High-Pressure Treatment of Non-Hydrated Flour Affects Structural Characteristics and Hydration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. High-Pressure Treatment
2.3. Molecular Structure of Starch
2.4. Crystalline Properties
2.5. Granular Structure
2.6. Starch Modification Degree
2.7. Hydration Properties
2.8. Statistical Evaluation
3. Results and Discussion
3.1. Molecular Alterations of Starch
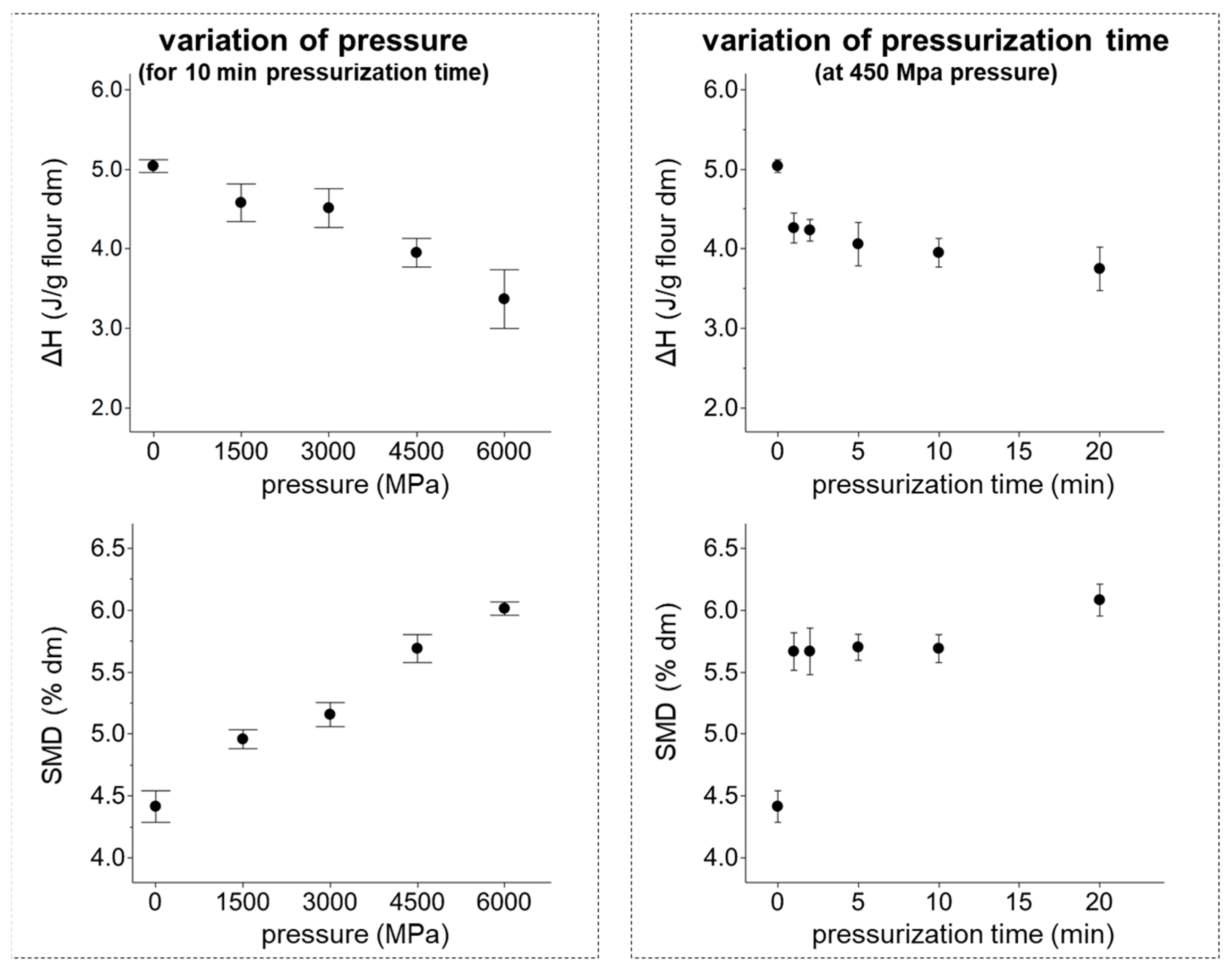
3.2. Alterations in Gelatinization Properties and Starch Modification Degree of Flours
3.3. Granular Modification of Wheat Flour Particles
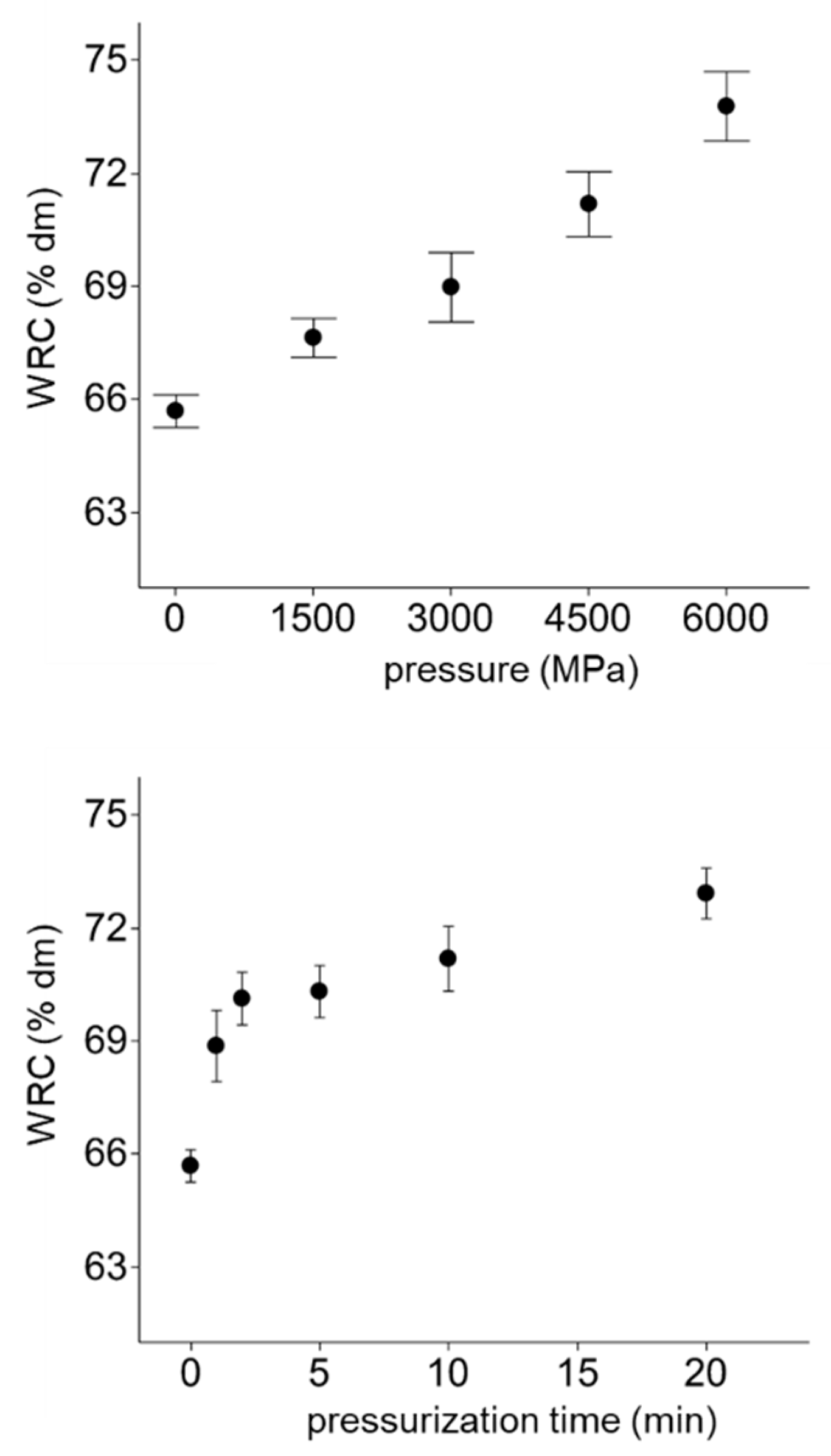
3.4. Facilitated Hydration of HPT Flours
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bull, M.K.; Zerdin, K.; Howe, E.; Goicoechea, D.; Paramanandhan, P.; Stockman, R.; Sellahewa, J.; Szabo, E.A.; Johnson, R.L.; Stewart, C.M. The effect of high pressure processing on the microbial, physical and chemical properties of Valencia and Navel orange juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 135–149. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Mastwijk, H.C.; Knol, J.J.; Quataert, M.C.J.; Vervoort, L.; Van der Plancken, I.; Hendrickx, M.E.; Matser, A.M. Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice. Part I: Impact on overall quality attributes. Innov. Food Sci. Emerg. Technol. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Barbosa-Cánovas, G.V.; Lelieveld, H.L.M. High Pressure Processing of Food; Food Engineering Series; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3233-7. [Google Scholar]
- Dunford, N.T. Food and Industrial Bioproducts and Bioprocessing; Wiley-Blackwell: Hoboken, NJ, USA, 2012; ISBN 9780813821054. [Google Scholar]
- Kim, H.-S.; Kim, B.-Y.; Baik, M.-Y. Application of Ultra High Pressure (UHP) in Starch Chemistry. Crit. Rev. Food Sci. Nutr. 2012, 52, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-L.; Hu, X.-S.; Shen, Q. Effect of high hydrostatic pressure on starches: A review. Starch-Stärke 2010, 62, 615–628. [Google Scholar] [CrossRef]
