Carbon Monoxide in Meat and Fish Packaging: Advantages and Limits
Abstract
:1. Introduction
2. Fresh Meat Packaging Methods
2.1. Raw Meat Spoilage-Associated Storage Conditions
2.1.1. Microbial Spoilage
2.1.2. Lipid Oxidation
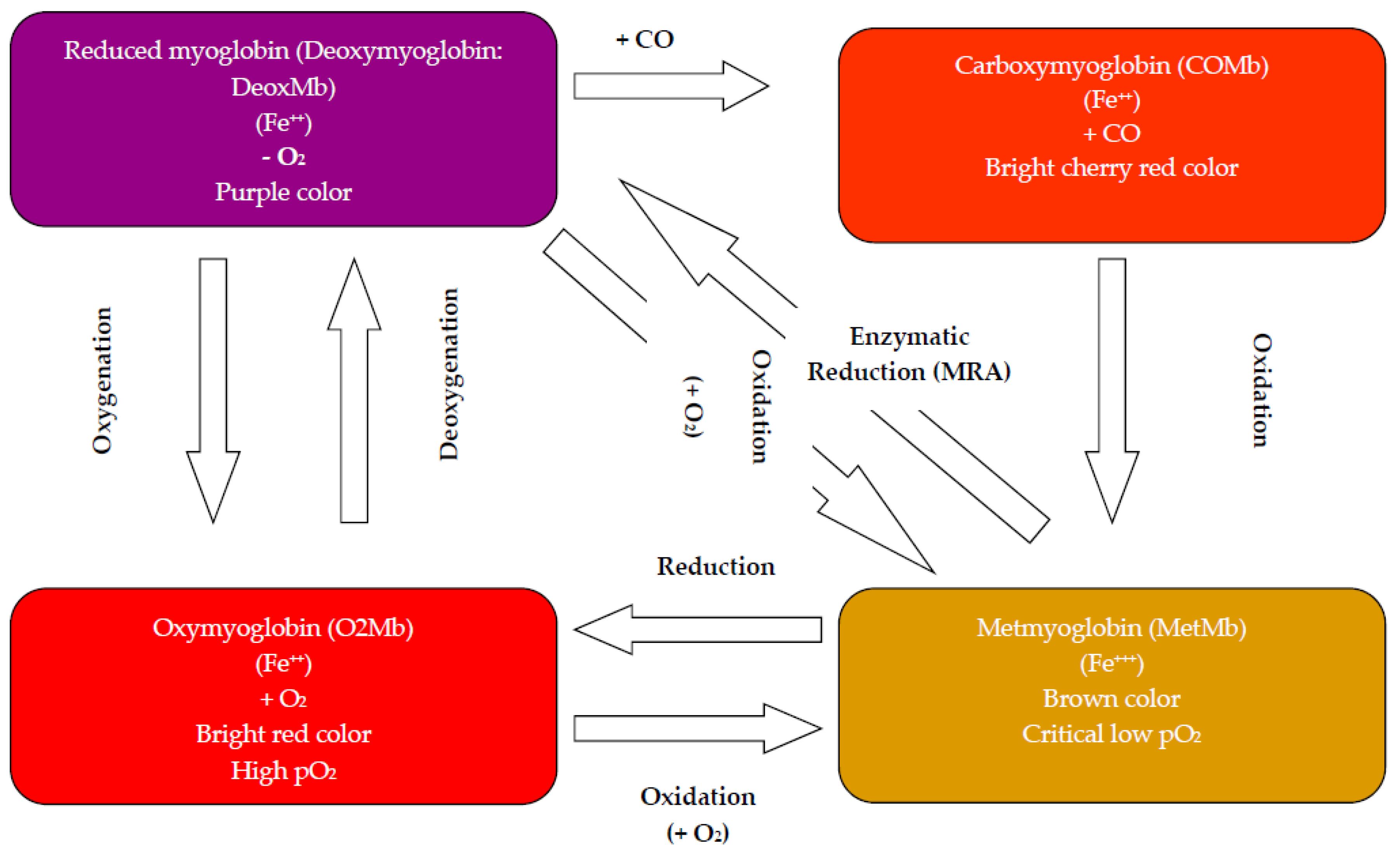
2.1.3. Pigment Oxidation
2.1.4. Photooxidation
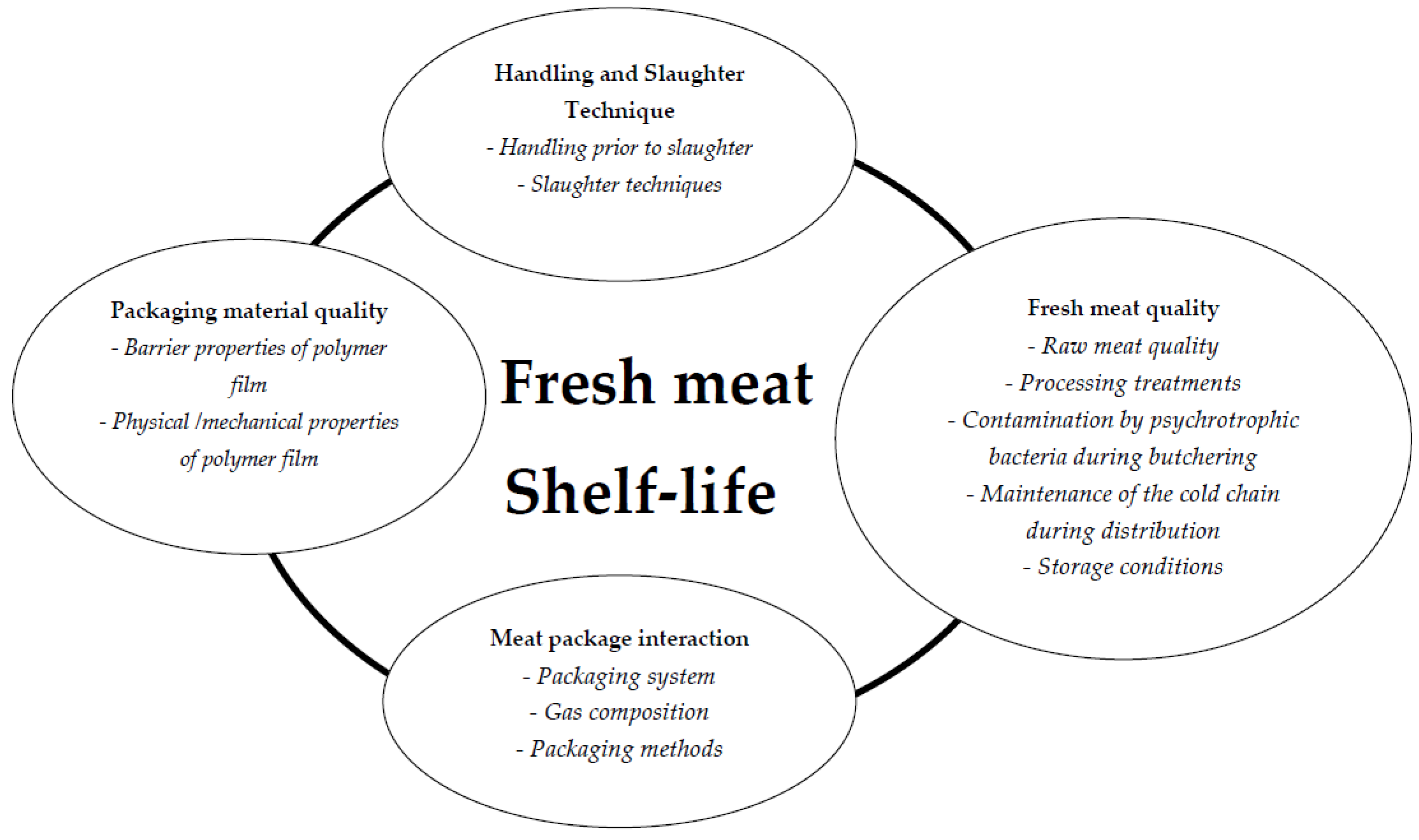
2.2. Fresh Meat Shelf-Life
2.3. Packaging Options
2.3.1. Emergence of MAP Case-Ready Meat Products
2.3.2. High O2 MAP
Gases Used in MAP
2.3.3. Vacuum Packaging
2.3.4. Safety of MAP
3. CO in Fresh Meat Packaging
3.1. What Is Carbon Monoxide?
3.2. Health Implications of CO
3.3. CO Application in Meat Packaging
3.3.1. Color Stabilizing and Shelf-Life Effects
3.3.2. Antimicrobial Effects
3.3.3. Other Effects
3.3.4. CO in Other Foods
Fruits and Vegetables
Seafood and Poultry
3.3.5. Co Pre-Treatments
4. Consumer’s Perceptions
5. Analysis of CO in Food
6. Safety Consideration of CO-Treated Meat
7. Regulatory Status for the Use of CO in MAP Systems
8. Could the Application of CO for Meat Packaging Be Re-Considered?
9. Conclusions
- The main disadvantage of using CO for meat packaging is the concern about masking of the microbiological risk by the formation of the stable and longer, bright red color.
