Protective Effect of Selenium-Enriched Ricegrass Juice against Cadmium-Induced Toxicity and DNA Damage in HEK293 Kidney Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Materials
2.3. Polyphenols Identification
2.4. Cell Culture Model
2.5. Cell Viability Assay
2.6. Anti-Cadmium Toxicity Properties
2.7. Determination of Lipid Peroxidation
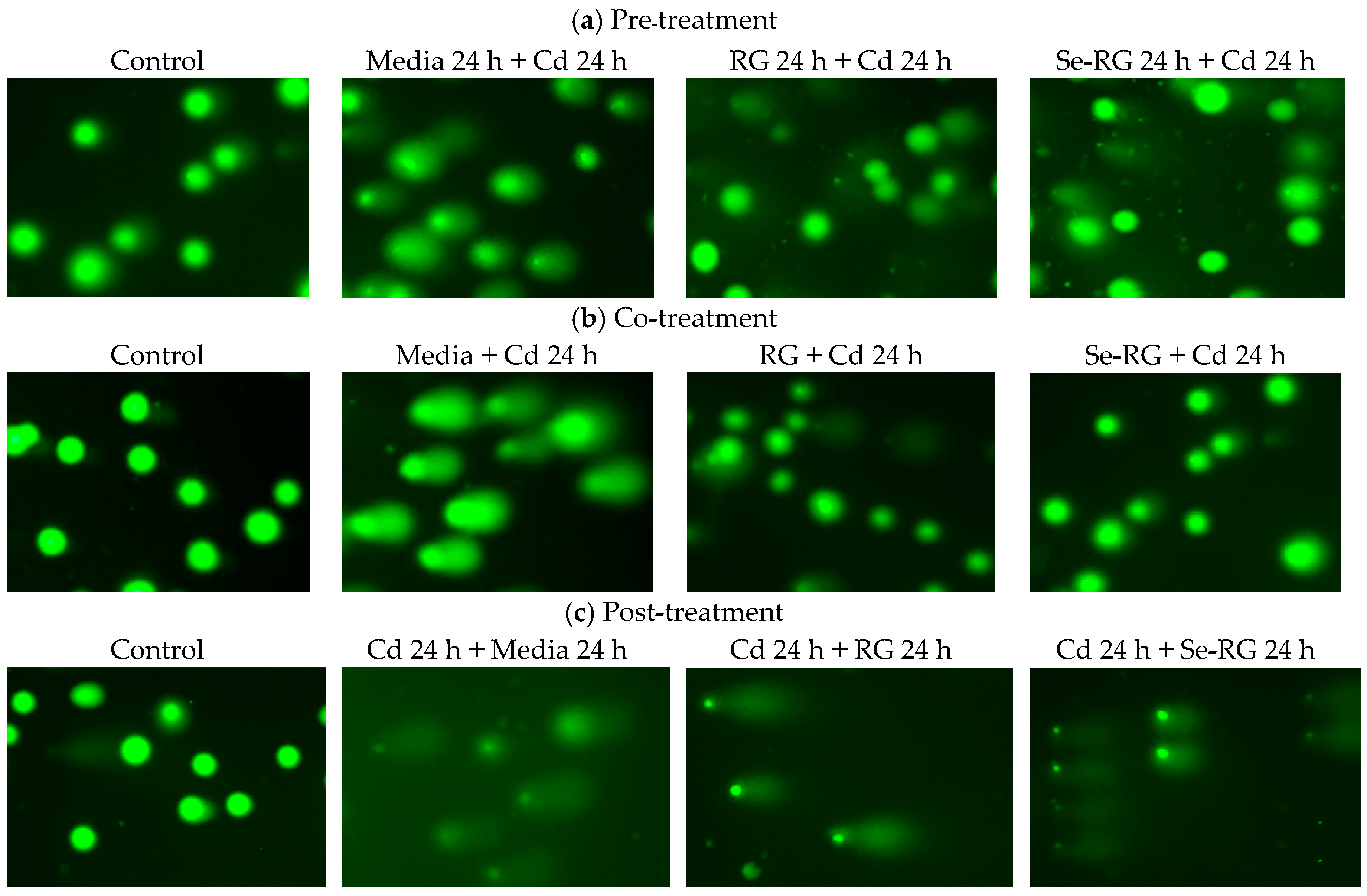
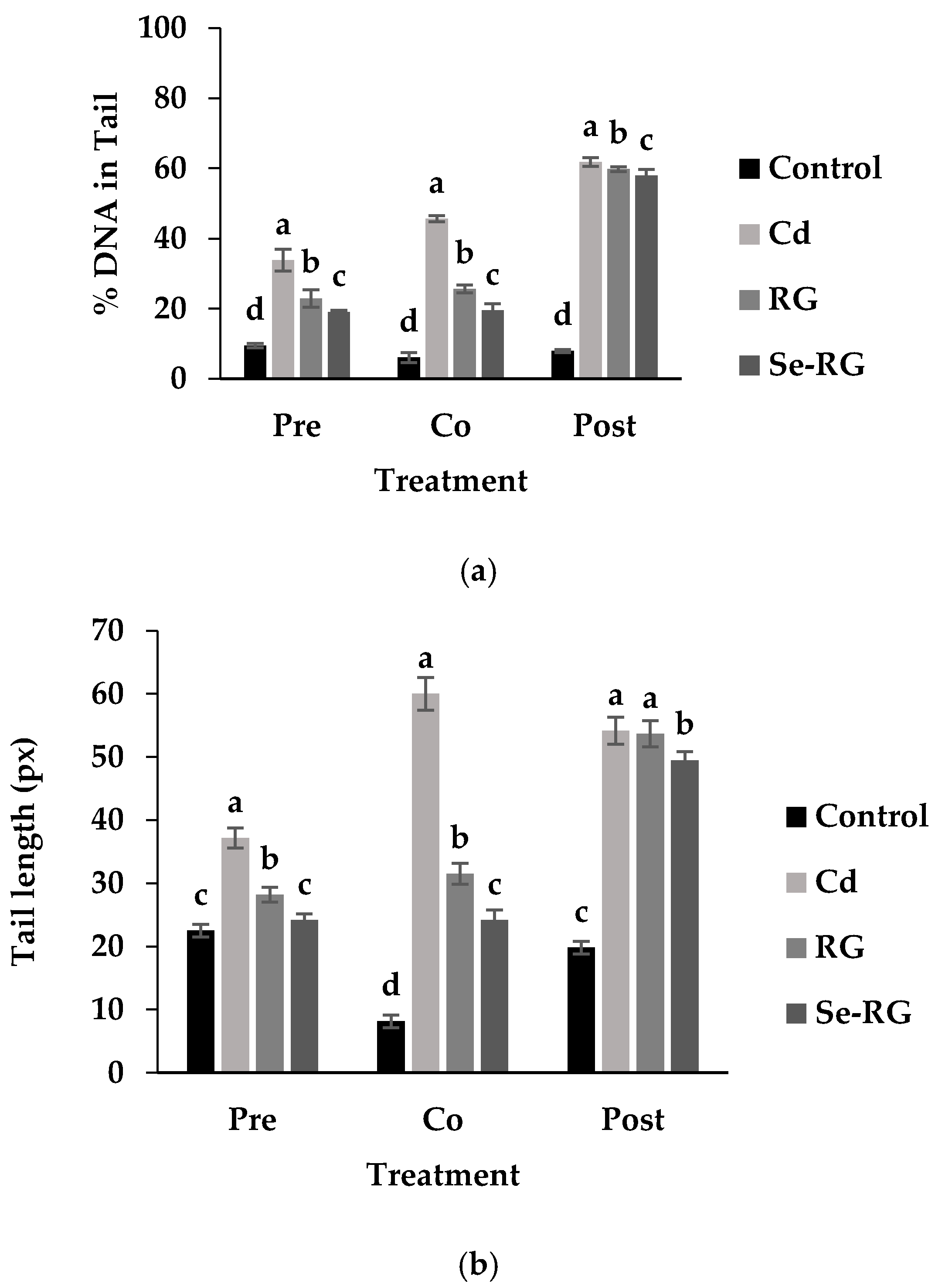
2.8. DNA Protective Properties Using Comet Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Polyphenols Identification Using Ultra High-Performance Liquid Chromatography–Electron Spray Ionization–Mass Detector (UHPLC–ESI–MS)
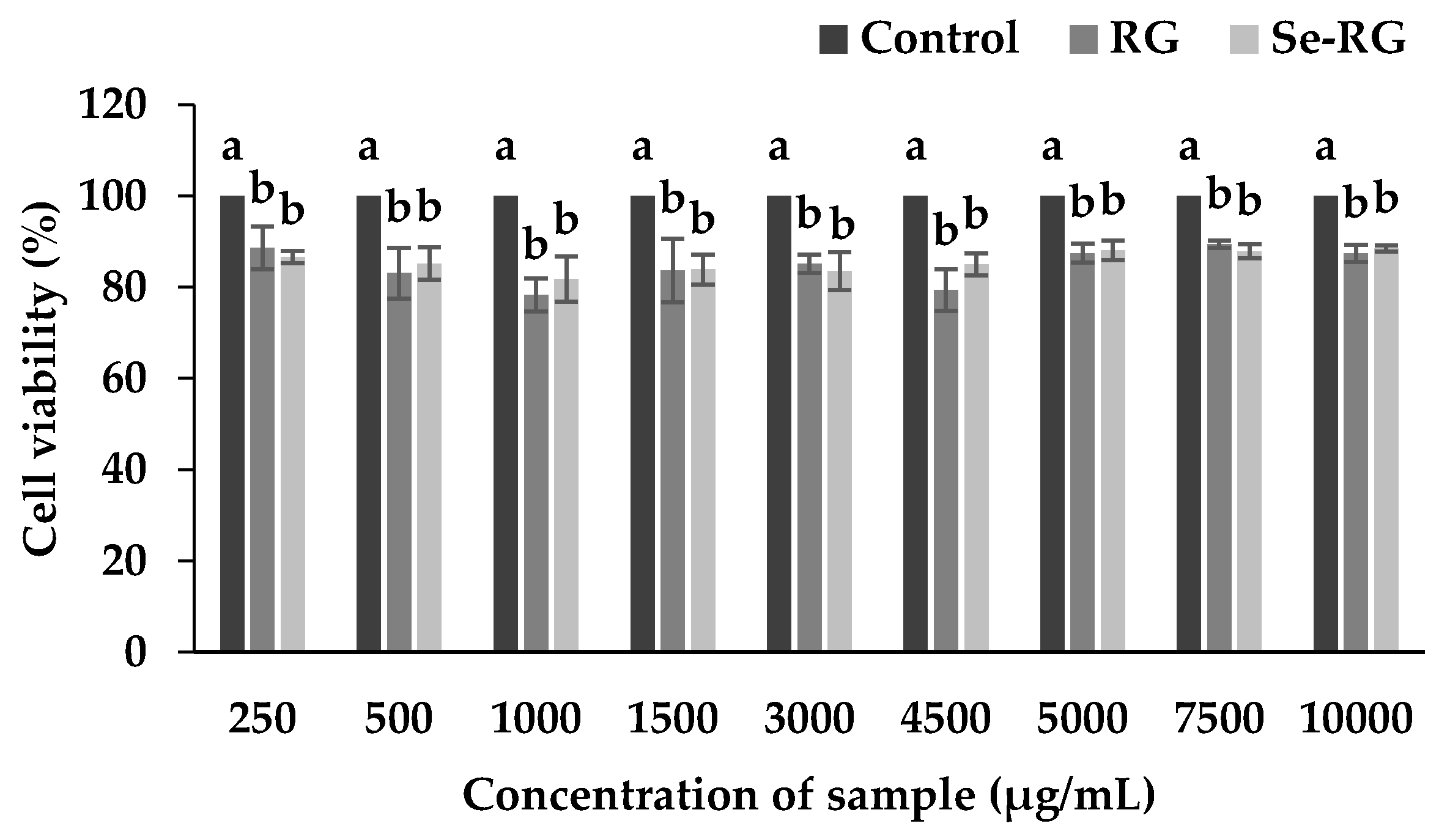
3.2. Cytotoxicity of Ricegrass Juice Extract (RG) and Se-Rich Ricegrass Juice Extract (Se-RG)
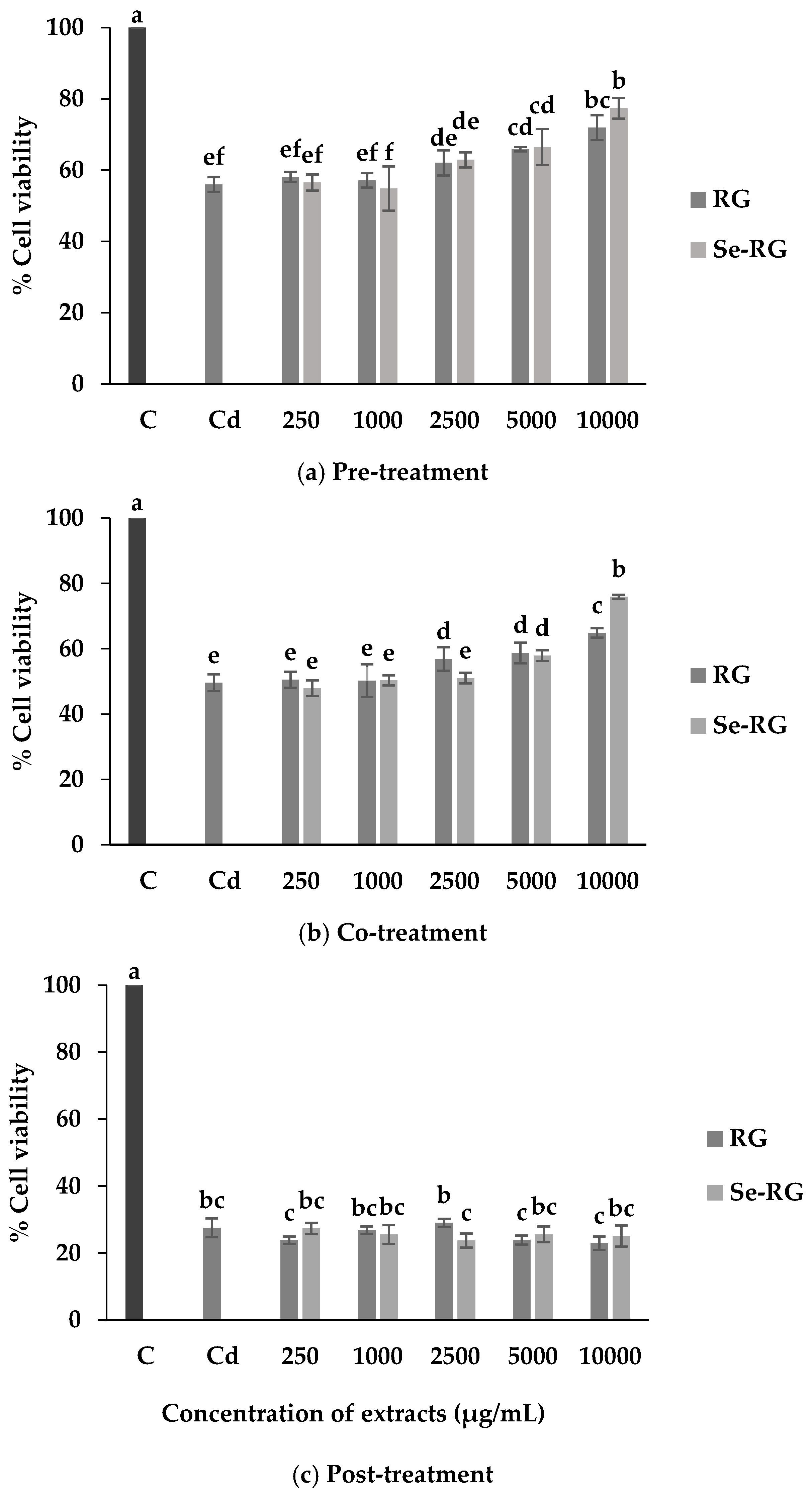
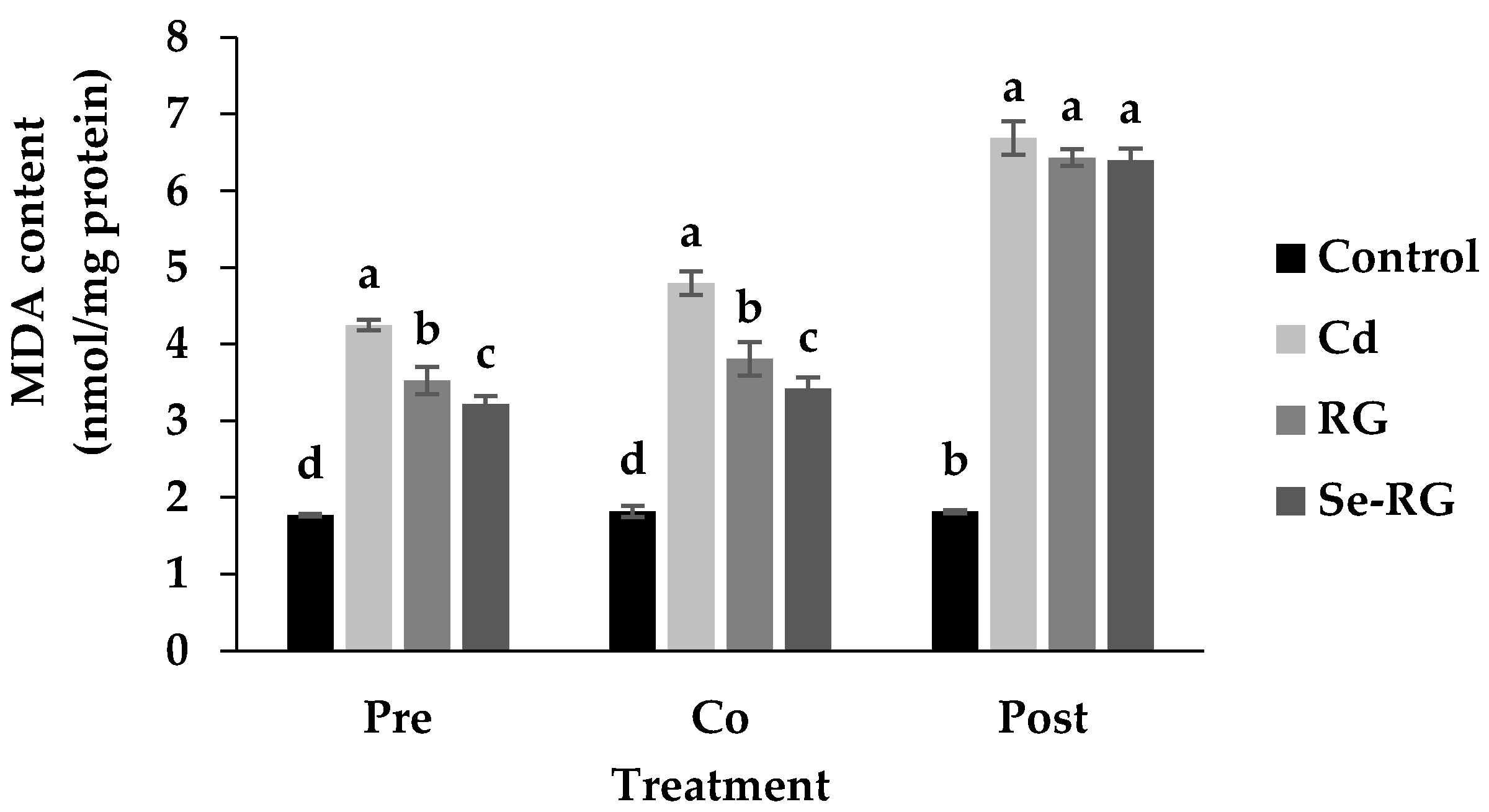
3.3. Anti-Cd Toxicity of RG and Se-RG and Effect on Lipid Peroxidation
3.4. DNA Protective Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology, Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Elinder, C.G.; Järup, L. Cadmium exposure and health risks: Recent findings. Ambio 1996, 25, 370–373. [Google Scholar]