- Cappa, C.; Barbosa-Cánovas, G.V.; Lucisano, M.; Mariotti, M. Effect of high pressure processing on the baking aptitude of corn starch and rice flour. LWT Food Sci. Technol. 2016, 73, 20–27. [Google Scholar] [CrossRef]
- Muntean, M.-V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High Pressure Processing in Food Industry—Characteristics and Applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef]
- Mu, T.-H.; Zhang, M.; Raad, L.; Sun, H.-N.; Wang, C. Effect of α-Amylase Degradation on Physicochemical Properties of Pre-High Hydrostatic Pressure-Treated Potato Starch. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.R.A.; Clark, R.; Ledward, D.A. Effects of high pressure on amylases and starch in wheat and barley flours. Food Chem. 1998, 63, 363–372. [Google Scholar] [CrossRef]
- Błaszczak, W.; Valverde, S.; Fornal, J. Effect of high pressure on the structure of potato starch. Carbohydr. Polym. 2005, 59, 377–383. [Google Scholar] [CrossRef]
- Oh, H.E.; Hemar, Y.; Anema, S.G.; Wong, M.; Neil Pinder, D. Effect of high-pressure treatment on normal rice and waxy rice starch-in-water suspensions. Carbohydr. Polym. 2008, 73, 332–343. [Google Scholar] [CrossRef]
- Leite, T.S.; de Jesus, A.L.T.; Schmiele, M.; Tribst, A.A.L.; Cristianini, M. High pressure processing (HPP) of pea starch: Effect on the gelatinization properties. LWT Food Sci. Technol. 2017, 76, 361–369. [Google Scholar] [CrossRef]
- Kudta, E.; Tomasik, P. The Modification of Starch by High Pressure. Part I: Air- and Oven-dried Potato Starch. Starch-Stärke 1992, 44, 167–173. [Google Scholar] [CrossRef]
- Kudla, E.; Tomasik, P. The Modification of Starch by High Pressure. Part II: Compression of Starch with Additives. Starch-Stärke 1992, 44, 253–259. [Google Scholar] [CrossRef]
- Stute, R.; Heilbronn, R.W.; Klingler, R.W.; Boguslawski, S.; Eshtiaghi, M.N.; Knorr, D. Effects of High Pressures Treatment on Starches. Starch-Starke 1996, 48, 399–408. [Google Scholar] [CrossRef]
- Oh, H.E.; Pinder, D.N.; Hemar, Y.; Anema, S.G.; Wong, M. Effect of high-pressure treatment on various starch-in-water suspensions. Food Hydrocoll. 2008, 22, 150–155. [Google Scholar] [CrossRef]