- A combination of low levels of CO with CO2 in high concentrations, which reduce the growth of microorganisms, is of key importance to justify approval by EU regulatory agencies.
- It is also imperative that CO risk management be implemented in the packaging operations. The supermarkets and consumers at home must handle meat with strict hygienic standards, and low storage temperatures must be kept in a continuous chill chain.
- CO followed by vacuum packaging is promising because of the possibility of better adjusting the color stability of the meat to the time of spoilage.
- By adopting CO for meat and fish MAP, the meat industry must label the packages with reliable times for maximum shelf-life (the use-by date).
- The EU considers packaging gases as additives, and CO is not on the list of such gases. In order for CO to be approved as an additive within the EU, the following criteria must be met according to Directive 94/34/EEC. “They present no hazard to the heath of consumer at the level of use proposed, so far as can be judged on the scientific evidence available; to provide aids in manufacture, processing, preparation, treatment, packing, transport or storage of food, provided that the additive is not used to disguise the effects of the use of faulty raw materials or of undesirable (including unhygienic) practices or techniques during the course of any of these activities and to assess the possible harmful effects by evaluation any cumulative, synergistic or potentiating effect of its use and the phenomenon of human intolerance to substances foreign to the body”.
- In the EU, current labeling regulations require packages with meat and meat products in MAP to be labeled with “Packaged in a protective atmosphere”. The specific gases do not need to be declared on the packages. Conventional gases will probably be the ones most concerned by this statement. Low concentrations of CO in food packaging systems could be required to appear on the product label to inform consumers of its use in the product.
- Analysis of CO can be used to control whether muscle food products have been treated with CO or not, despite not having been labeled as such. These considerations underline the suitability to develop better alternative fast methods to detect even small amounts of the CO-Mb adduct in muscle tissues, in regard to the fraudulent treatment of meat and fish in the MAP systems.
Acknowledgments
Author’s Contributions
Conflicts of Interest
References
- Renerre, M.; Labadie, J. Fresh meat packaging and meat quality. In Proceedings of the 39th International Congress of Meat Science and Technology, Calgary, AB, Canada, 1–6 August 1993. Session 8. [Google Scholar]
- Jakobsen, M.; Bertelsen, G. Color stability and lipid oxidation of fresh beef—Development of a response surface model for predicting the effects of temperature, storage time, and modified atmosphere composition. Meat Sci. 2000, 54, 49–57. [Google Scholar] [CrossRef]
- Jayasingh, P.; Cornforth, D.P.; Brennand, C.P.; Carpenter, C.E.; Whittier, D.R. Sensory evaluation of ground beef stored in high-oxygen modified atmosphere packaging. J. Food Sci. 2002, 67, 3493–3496. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine Longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, G.; Lagerstedt, A.; Ertbjerg, P.; Sampels, S.; Lundström, K. Ageing of large cuts of beef loin in vacuum or high oxygen modified atmosphere, effect on shear force, calpain activity, desmin degradation and protein oxidation. Meat Sci. 2010, 85, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Hague, M.A.; Warren, K.E.; Hunt, M.C.; Kropf, D.H.; Kastner, C.L.; Stroda, S.L.; Johnson, D.E. Endpoint temperature, internal cooked color, and expressible juice color relationships in ground beef patties. J. Food Sci. 1994, 59, 465–470. [Google Scholar] [CrossRef]
- Killinger, K.M.; Hunt, M.C.; Campbell, R.E.; Kropf, D.H. Factors affecting premature browning during cooking of store-purchased ground beef. J. Food Sci. 2000, 65, 585–587. [Google Scholar] [CrossRef]
- Warren, K.E.; Hunt, M.C.; Kropf, D.H. Myoglobin oxidative state affects internal cooked color development in ground beef patties. J. Food Sci. 1996, 61, 513–515. [Google Scholar] [CrossRef]
- Lyon, B.G.; Berry, B.W.; Soderberg, D.; Clinch, N. Visual color and doneness indicators and the incidence of premature brown color in beef patties cooked to four end point temperatures. J. Food Prot. 2000, 63, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- USFDA. GRAS Notice Number GRN 000143; United States Food and Drug Administration: Washington, DC, USA, 2004.
