2.2. Profile by UPLC-DAD-ESI-TQ-MS Analysis
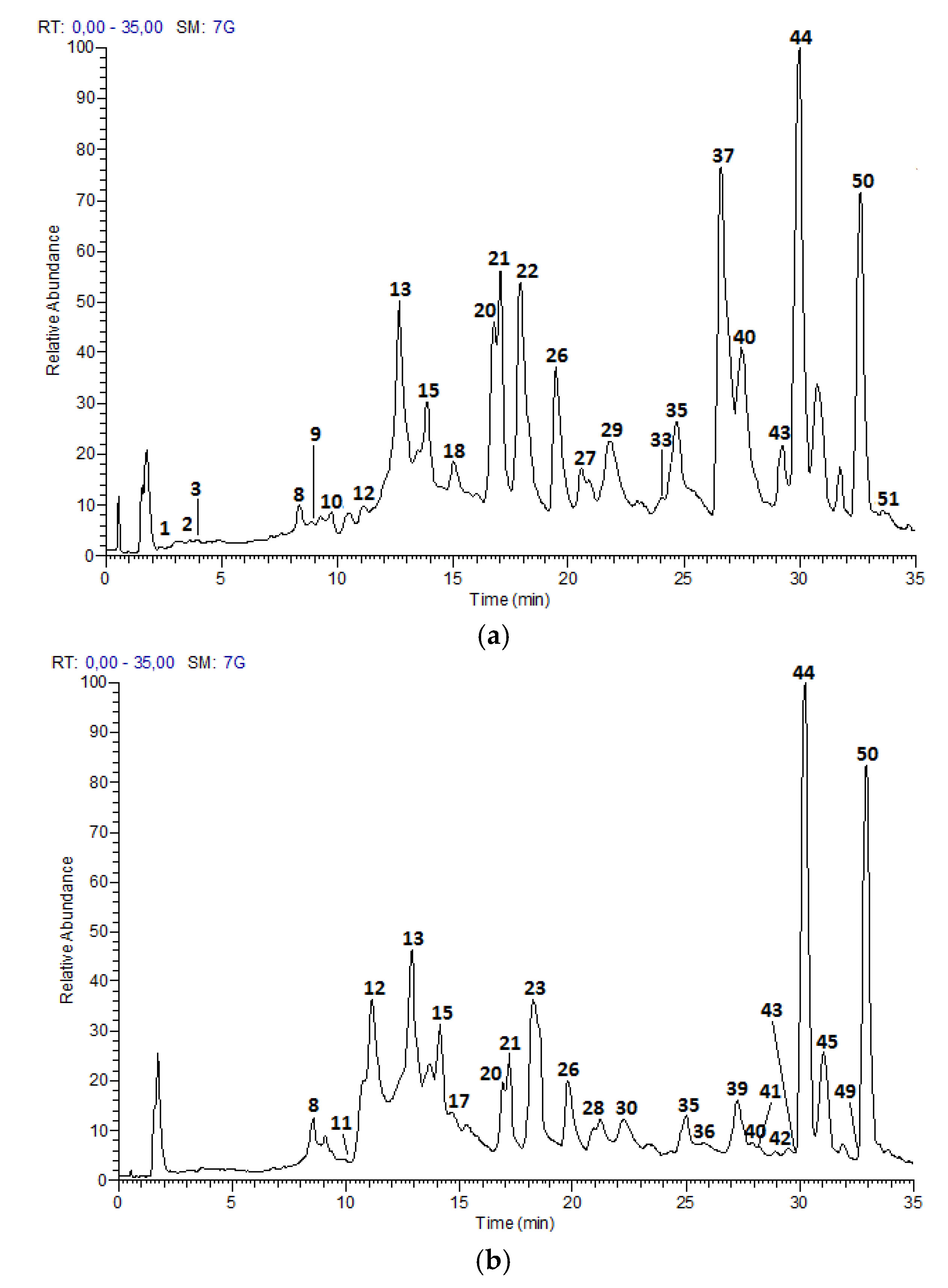
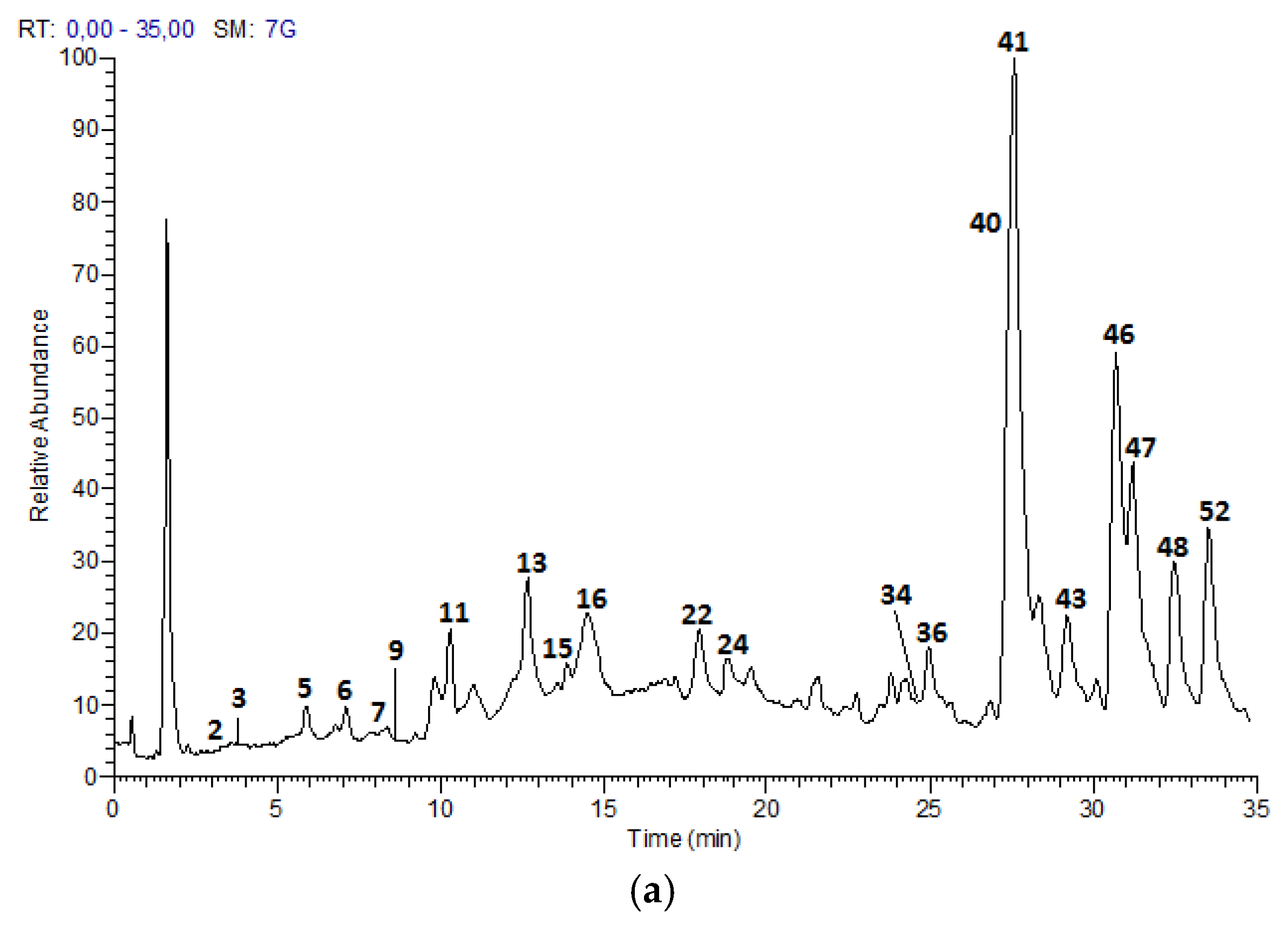
The UPLC-DAD-ESI-MS/MS analysis described in the Materials and Methods section, allowed to identify 52 compounds, including 21 proanthocyanidins and flavan-3-ol monomers, 15 glycosylated flavonols and nine acids and derivatives in Costa Rican apple (Anna cultivar) and plum (Satsuma cultivar) only commercial cultivars; as well as five hydroxy chalcones and two glycosylated isoprenoids characteristic of apple fruits.
Figure 1 and
Figure 2 show the chromatograms of the samples and
Table 2 summarizes the analysis results for the 52 compounds.
The first common group of compounds, proanthocyanidins, corresponded to oligomers of flavan-3-ols catechin and epicatechin. The monomeric units of these proanthocyanidins are linked through a C4-C8 or C4-C6 bond (B-type), which coexist with an additional C2-O-C7 linkage (A-type) [
28]. Peaks 9 (Rt = 8.87 min) and 15 (Rt = 13.97 min) showed a [M − H]
− at
m/
z 289.0710 (C
15H
14O
6) that correspond to monomers catechin or epicatechin. The main MS
2 fragments at
m/
z 245 and 205, occur through the loss of C
2H
4O and C
4H
4O
2 due to retro-Diels-Alder fission (RDA) of ring A [
29].