- Simonin, H.; Marzouki, S.; Guyon, C.; Orlowska, M.; Le-Bail, A.; de Lamballerie, M. Pasting properties of high-pressure-treated starch suspensions. High Press. Res. 2009, 29, 726–731. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Liu, Y.N.; Li, H.; Shen, R.L.; Li, X.H.; Yang, L.Z. The Study on Gelatinization Pressure of Starch by Ultra-High Pressure Processing. Adv. Mater. Res. 2011, 295–297, 131–134. [Google Scholar] [CrossRef]
- Kawai, K.; Fukami, K.; Yamamoto, K. Effect of temperature on gelatinization and retrogradation in high hydrostatic pressure treatment of potato starch–water mixtures. Carbohydr. Polym. 2012, 87, 314–321. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Flanagan, B.M.; Hasjim, J.; Gidley, M.J. Cryo-milling of starch granules leads to differential effects on molecular size and conformation. Carbohydr. Polym. 2011, 84, 1133–1140. [Google Scholar] [CrossRef]
- Jakobi, S.; Jekle, M.; Becker, T. Direct link between specific structural levels of starch and hydration properties. Carbohydr. Polym. 2018, 181, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hasjim, J.; Xie, F.; Halley, P.J.; Gilbert, R.G. Shear degradation of molecular, crystalline, and granular structures of starch during extrusion. Starch-Stärke 2014, 66, 595–605. [Google Scholar] [CrossRef]
- Liu, M.; Wu, N.-N.; Yu, G.-P.; Zhai, X.-T.; Chen, X.; Zhang, M.; Tian, X.-H.; Liu, Y.-X.; Wang, L.-P.; Tan, B. Physicochemical properties, structural properties, and in vitro digestibility of pea starch treated with high hydrostatic pressure. Starch-Stärke 2018, 70. [Google Scholar] [CrossRef]
- Douzals, J.P.; Marechal, P.A.; Coquille, J.C.; Gervais, P. Microscopic Study of Starch Gelatinization under High Hydrostatic Pressure. J. Agric. Food Chem. 1996, 44, 1403–1408. [Google Scholar] [CrossRef]
- Bauer, B.A.; Knorr, D. The impact of pressure, temperature and treatment time on starches: Pressure-induced starch gelatinisation as pressure time temperature indicator for high hydrostatic pressure processing. J. Food Eng. 2005, 68, 329–334. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. Particle size, rheological and structural properties of whole wheat flour doughs as treated by high pressure. Int. J. Food Prop. 2017, 20, 1829–1842. [Google Scholar] [CrossRef]