- Cornforth, D.P.; Hunt, M.C. Low-oxygen packaging of fresh meat with carbon monoxide: Meat quality, microbiology, and safety. In The American Meat Science Association (AMSA) White Paper Series Number 2; American Meat Science Association: Savoy, IL, USA, 2008. [Google Scholar]
- Brewer, M.S.; Wu, S.; Field, R.A.; Ray, B. Carbon monoxide effects on color and microbial counts of vacuum packaged beef steaks in refrigerated storage. J. Food Qual. 1994, 17, 231–236. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Lanier, T.C.; Carpenter, J.A.; Toledo, R.T.; Regan, J.O. Metmyoglobin reduction in beef systems as affected by aerobic, anaerobic, and carbon monoxide-containing environments. J. Food Sci. 1978, 43, 1788–1792. [Google Scholar] [CrossRef]
- Meischen, H.W.; Huffman, D.L.; Davis, G.W. Branded beef—Product of tomorrow today. In Proceedings of the 40th Reciprocal Meat Conference, Saint Paul, MN, USA, 28 June–1 July 1987; pp. 37–46. [Google Scholar]
- Sørheim, O.; Nissen, H.; Nesbakken, T. The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide. Meat Sci. 1999, 52, 157–164. [Google Scholar] [CrossRef]
- Grebitus, C.; Jensen, H.H.; Roosen, J. US and German consumer preferences for ground beef packaged under a modified atmosphere—Different regulations, different behaviour? Food Policy 2013, 40, 109–118. [Google Scholar] [CrossRef]
- Djenane, D.; Beltrán, J.A.; Camo, J.; Roncalés, P. Influence of vacuum at different ageing times and subsequent retail display on shelf life of beef cuts packaged with active film under high O2. J. Food Sci. Technol. 2016, 53, 4244–4257. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Sanchéz, A.; Beltrán, J.A.; Roncalés, P. The shelf life of beef steaks treated with DL-lactic acid and antioxidants and stored under modified atmospheres. Food Microbiol. 2003, 20, 1–7. [Google Scholar] [CrossRef]
- Sanchéz, A.; Djenane, D.; Torrescano, G.; Beltran, J.A.; Roncalés, P. The effects of ascorbic acid, taurine, carnosine and rosemary powder on color and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001, 58, 421–429. [Google Scholar] [CrossRef]
- Djenane, D.; Sanchéz, A.; Beltrán, J.A.; Roncalés, P. Ability of α-tocopherol, taurine and rosemary, in combination with vitamin C, to increase the oxidative stability of beef steaks packaged in modified atmosphere. Food Chem. 2002, 76, 407–415. [Google Scholar] [CrossRef]
- Ledward, D.A. Metmyoglobin formation in beef stored in carbon dioxide enriched and oxygen depleted atmospheres. J. Food Sci. 1970, 35, 33–37. [Google Scholar] [CrossRef]
- Kropf, D.H. Effects of retail display conditions on meat color. Recipr. Meat Conf. Proc. 1980, 33, 15–32. [Google Scholar]
- Zakrys, P.I.; Hogan, S.A.; O’Sullivan, M.G.; Allen, P.; Kerry, J.P. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 2008, 79, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Wongwichian, C.; Klomklao, S.; Panpipat, W.; Benjakul, S.; Chaijan, M. Interrelationship between myoglobin and lipid oxidations in oxeye scad (Selar boops) muscle during iced storage. Food Chem. 2015, 174, 279–285. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, D.B. Changes in the colour and opacity of meat. Food Chem. 1982, 9, 75–88. [Google Scholar] [CrossRef]
- Djenane, D.; Sanchéz, A.; Beltrán, J.A.; Roncalés, P. Extension of the retail display life of fresh beef packaged in modified atmosphere by varying lighting conditions. J. Food Sci. 2001, 66, 181–185. [Google Scholar] [CrossRef]
- Walker, H.W. Effects of Microflora on Fresh Meat Color. Recipr. Meat Conference Proc. 1980, 33, 33–36. [Google Scholar]
- Bertelsen, G.; Boegh-Soerensen, L. The effect of lighting on color of beef. In Proceedings of the Institutional Investors Roundtable (IIR)–Meeting (Commission C2), Bristol, UK, 10–12 September 1986; pp. 433–438. [Google Scholar]