- Wang, X.Q.; Xu, D.; Lü, M.K.; Yuan, D.R.; Yin, X.; Zhang, G.H.; Xu, S.X.; Lu, G.W.; Song, C.F.; Guo, S.Y. Crystal growth and physical properties of UV nonlinear optical crystal zinc cadmium thiocyanate, ZnCd(SCN)4. Chem. Phys. Lett. 2001, 346, 393–406. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, Z.A.; Vu, T.T.; Zaman, K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Nahak, G.; Suar, M.; Sahu, R.K. Antioxidant Potential and Nutritional Values of Vegetables: A Review. Res. J. Med. Plant 2014, 8, 50–81. [Google Scholar] [CrossRef]
- Chomchan, R.; Siripongvutikorn, S.; Puttarak, P.; Rattanapon, R. Investigation of phytochemical constituents, phenolic profiles and antioxidant activities of ricegrass juice compared to wheatgrass juice. Funct. Foods Health Dis. 2016, 6, 822–835. [Google Scholar]
- Sandbichler, A.M.; Höckner, M. Cadmium protection strategies—A hidden trade-off? Int. J. Mol. Sci. 2016, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Sharma, A. Effects of cadmium and selenium on cell division and chromosomal aberrations in Allium sativum L. Water Air Soil Pollut. 1988, 37, 433–438. [Google Scholar] [CrossRef]
- Chomchan, R.; Siripongvutikorn, S.; Puttarak, P. Selenium bio-fortification: An alternative to improve phytochemicals and bioactivities of plant foods. Funct. Foods. Health Dis. 2017, 7, 263–279. [Google Scholar]