- Dhakal, S.; Giusti, M.M.; Balasubramaniam, V. Effect of high pressure processing on dispersive and aggregative properties of almond milk. J. Sci. Food Agric. 2016, 96, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Reineke, K.; Weich, H.; Knorr, D. The Influence of Sugars on Pressure Induced Starch Gelatinization. Procedia Food Sci. 2011, 1, 2040–2046. [Google Scholar] [CrossRef]
- Douzals, J.P.; Perrier Cornet, J.M.; Gervais, P.; Coquille, J.C. High-Pressure Gelatinization of Wheat Starch and Properties of Pressure-Induced Gels. J. Agric. Food Chem. 1998, 46, 4824–4829. [Google Scholar] [CrossRef]
- Douzals, J.P.; Perrier-Cornet, J.M.; Gervais, P.; Coquille, J.C. Hydration and Pressure-Temperature Phase Diagrams of Wheat Starch. In Advances in High Pressure Bioscience and Biotechnology; Springer: Berlin/Heidelberg, Germany, 1999; pp. 333–336. ISBN 978-3-642-60196-5. [Google Scholar]
- Cappa, C.; Lucisano, M.; Barbosa-Cánovas, G.V.; Mariotti, M. Physical and structural changes induced by high pressure on corn starch, rice flour and waxy rice flour. Food Res. Int. 2016, 85, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A. Impact of High Hydrostatic Pressure on Wheat, Tapioca, and Potato Starches. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, August 2005. [Google Scholar]
| Pressure (MPa) | Pressurization Time (min) | Pressure Build-Up (min) |
|---|---|---|
| 0 | 10 | 0 |
| 150 | 10 | 0.75 |
| 300 | 10 | 1.50 |
| 450 | 10 | 2.25 |
| 600 | 10 | 3.00 |
| 450 | 1 | 2.25 |
| 450 | 2 | 2.25 |
| 450 | 5 | 2.25 |
| 450 | 10 | 2.25 |
| 450 | 20 | 2.25 |
| Variation of Pressure (for 10 min Pressurization Time) | Variation of Pressurization Time (at 450 MPa) | ||||||
|---|---|---|---|---|---|---|---|
| Pressure (MPa) | Maltose (mg/g Flour) | Amylose (% of Total Starch) | Total Starch (% DM) | Pressurization Time (min) | Maltose (mg/g Flour) | Amylose (% of Total Starch) | Total Starch (% DM) |
| 0 | 0.8 ± 0.1 A | 31.7 ± 1.7 C | 77.7 ± 0.8 A | 0 | 0.8 ± 0.1 A | 31.7 ± 1.7 C | 77.7 ± 0.8 A |