- Andersen, H.J.; Skibsted, L.H. Oxidative stability of frozen pork patties, effect of light and added salt. J. Food Sci. 1991, 56, 1182–1184. [Google Scholar] [CrossRef]
- Sánchez-Escalante, A.; Torrescano, G.; Djenane, D.; Beltrán, J.A.; Giménez, B.; Roncalés, P. Effect of antioxidants and lighting conditions on color and lipid stability of beef patties packaged in high-oxygen modified atmosphere. CyTA J. Food. 2011, 9, 49–57. [Google Scholar] [CrossRef]
- Martínez, L.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of illumination on the display life of fresh pork sausages packaged in modified atmosphere. Influence of the addition of rosemary, ascorbic acid and black pepper. Meat Sci. 2007, 75, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.E. Factors affecting the rate of metmyoglobin accumulation in pre-packaged beef. Meat Sci. 1980, 4, 247–265. [Google Scholar] [CrossRef]
- Aurand, L.W.; Boone, N.H.; Gidding, G.G. Superoxide and singlet oxygen in milk lipid peroxidation. J. Dairy Sci. 1977, 60, 363–369. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Dugan, L.R. Studies in photooxidation of olive oil. J. Am. Oil Chem. Soc. 1985, 62, 892–896. [Google Scholar] [CrossRef]
- Luby, J.M.; Gray, J.I.; Harte, B.R. Effects of Packaging and Light Source on the Oxidative Stability of Cholesterol in butter. J. Food Sci. 1986, 51, 908–911. [Google Scholar] [CrossRef]
- Whang, K.; Peng, I.C. Photosensitized Lipid Peroxidation in Ground Pork and Turkey. J. Food Sci. 1988, 53, 1596–1598. [Google Scholar] [CrossRef]
- Lee, C.K.; Li, S.; Hui, S.Y. A design methodology for smart LED lighting systems powered by weakly regulated renewable power grids. IEEE Trans. Smart Grid 2011, 2, 548–554. [Google Scholar] [CrossRef]
- Mahalik, N.P. Advances in Packaging Methods, Processes and Systems. Challenges 2014, 5, 374–389. [Google Scholar] [CrossRef]
- Djenane, D.; Sanchéz, A.; Beltrán, J.A.; Roncalés, P. Extension of the shelf life of beef steaks packaged in a modified atmosphere by treatment with rosemary and display under UV-free lighting. Meat Sci. 2003, 64, 417–426. [Google Scholar] [CrossRef]
- Siegel, D.G. Case-ready concepts: Packaging technologies. In Proceedings of the Western Science Research Update Conference on Technologies for Improving the Quality and Safety of Case-Ready Products, Annual Meeting of the National Meat Association, Las Vegas, NV, USA, 22 February 2001. [Google Scholar]
- Gill, C. The solubility of carbon dioxide in meat. Meat Sci. 1988, 22, 65–71. [Google Scholar] [CrossRef]
- Gee, D.L.; Brown, W.D. Extension of shelf life in refrigerated ground beef stored under an atmosphere containing carbon dioxide and carbon monoxide. J. Agric. Food Chem. 1978, 26, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Seideman, S.C.; Durland, P.R. The utilization of modified gas atmosphere packaging for fresh meat: A review. J. Food Qual. 1984, 6, 239–252. [Google Scholar] [CrossRef]
- Wolfe, S.K. Use of CO- and CO2-enriched atmospheres for meats, fish, and produce. Food Technol. 1980, 34, 55–58. [Google Scholar]
- Jongberg, S.; Wen, J.; Tørngren, M.A.; Lund, M.N. Effect of high-oxygen atmosphere packaging on oxidative stability and sensory quality of two chicken muscles during chill storage. Food Packag. Shelf Life 2014, 1, 38–48. [Google Scholar] [CrossRef]
- Brewer, M.S.; Wu, S.Y. Display, packaging and meat block location effects on color and lipid oxidation of frozen lean ground beef. J. Food Sci. 1993, 58, 1219–1223. [Google Scholar] [CrossRef]