- Chomchan, R.; Siripongvutikorn, S.; Puttarak, P.; Rattanapon, R. Influence of selenium bio-fortification on nutritional compositions, bioactive compounds content and anti-oxidative properties of young ricegrass (Oryza sativa L.). Funct. Food Health. Dis. 2017, 7, 195–209. [Google Scholar]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. In Mammalian Cell Viability. Methods in Molecular Biology (Methods and Protocols); Stoddart, M., Ed.; Humana Press: New York, NY, USA, 2011; Volume 740, pp. 7–12. ISBN 978-1-61779-107-9. [Google Scholar]
- Du, Y.; Esfandi, R.; Willmore, W.G.; Tsopmo, A. Antioxidant activity of oat proteins derived peptides in stressed hepatic HepG2 cells. Antioxidants 2016, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Jiao, H.; Zhao, B. Tea catechins protect against lead-induced cytotoxicity, lipid peroxidation, and membrane fluidity in HepG2 cells. Toxicol. Sci. 2002, 69, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T.; Barnes, D. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P. Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat. Res. 2000, 455, 111–127. [Google Scholar] [CrossRef]
- Besson, E.; Dellamonica, G.; Chopin, J.; Markham, K.R.; Kim, M.; Koh, H.-S.; Fukami, H. C-glycosylflavones from Oryza sativa. Phytochemisty 1985, 24, 1061–1064. [Google Scholar] [CrossRef]