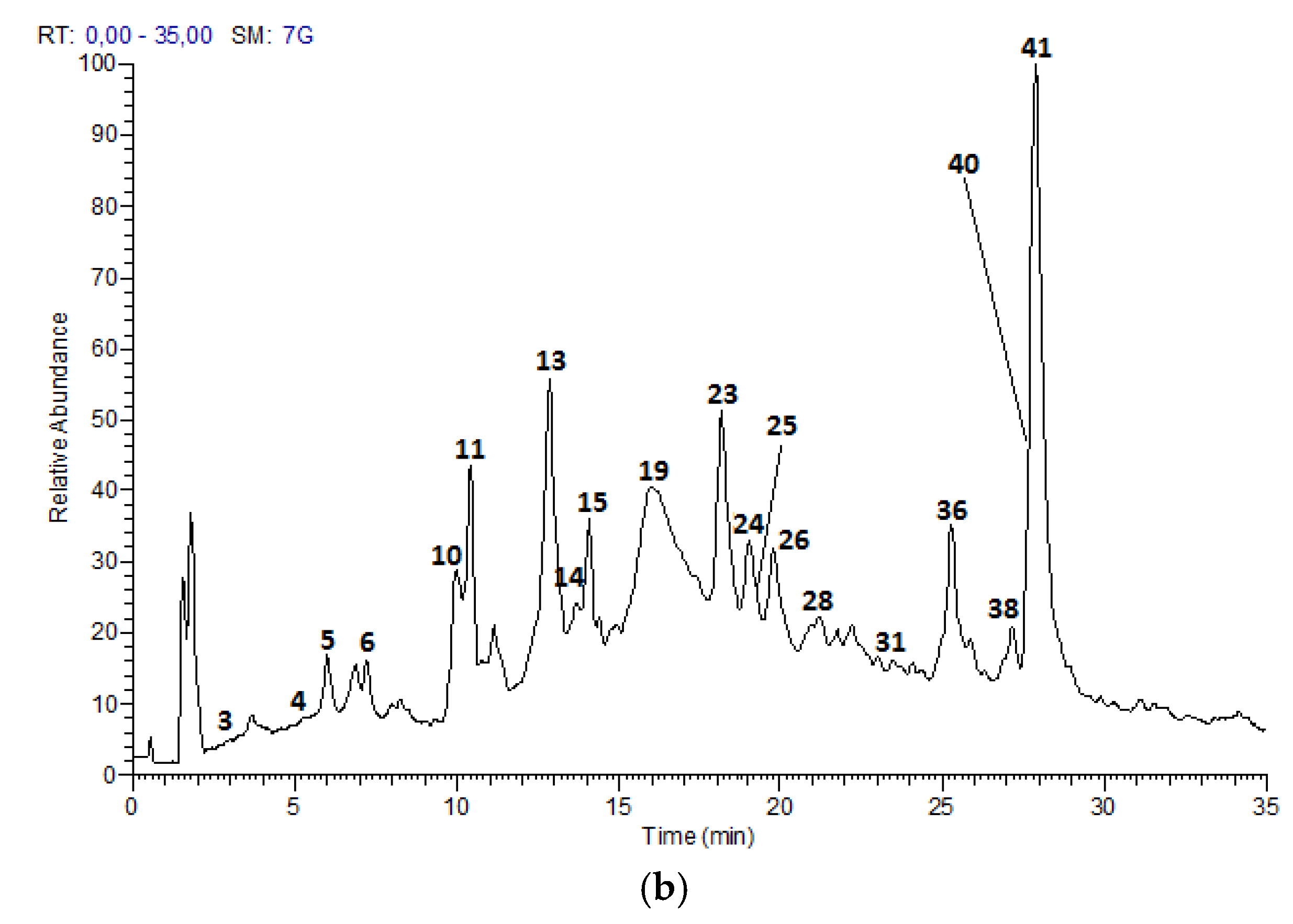
On the other hand, peaks 3 (Rt = 3.96 min), 4 (Rt = 5.40 min), whose [M − H]
− is at
m/
z 441.0819 (C
22H
18O
10) correspond to (epi)catechin-3-
O-gallate (
Figure 3). The main fragment at
m/
z 315 [M – H − 126]
− is due to the elimination of a phloroglucinol, and fragments at
m/
z 289 and 153 are both residuals from the cleavage of the ester group [
30].
Peak 36 (Rt = 25.19 min) shows a [M − H]
− at
m/
z 575.1185 (C
30H
24O
12) and main MS
2 ion at
m/
z 449, which indicate the presence of a procyanidin A-type dimer. The base ion at
m/
z 449 [M – H − 126]
−, corresponds to the elimination of a phloroglucinol molecule from this A-type dimer [
31]. Peaks 24 (Rt = 18.98 min), 25 (Rt = 19.37 min) and 31 (Rt = 23.54 min) show a [M − H]
− at
m/
z 863.1798 (C
45H
36O
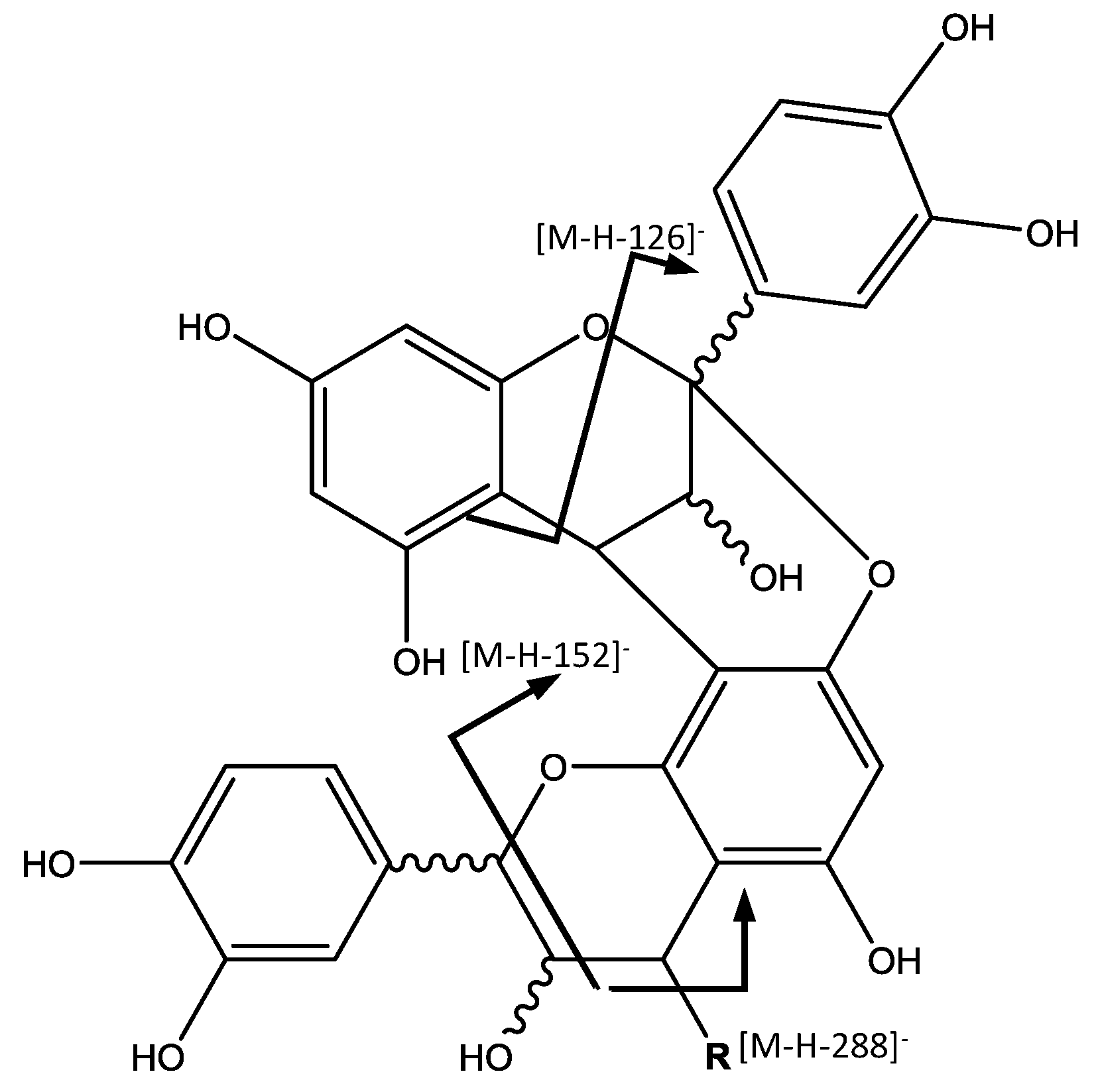
18), revealing the presence of a procyanidin trimer with A-type interflavan linkage (
Figure 4). In the MS
2 spectrum, fragment ions at
m/
z 711 [M – H − 152]
− and 575 [M – H − 288]
− observed, result from the RDA fission and quinone-methide (QM) cleavage, respectively [
32].
Peaks 1 (Rt = 2.69 min), 8 (Rt = 8.44 min), 13 (Rt = 12.75 min), 38 (Rt = 27.21 min) and 42 (Rt = 28.62 min) show [M − H]
− at
m/
z 577.1344 (C
30H
26O
12), corresponding to procyanidins with B-type linkage (
Figure 5), 2 amu (atomic mass units) higher than that of the A-type procyanidin, and major ions containing the structural information at
m/
z 559, 451, 425, 407 and 289. The ion at
m/
z 559 [M – H − 18]
− originates from water loss. The ion at
m/
z 451 [M – 126 − H]
− results from the elimination of the phloroglucinal as in A-type dimers. The fragment ions at
m/
z 425 [M – H − 152]
− and 407 [M – H − 170]
− come from RDA, while the ion at
m/
z 289 originates from QM resulting in the ion of the monomer [
31].
