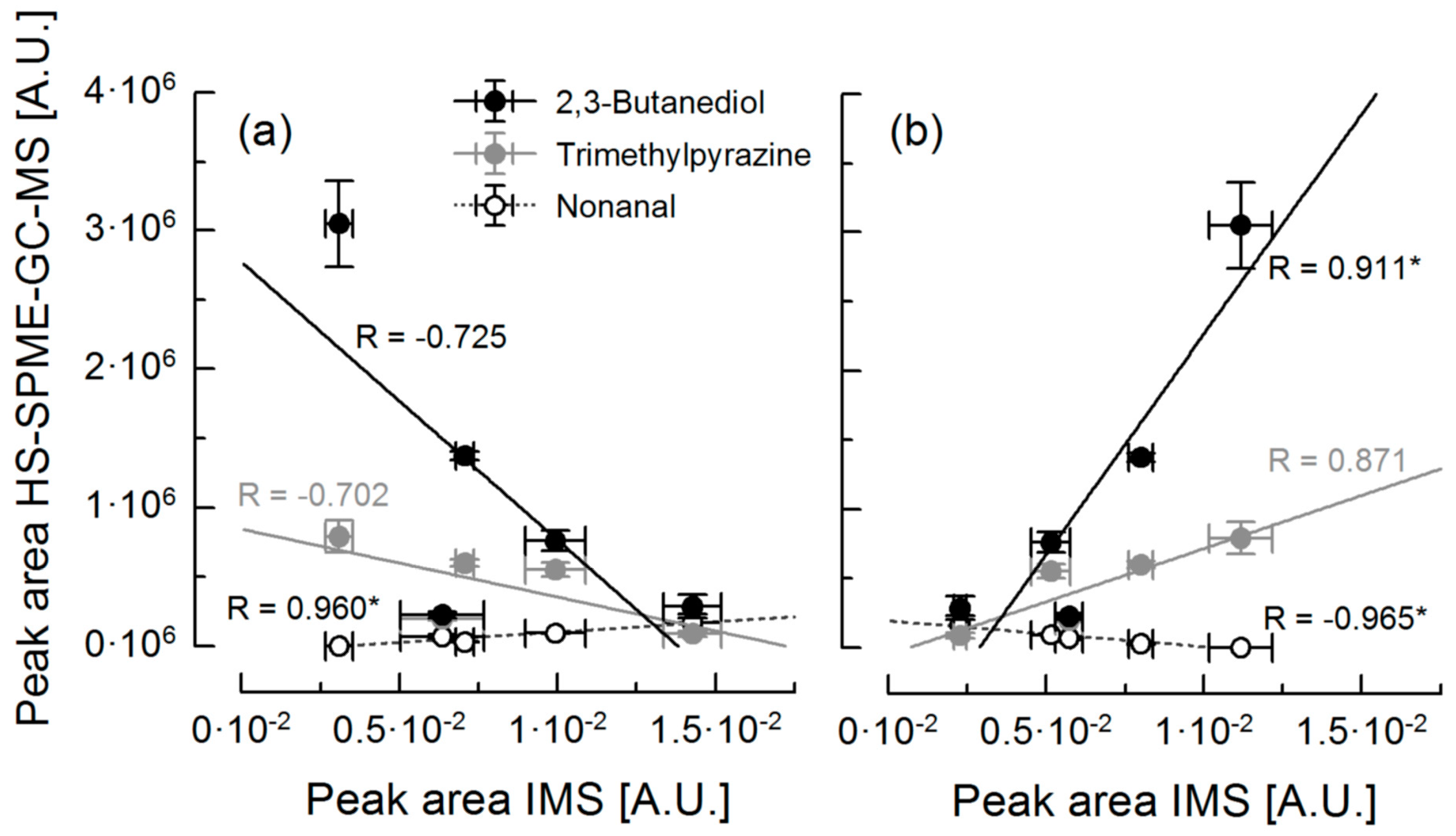
| Compound | Rpeak 1 | Rpeak 2 | Rpeak 3 | Rpeak 4 | Rpeak 5 | Rpeak 6 | Rpeak 7 |
|---|---|---|---|---|---|---|---|
| 2,3-Butanediol | −0.725 | −0.556 | 0.080 | 0.904 * | −0.094 | −0.232 | 0.911 * |
| 1-Butanol-3-methyl-acetate | −0.006 | −0.152 | −0.983 * | 0.292 | 0.540 | 0.776 | 0.000 |
| Benzaldehyde | −0.323 | 0.266 | −0.146 | −0.338 | 0.665 | −0.244 | −0.034 |
| Trimethylpyrazine | −0.702 | −0.090 | 0.331 | 0.654 | 0.057 | −0.479 | 0.871 |
| Acetophenone | 0.166 | −0.513 | −0.944 * | 0.310 | 0.154 | 0.875 | −0.152 |
| Tetramethypyrazine | −0.619 | 0.363 | 0.543 | 0.250 | 0.237 | −0.713 | 0.681 |
| Linalool | −0.156 | 0.677 | 0.041 | −0.610 | 0.718 | −0.355 | −0.190 |
| Nonanal | 0.960 * | 0.065 | −0.018 | −0.665 | −0.522 | 0.438 | −0.965 * |
| Phenethyl alcohol | −0.483 | 0.227 | −0.004 | 0.502 | 0.354 | −0.153 | 0.658 |
| Phenethyl acetate | −0.338 | 0.858 | 0.349 | −0.280 | 0.621 | −0.577 | 0.234 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, C.; Jaros, D.; Rohm, H. Ion Mobility Spectrometry as a Potential Tool for Flavor Control in Chocolate Manufacture. Foods 2019, 8, 460. https://doi.org/10.3390/foods8100460
Schmidt C, Jaros D, Rohm H. Ion Mobility Spectrometry as a Potential Tool for Flavor Control in Chocolate Manufacture. Foods. 2019; 8(10):460. https://doi.org/10.3390/foods8100460
Chicago/Turabian StyleSchmidt, Carolin, Doris Jaros, and Harald Rohm. 2019. "Ion Mobility Spectrometry as a Potential Tool for Flavor Control in Chocolate Manufacture" Foods 8, no. 10: 460. https://doi.org/10.3390/foods8100460
APA StyleSchmidt, C., Jaros, D., & Rohm, H. (2019). Ion Mobility Spectrometry as a Potential Tool for Flavor Control in Chocolate Manufacture. Foods, 8(10), 460. https://doi.org/10.3390/foods8100460