- Yang, Z.; Nakabayashi, R.; Okazaki, Y.; Mori, T.; Takamatsu, S.; Kitanaka, S.; Kikuchi, J.; Saito, K. Toward better annotation in plant metabolomics: Isolation and structure elucidation of 36 specialized metabolites from Oryza sativa (rice) by using MS/MS and NMR analyses. Metabolomics 2014, 10, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cheon, Y.M.; Kim, B.G.; Ahn, J.H. Analysis of flavonoids and characterization of the OsFNS gene involved in flavone biosynthesis in rice. J. Plant Biol. 2008, 51, 97–101. [Google Scholar] [CrossRef]
- Pourrut, B.; Pinelli, E.; Celiz Mendiola, V.; Silvestre, J.; Douay, F. Recommendations for increasing alkaline comet assay reliability in plants. Mutagenesis 2015, 30, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Henkler, F.; Brinkmann, J.; Luch, A. The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers 2010, 2, 376–396. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.R.; Mulgrew, J.; Paolini, G.V.; van Hoorn, W.P. Predicting cytotoxicity from heterogeneous data sources with Bayesian learning. J. Cheminform. 2010, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Eneman, J.; Potts, R.; Osier, M.; Shukla, G.; Lee, C.; Chiu, J.; Hart, B. Suppressed oxidant-induced apoptosis in cadmium adapted alveolar epithelial cells and its potential involvement in cadmium carcinogenesis. Toxicology 2000, 147, 215–228. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Rahn, R.O.; Zhang, R. Dietary flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett. 1997, 119, 99–107. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.Y.; Yang, D.J.; Chang, M.H.; Chen, Y.C. Alleviative effects of litchi (Litchi chinensis Sonn.) flower on lipid peroxidation and protein degradation in emulsified pork meatballs. J. Food Drug Anal. 2015, 23, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp. Toxicol. Pathol. 2010, 62, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Rhee, I.K.; Park, K.Y.; Park, K.Y.; Kim, J.K.; Rhee, S.J. Action of green tea catechin on bone metabolic disorder in chronic cadmium-poisoned rats. Life Sci. 2003, 73, 1479–1489. [Google Scholar] [CrossRef]
- El-Sharaky, A.; Newairy, A.; Badreldeen, M.; Eweda, S.; Sheweita, S. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 2007, 235, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, B.; Cheng, Y.; Lin, H. Ameliorative effects of selenium on cadmium-induced oxidative stress and endoplasmic reticulum stress in the chicken kidney. Biol. Trace Elem. Res. 2015, 167, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, B.; Markovic, S.; Pavlovic, S.; Zikic, R.; Stajn, A.; Saicic, Z. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: Protective effect of selenium. Physiol. Res. 2008, 57, 403–411. [Google Scholar] [PubMed]