- Li, X.; Lindahl, G.; Zamaratskaia, G.; Lundström, K. Influence of vacuum skin packaging on color stability of beef Longissimus lumborum compared with vacuum and high-oxygen modified atmosphere packaging. Meat Sci. 2012, 92, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, Å.; Ahnström, M.L.; Lundström, K. Vacuum skin pack of beef—A consumer friendly alternative. Meat Sci. 2011, 88, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.E.; Kropf, D.H. Packaging Vacuum. In Encyclopedia of Meat Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 26–33. [Google Scholar]
- Djenane, D.; Martinez, L.; Sanchéz, A.; Beltrán, J.A.; Roncalés, P. Antioxidant effect of carnosine and carnitine in fresh beef steaks stored under modified atmosphere. Food Chem. 2004, 85, 453–459. [Google Scholar] [CrossRef]
- Sanchéz-Escalante, A.; Djenane, D.; Torrescano, G.; Beltrán, J.A.; Roncalés, P. Antioxidant action of borage, rosemary, oregano and ascorbic acid in beef patties packaged in modified atmosphere. J. Food Sci. 2003, 68, 339–344. [Google Scholar] [CrossRef]
- Sørheim, O.; Aune, T.; Nesbakken, T. Technological, hygienic and toxicological aspects of carbon monoxide used in modified-atmosphere packaging of meat. Trends Food Sci. Technol. 1997, 8, 307–312. [Google Scholar] [CrossRef]
- Ishiwata, H.; Takeda, Y.; Kawasaki, Y.; Yoshida, R.; Sugita, T.; Sakamoto, S. Concentration of carbon monoxide in commercial fish flesh exposed to carbon monoxide gas for colour fixing. J. Food Hyg. Soc. Jpn. 1996, 37, 83–90. [Google Scholar] [CrossRef]
- Durante, W.; Schafer, A.I. Carbon monoxide and vascular cell function. Int. J. Mol. Med. 1998, 2, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Dauphin, J.F.; Saint Lebe, L.R. Radiation chemistry of carbohydrates. In Radiation Chemistry of Major Food Components; Elias, P.S., Cohen, A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 131–185. [Google Scholar]
- Nam, K.C.; Ahn, D.U. Carbon monoxide-heme pigment is responsible for the pink color in irradiated raw turkey breast meat. Meat Sci. 2002, 60, 25–33. [Google Scholar] [CrossRef]
- Nam, K.C.; Ahn, D.U. Mechanisms of pink color formation in irradiated precooked turkey breast meat. J. Food Sci. 2002, 67, 600–607. [Google Scholar] [CrossRef]
- Kim, Y.H.; Nam, K.C.; Ahn, D.U. Color, Oxidation-Reduction Potential, and Gas Production of Irradiated Meats from Different Animal Species. J. Food Sci. 2002, 67, 1692–1695. [Google Scholar] [CrossRef]
- Ismail, H.A.; Lee, E.J.; Ko, K.Y.; Paik, H.D.; Ahn, D.U. Effect of Antioxidant Application Methods on the Color, Lipid Oxidation, and Volatiles of Irradiated Ground Beef. J. Food Sci. 2009, 74, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Ahn, D.U. Sources and Mechanisms of Carbon Monoxide Production by Irradiation. J. Food Sci. 2004, 69, C485–C490. [Google Scholar] [CrossRef]
- Lee, E.J.; Ahn, D.U. Sources and mechanisms of carbon monoxide production by irradiation. In Proceedings of the Annual meeting of the Institute of Food Technologists, Chicago, IL, USA, 16–20 June 2003. Session 76E. [Google Scholar]
- Furuta, M.; Dohmaru, T.; Katayama, T.; Torantoni, H.; Takeda, A. Detection of irradiated frozen meat and poultry using carbon monoxide gas as a probe. J. Agric. Food Chem. 1992, 40, 1099–1100. [Google Scholar] [CrossRef]
- Haldane, J. The relation of the action of carbonic oxide to oxygen tension. J. Physiol. 1895, 18, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Lambooy, E.; Spanjaard, W. Euthanasia of young pigs with carbon monoxide. Vet. Rec. 1980, 107, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.W.; Nañagas, K.A. Carbon monoxide poisoning. Med. Clin. N. Am. 2005, 89, 1161–1194. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.W.; Nañagas, K.A. Carbon monoxide poisoning. Emerg. Med. Clin. N. Am. 2004, 22, 985–1018. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.R.; Lin, Y.T.; Hwang, B.F. Air pollution and newly diagnostic autism spectrum disorders: A population-based cohort study in Taiwan. PLoS ONE 2013, 8, e75510. [Google Scholar] [CrossRef] [PubMed]