| 150 | 0.8 ± 0.1 A,B | 33.2 ± 1.2 B,C | 76.8 ± 0.8 A,B | 1 | 0.7 ± 0.1 A | 34.2 ± 1.0 B,C | 76.2 ± 0.9 A,B |
| 300 | 0.6 ± 0.1 B | 35.2 ± 0.5 B,C | 74.0 ± 0.5 B,C | 2 | 0.6 ± 0.1 A | 35.7 ± 0.7 B | 74.7 ± 0.5 B,C |
| 450 | 0.6 ± 0.0 B | 37.0 ± 1.3 A,B | 71.7 ± 0.7 C | 5 | 0.7 ± 0.2 A | 36.2 ± 0.6 B | 73.7 ± 0.3 C,D |
| 600 | 0.6 ± 0.1 B | 40.3 ± 1.2 A | 67.6 ± 1.7 D | 10 | 0.6 ± 0.0 A | 37.0 ± 1.3 A,B | 71.7 ± 0.7 D |
| 20 | 0.6 ± 0.1 A | 40.4 ± 0.7 A | 69.4 ± 0.4 E | ||||
| Variation of Pressure (10 min Pressurization Time) | Variation of Pressurization Time (450 MPa) | ||||||
|---|---|---|---|---|---|---|---|
| Pressure (MPa) | D3,10 (µm) | D3,50 (µm) | D3,90 (µm) | Pressurization Time (min) | D3,10 (µm) | D3,50 (µm) | D3,90 (µm) |
| 0 | 24.2 ± 0.4 A | 90.4 ± 0.8 A | 185.2 ± 5.9 A | 0 | 24.2 ± 0.4 A | 90.4 ± 0.8 A | 185.2 ± 5.9 A |
| 150 | 19.8 ± 0.4 A | 81.5 ± 1.7 B | 184.5 ± 7.3 B | 1 | 17.6 ± 1.1 C | 74.0 ± 4.7 B | 182.8 ± 4.5 A,B,C |
| 300 | 19.9 ± 0.6 A | 82.2 ± 1.5 B | 184.4 ± 2.3 B | 2 | 17.4 ± 1.3 C | 73.5 ± 5.1 B | 179.0 ± 3.3 B,C |
| 450 | 20.2 ± 2.1 A | 81.0 ± 6.5 B | 184.3 ± 6.1 B | 5 | 18.0 ± 1.4 C | 75.9 ± 5.2 B | 177.9 ± 3.0 C |
| 600 | 21.1 ± 2.0 A | 83.4 ± 7.1 B | 191.7 ± 9.9 B | 10 | 20.2 ± 2.1 B | 77.1 ± 4.9 B | 184.3 ± 6.1 A,B |
| 20 | 17.0 ± 0.9 C | 71.7 ± 3.8 B | 179.6 ± 3.7 A,B,C | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobi, S.; Jekle, M.; Becker, T. High-Pressure Treatment of Non-Hydrated Flour Affects Structural Characteristics and Hydration. Foods 2018, 7, 78. https://doi.org/10.3390/foods7050078
Jakobi S, Jekle M, Becker T. High-Pressure Treatment of Non-Hydrated Flour Affects Structural Characteristics and Hydration. Foods. 2018; 7(5):78. https://doi.org/10.3390/foods7050078
Chicago/Turabian StyleJakobi, Sabina, Mario Jekle, and Thomas Becker. 2018. "High-Pressure Treatment of Non-Hydrated Flour Affects Structural Characteristics and Hydration" Foods 7, no. 5: 78. https://doi.org/10.3390/foods7050078
APA StyleJakobi, S., Jekle, M., & Becker, T. (2018). High-Pressure Treatment of Non-Hydrated Flour Affects Structural Characteristics and Hydration. Foods, 7(5), 78. https://doi.org/10.3390/foods7050078