- Zhou, Y.J.; Zhang, S.P.; Liu, C.W.; Cai, Y.Q. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK1 cells. Toxicol. In Vitro 2009, 23, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, N.; Sudheer, A.R.; Srinivasan, M.; Menon, V.P. Quercetin ameliorates gamma radiation-induced DNA damage and biochemical changes in human peripheral blood lymphocytes. Mutat. Res. 2008, 654, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.L.; Lancia, J.K.; Mathur, A.; Smith, M.L. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 2006, 26, 899–904. [Google Scholar] [PubMed]


| Time (min) | % Solvent B |
|---|---|
| 0.00–10.00 | 0.00–10.00 |
| 10.01–15.00 | 10.00 |
| 15.01–20.00 | 10.00–15.00 |
| 20.01–30.00 | 15.00–25.00 |
| 30.01–35.00 | 25.00 |
| 35.01–45.00 | 25.00–100.00 |
| Control | Negative Control | Sample | ||||
|---|---|---|---|---|---|---|
| Time | 24 h + 24 h | 24 h + 24 h | 24 h + 24 h | |||
| Pre-incubation | Media | Media | Media | CdCl2 * | Extracts ** | CdCl2 |
| Co-incubation | Media | - | Media + CdCl2 | - | Extracts + CdCl2 | - |
| Post-incubation | Media | Media | CdCl2 | Media | CdCl2 | Extracts |
| Tentative Compounds | MW * | [M − H]+ (m/z) | References |
|---|---|---|---|
| Tricin | 330 | 329 | [22,23] |
| 1-O-Sinapoyl-β-d-glucose | 386 | 385 | [22] |
| 3-O-Feruloylquinic acid | 368 | 367 | [22,24] |