- Frydman, M. The smoking addiction of pregnant women and the consequences on their offspring’s intellectual development. J. Environ. Pathol. Toxicol. Oncol. 1996, 15, 169–172. [Google Scholar] [PubMed]
- Brunori, M.; Vallone, B. A globin for the brain. FASEB J. 2006, 20, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Barinaga, M. Carbon monoxide: Killer to brain messenger in one step. Science 1993, 15, 259–309. [Google Scholar] [CrossRef]
- Verma, A.; Hirsch, D.J.; Glatt, C.E.; Ronnett, G.V.; Snyder, S.H. Carbon monoxide: A putative neural messenger. Science 1993, 259, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Bani-Hani, M.G.; Motterlini, R. Use of carbon monoxide as a therapeutic agent: Promises and challenges. Intensiv. Care Med. 2008, 34, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Zuckerbraun, B.S.; Haga, M.; Liu, F.; Song, R.; Usheva, A.; Stachulak, C.; Bodyak, N.; Smith, R.N.; Csizmadia, E.; et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat. Med. 2003, 9, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Mitchell-Flack, M.J.; Wang, A.; Levy, R.J. Carbon monoxide modulates cytochrome oxidase activity and oxidative stress in the developing murine brain during isoflurane exposure. Free Radic. Biol. Med. 2015, 86, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Pannen, B.H. Carbon monoxide—From mitochondrial poisoning to therapeutic use. Crit. Care 2009, 13, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hsu, A.; Moore, P.K. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation—A tale of three gases! Pharmacol. Ther. 2009, 123, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Soni, H.; Pandya, G.; Patel, P.; Acharya, A.; Jain, M.; Mehta, A.A. Beneficial effects of carbon monoxide-releasing molecule-2 (CORM-2) on acute doxorubicin cardiotoxicity in mice: Role of oxidative stress and apoptosis. Toxicol. Appl. Pharmacol. 2011, 253, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Roderique, J.D.; Josef, C.S.; Feldman, M.J.; Spiess, B.D. A modern literature review of carbon monoxide poisoning theories, therapies, and potential targets for therapy advancement. Rev. Toxicol. 2015, 334, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Joe, Y.; Yu, J.K.; Chen, Y.; Jeong, S.O.; Mani, N.; Cho, G.J.; Pae, H.O.; Ryter, S.W.; Chung, H.T. Carbon monoxide protects against hepatic ischemia/reperfusion injury by modulating the miR-34a/SIRT1 pathway. Biochim. Biophys. Acta 2015, 1852, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Onyiah, J.C.; Sheikh, S.Z.; Maharshak, N.; Steinbach, E.C.; Russo, S.M.; Kobayashi, T.; Mackey, L.C.; Hansen, J.J.; Moeser, A.J.; Rawls, J.F.; et al. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology 2013, 144, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Steiger, C.; Lühmann, T.; Meinel, L. Oral drug delivery of therapeutic gases-Carbon monoxide release for gastrointestinal diseases. J. Control. Release 2014, 189, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, B.H.P.; Janz, J.A.M.; Morel, P.C.H.; Purchas, R.W.; Hendriks, W.H. The effect of modified atmosphere packaging with carbon monoxide on the storage quality of master-packaged fresh pork. Meat Sci. 2006, 73, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Sørheim, O.; Nissen, H.; Aune, T.; Nesbakken, T. Use of carbon monoxide in retail meat packaging. In Proceedings of the 54th Reciprocal Meat Conference, Indianapolis, IN, USA, 24–28 July 2001; pp. 47–51. [Google Scholar]