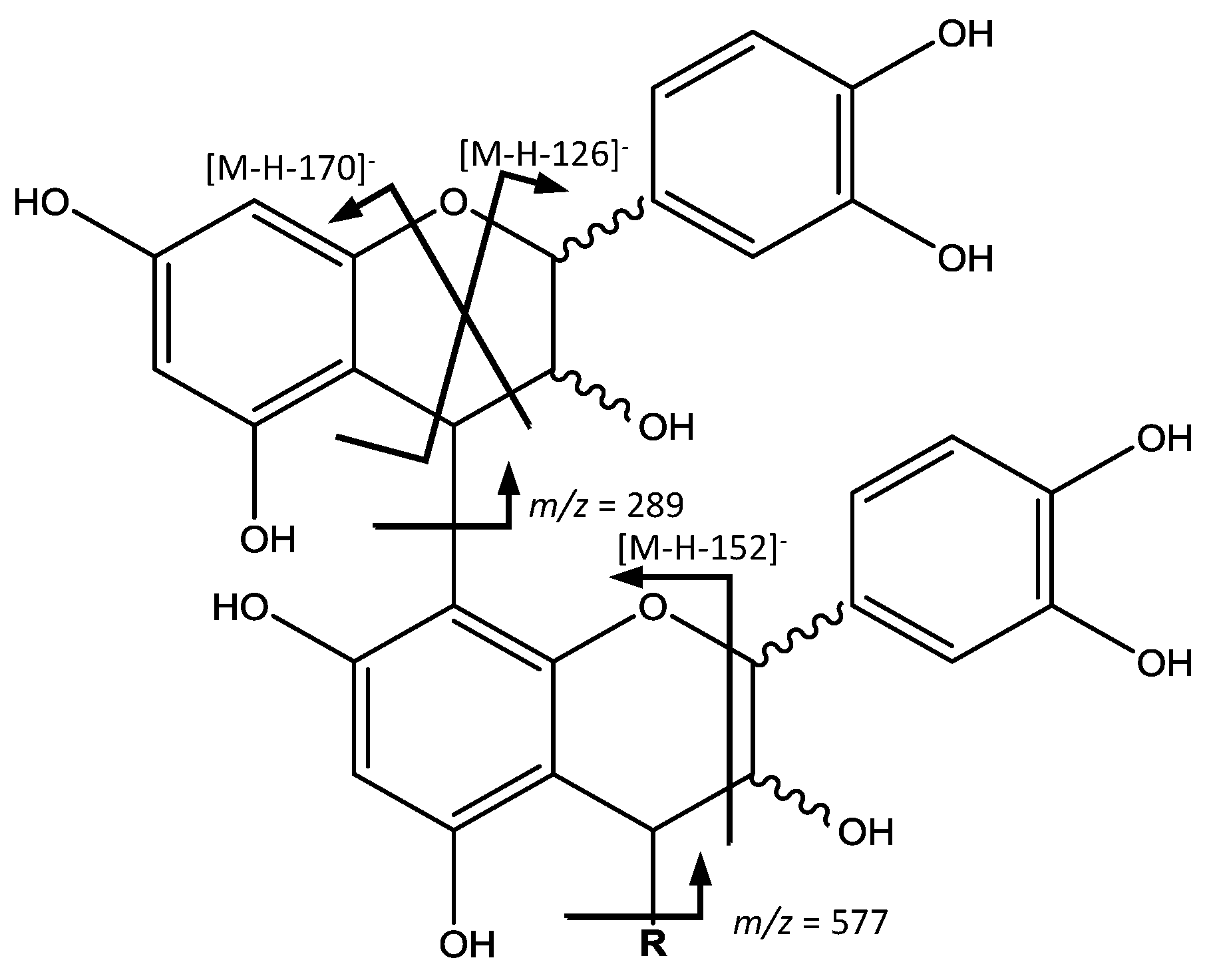
On the other hand (
Figure 5), peaks 14 (Rt = 13.20 min), 22 (Rt = 17.88 min), 23 (Rt = 18.16 min), 28 (Rt = 21.23 min) and 30 (Rt = 22.21 min) with ([M − H]
−) at
m/
z 865.1956 (C
45H
38O
18) were tentatively identified to be procyanidin B-type trimers. Their fragmentation behaviors seem to be similar to that of dimers, with ion fragments at
m/
z 695 [M – H − 170]
−, 713 [M – H − 152]
− and 739 [M – H − 126]
−. The QM cleavage of the interflavan bond mainly produced the ions at
m/
z 289 and 577, indicating the cleavage happens in upper interflavan bond [
31].
In a similar way (
Figure 5), peak 26 (Rt = 19.70 min), [M − H]
− at
m/
z 1153.2603 (C
60H
50O
24) was identified as a procyanidin B-type tetramer, with fragments at
m/
z 1027 [M – H − 126]
−, 1001 [M – H − 152]
−, 983 [M – H − 170]
−, 865 and 577. Also, peaks 27 (Rt = 20.54 min) and 29 (Rt = 21.83 min), with [M − H]
− at
m/
z 1441.3229 (C
75H
62O
30), were identified as two procyanidin B-type pentamers with a characteristic fragment at
m/
z 1315 [M – H − 126]
−, and also those derived from QM cleavage at
m/
z 1151, 865, 577 and 289 [
33].