| Chrysoeriol arabinosyl arabinoside | 564 | 563 | [22,24] |
| Swertisin | 446 | 445 | [22] |
| Tricin-7-O-β-d-glucopyranoside | 492 | 491 | [25] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chomchan, R.; Siripongvutikorn, S.; Maliyam, P.; Saibandith, B.; Puttarak, P. Protective Effect of Selenium-Enriched Ricegrass Juice against Cadmium-Induced Toxicity and DNA Damage in HEK293 Kidney Cells. Foods 2018, 7, 81. https://doi.org/10.3390/foods7060081
Chomchan R, Siripongvutikorn S, Maliyam P, Saibandith B, Puttarak P. Protective Effect of Selenium-Enriched Ricegrass Juice against Cadmium-Induced Toxicity and DNA Damage in HEK293 Kidney Cells. Foods. 2018; 7(6):81. https://doi.org/10.3390/foods7060081
Chicago/Turabian StyleChomchan, Rattanamanee, Sunisa Siripongvutikorn, Pattaravan Maliyam, Bandhita Saibandith, and Panupong Puttarak. 2018. "Protective Effect of Selenium-Enriched Ricegrass Juice against Cadmium-Induced Toxicity and DNA Damage in HEK293 Kidney Cells" Foods 7, no. 6: 81. https://doi.org/10.3390/foods7060081
APA StyleChomchan, R., Siripongvutikorn, S., Maliyam, P., Saibandith, B., & Puttarak, P. (2018). Protective Effect of Selenium-Enriched Ricegrass Juice against Cadmium-Induced Toxicity and DNA Damage in HEK293 Kidney Cells. Foods, 7(6), 81. https://doi.org/10.3390/foods7060081