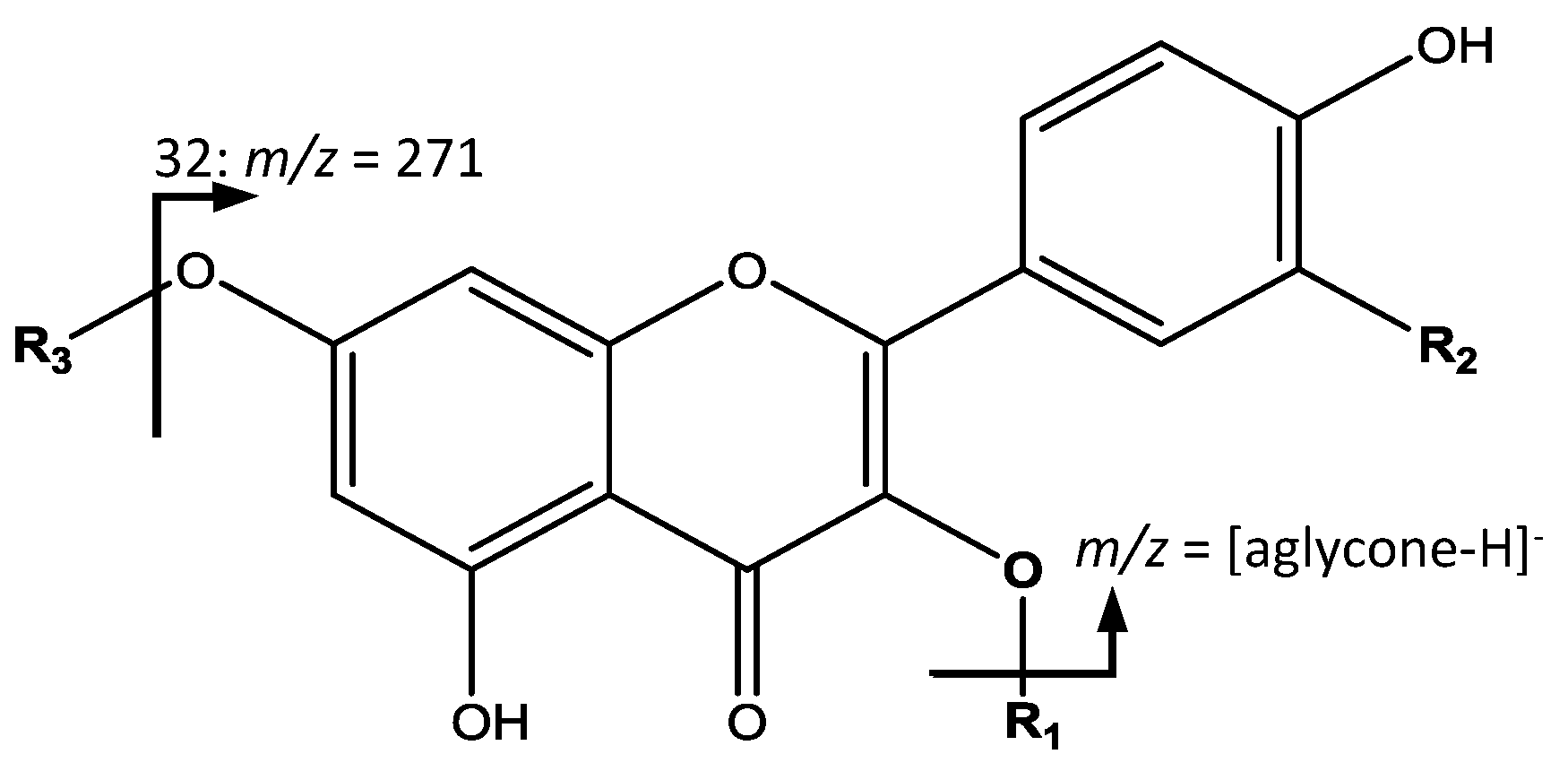
The second group of common compounds, glycosylated flavonol derivatives were elucidated based in the fragmentation pattern from the aglycone due to the loss of glycosides (
Figure 6). For instance, peaks 18 (Rt = 14.97 min) and 19 (Rt = 15.90 min) had [M − H]
− at
m/
z 447.0922 (C
21H
20O
11) were identified as kaempferol-hexoside isomers with a main fragment at
m/
z 285 corresponding to kaempferol [
34]. Peak 32 (Rt = 23.97 min) with [M − H]
− at
m/
z 433.1131 (C
21H
22O
10) showed its main fragment at
m/
z 271, corresponding to naringenin-hexoside [
35].
All the remaining flavonol derivatives presented their main fragment at
m/
z 301, which corresponds to the quercetin aglycone. They differ in the bonded glycoside with some variations among them. For instance, peaks 33 (Rt = 24.12 min) and 34 (Rt = 24.72 min) had a [M − H]
− at
m/
z 595.1284 (C
26H
28O
16), were assigned to quercetin-pentosylhexoside isomers. Peaks 37 (Rt = 26.57 min) and 41 (Rt = 27.79 min), with [M − H]
− at
m/
z 463.0875 (C
21H
20O
12) correspond to quercetin-hexoside isomers. Peak 40 (Rt = 27.60 min), with [M − H]
− at
m/
z 609.1440 (C
27H
30O
16) was identified as quercetin-rutinoside. Peaks 43 (Rt = 29.28 min) and 46 (Rt = 30.69 min) had [M − H]
− at
m/
z 433.0769 (C
20H
18O
11), coincident with isomers of quercetin-pentoside [
36].
Peak 47 (Rt = 31.21 min) had [M − H]
− at
m/
z 565.1184 (C
25H
26O
15), were identified as quercetin-pentosyl-pentoside. Peaks 48 (Rt = 32.46 min), 49 (Rt = 32.57 min) and 51 (Rt = 33.27 min) with [M − H]
− at
m/
z 447.0922 (C
21H
20O
11) were assigned as quercetin-deoxyhexoside isomers. Finally, peak 52 (Rt = 33.51 min) with [M − H]
− at
m/
z 505.0975 (C
23H
22O
13), was identified as quercetin-acetylhexoside [
16].
Among the third group of common compounds, acids and derivatives, two small acids correspond (
Figure 7) to peak 2 (Rt = 3.36 min) with [M − H]
− at
m/
z 153.0191 (C
7H
6O
4) and a main fragment at
m/
z 109 [M – H − 44]
− due to the loss of CO
2 from a carboxylic acid [
37] identified as protocatechuic acid, and peak 16 (Rt = 14.44 min), with [M − H]
− at
m/
z 173.0454 (C
7H
10O
5) and main fragments at
m/
z 111 generated from RDA fission, and 93 from subsequent loss of water assigned to shikimic acid [
38].
On the other hand, a series of
p-coumaric acid derivatives was identified, as shown in
Figure 8. For instance, peaks 5 (Rt = 5.95 min) and 12 (Rt = 11.10 min), with [M − H]
− at
m/
z 353.0869 (C
16H
18O
9), are identified as caffeoylquinic acid isomers, with main fragments at
m/
z 191 [quinic acid−H]
−, 179 [caffeic acid−H]
−, and 145 due to the loss of CO
2 from the quinic acid ion. Peak 6 (Rt = 7.23 min) shows [M − H]
− at
m/
z 341.0872 (C
15H
18O
9), with main fragments at
m/
z 179 [caffeic acid − H]
− and 161 [M – H − 179]
−, corresponding to caffeoyl-hexoside. Peak 7 (Rt = 8.30 min) with [M − H]
− at
m/
z 163.0398 (C
9H
6O
3), is identified as coumaric acid due to the fragment at 119 [M – H − CO
2]
−. Peaks 10 (Rt = 9.94 min) and 11 (Rt = 10.27 min), with [M − H]
− at
m/
z 325.0921 (C
15H
18O
8) are assigned to coumaroyl-hexoside isomers, due to fragments at
m/
z 163 [coumaric acid−H]
− and 145 [coumaric acid−H−H
2O]
−. Another cinnamic acid derivative was found in peak 17 (Rt = 14.49 min) with [M − H]
− at
m/
z 337.0927 (C
16H
18O
8), and a main fragment at
m/
z 173 due to the loss of water of the quinic acid ion, thus corresponding to
p-coumaroylquinic acid [
16].
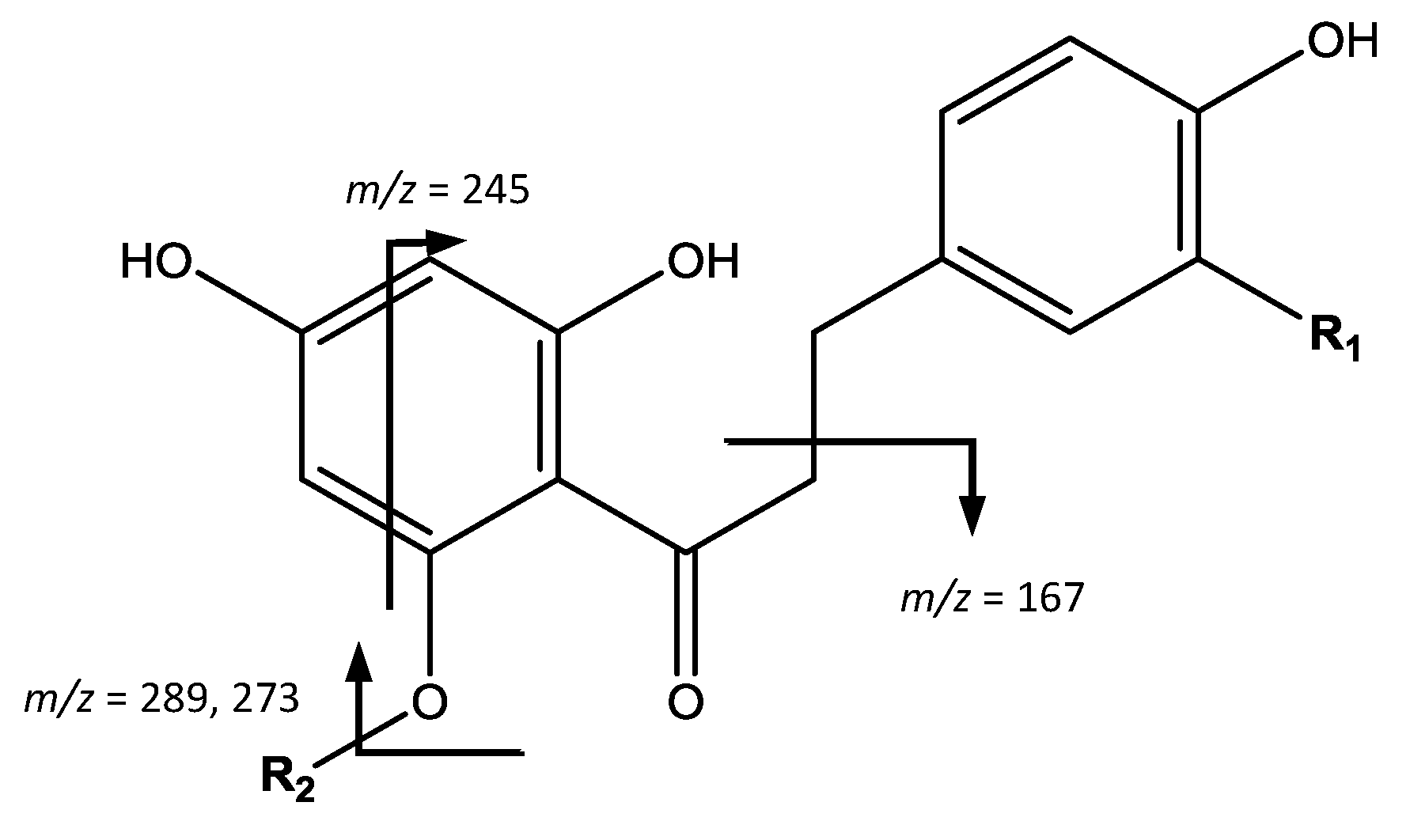
The fourth group of compounds, chalcones, shown in
Figure 9, were found only in apples. For instance, peak 35 (Rt = 24.87 min) shows [M − H]
− at
m/
z 583.1660 (C
26H
32O
15) and the main fragment at
m/
z 289, which correspond to 3-hydroxyphloretin aglycone, allowing to identify the compound as 3-hydroxyphloretin-pentosylhexoside [
11]. Peak 39 (Rt = 27.29 min) shows [M − H]
− at
m/
z 289.0716 (C
15H
14O
6), the same mass and molecular formula of flavan-3-ols, but differs in the fragmentation that occurs at
m/
z 271 [M – H − H
2O]
− due to the loss of water, 245 coming from RDA, and 167 due to α-cleavage of the carbonyl group [
39] therefore being assigned as 3-hydroxyphloretin.
On the other hand, peaks 44 (Rt = 30.11 min) and 45 (Rt = 31.04 min) with [M − H]
− at
m/
z 567.1704 (C
26H
32O
14) were identified as phloretin-pentosylhexoside isomers, due to the main fragment at
m/
z 273 which corresponds to the phloretin ion generated from loss of glycosides. Finally, peak 50 (Rt = 32.78 min), [M − H]
− at
m/
z 273.0767 (C
15H
14O
5) is assigned as phloretin, with a main fragment at
m/
z 167 due to α-cleavage of the carbonyl group [
11,
39].
The fifth group of compounds, glycosylated isoprenoid derivatives, shown in
Figure 10, was found only in apple. Peaks 20 (Rt = 16.80 min) and 21 (Rt = 17.10 min) showed [M − H]
− at
m/
z 517.2280 (C
24H
38O
12). Their main fragments at
m/
z 385 [M – H − 132]
− and 205 [M – H − 312]
− correspond to the loss of a pentoside and a pentosylhexoside respectively. The resulting ion is coincident with vomifoliol, allowing the peaks to be assigned to vomifoliol-pentosylhexoside isomers [
40].
Polyphenol profiling reveals great similarities between skins and flesh, with a high number of compounds and high diversity for both fruits. When comparing the reports for apple cultivars in the literature, our results for Anna cultivar from Costa Rica show greater number and diversity of polyphenols than the findings on sixteen cultivars from Norway, Italy, Canada and United States [
7,
8,
9,
10], and similar to only two cultivars, namely Golden Delicious and Braeburn from Slovenia [
11]. Further, findings on proanthocyanidins indicate better results both in total occurrence and in greater polymerization degree, for instance procyanidin tetramers and pentamers in the skins of Costa Rican Anna apple cultivar.
In the case of plums, when comparing the reports of twenty-four cultivars from United States, Germany and Portugal [
15,
16,
17,
18], greater number and diversity of polyphenols is observed in the skins of Satsuma cultivar from Costa Rica. Likewise, results for Satsuma flesh are also superior to twenty-three of the cultivars, being similar to President cultivar from Germany. Of special interest is the presence of a greater number of procyanidins oligomers, such as procyanidin trimers and one tetramer as well as of glycosylated flavonols, showing different quercetin derivatives not reported in previous characterizations of plum skins.
It is important to highlight the characterization of proanthocyanidin oligomers present in both fruits, which is of special interest due to procyanidins antioxidant results having been reported to increase with higher degree of polymerization, for instance trimers, tetramers and pentamers showing better results than dimers or flavan-3-ol monomers [
20,
21]. The detailed characterization of these fruit cultivars provides an important contribution expanding the knowledge for their exploitation as available sources of such diversity of polyphenols, which in turn can be of interest for further research due to their potential biological activities.