- De Santos, F.; Rojas, M.; Lockhorn, G.; Brewer, M.S. Effect of carbon monoxide in modified atmosphere packaging, storage time and endpoint cooking temperature on the internal color of enhanced pork. Meat Sci. 2007, 77, 520–528. [Google Scholar] [CrossRef] [PubMed]
- El-Badawi, A.A.; Cain, R.F.; Samuels, C.E. Anglemeier, A.F. Color and pigment stability of packaged refrigerated beef. Food Technol. 1964, 18, 159–163. [Google Scholar]
- Luño, M.; Roncalés, P.; Djenane, D.; Beltrán, J.A. Beef shelf life in low O2 and high CO2 atmospheres containing different low CO concentrations. Meat Sci. 2000, 55, 413–419. [Google Scholar] [CrossRef]
- Nissen, H.; Alvseike, O.; Bredholt, S.; Hoick, A.; Nesbakken, T. Comparison between the growth of Yersinia enterocolitica, Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella spp. in ground beef packaged by three commercially used packaging techniques. Int. J. Food Microbiol. 2000, 59, 211–220. [Google Scholar] [CrossRef]
- Jayasingh, P.; Cornforth, D.P.; Carpenter, C.E.; Whittier, D. Evaluation of carbon monoxide treatment in modified atmosphere packaging or vacuum packaging to increase color stability of fresh beef. Meat Sci. 2001, 59, 317–324. [Google Scholar] [CrossRef]
- Kusmider, E.A.; Sebranek, J.G.; Lonergan, S.M.; Honeyman, M.S. Effects of carbon monoxide packaging on color and lipid stability of irradiated ground beef. J. Food Sci. 2002, 67, 3463–3468. [Google Scholar] [CrossRef]
- Krause, T.R.; Sebranek, J.G.; Rust, R.E.; Honeyman, M.S. Use of carbon monoxide packaging for improving the shelf life of pork. J. Food Sci. 2003, 68, 2596–2603. [Google Scholar] [CrossRef]
- John, L.; Cornforth, D.P.; Carpenter, C.E.; Sørheim, O.; Pettee, B.; Whittier, D.R. Comparison of color and thiobarbituric acid (TBA) values of cooked hamburger patties after storage of fresh beef chubs in modified atmospheres. J. Food Sci. 2004, 69, 608–614. [Google Scholar] [CrossRef]
- John, L.; Cornforth, J.; Carpenter, C.E.; Sørheim, O.; Pettee, B.C.; Whittier, D.R. Color and thiobarbituric acid values of cooked top sirloin steaks packaged in modified atmospheres of 80% oxygen, or 0.4% carbon monoxide, or vacuum. Meat Sci. 2005, 69, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Djenane, D.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of different concentrations of carbon dioxide and low concentration of carbon monoxide on the shelf-life of fresh pork sausages packaged in modified atmosphere. Meat Sci. 2005, 71, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Wicklund, R.A.; Paulson, D.D.; Tucker, E.M.; Stetzer, A.J.; De Santos, F.; Rojas, M.; MacFarlane, B.J.; Brewer, M.S. Effect of carbon monoxide and high oxygen modified atmosphere packaging and phosphate enhanced, case-ready pork chops. Meat Sci. 2006, 74, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Sørheim, O.; Langsrud, Ø.; Cornforth, D.P.; Johannessen, T.C.; Slinde, E.; Berg, P.; Nesbakken, T. Carbon Monoxide as a Colorant in Cooked or Fermented Sausages. J. Food Sci. 2006, 71, 549–555. [Google Scholar] [CrossRef]
- Stetzer, A.J.; Wicklund, R.A.; Paulson, D.D.; Tucker, E.M.; Macfarlane, B.J.; Brewer, M.S. Effect of carbon monoxide and high oxygen modified atmosphere packaging (MAP) on quality characteristics of beef strip steaks. J. Muscle Foods 2007, 18, 56–66. [Google Scholar] [CrossRef]
- Aspé, E.; Roeckel, M.; Martí, M.C.; Jiménez, R. Effect of pre-treatment with carbon monoxide and film properties on the quality of vacuum packaging of beef chops. Packag. Technol. Sci. 2008, 21, 395–404. [Google Scholar] [CrossRef]
- Mantilla, D.; Kristinsson, H.G.; Balaban, M.O.; Otwell, W.S.; Chapman, F.A.; Raghavan, S. Color stability of frozen whole tilapia exposed to pre-mortem treatment with carbon monoxide. J. Sci. Food Agric. 2008, 88, 1394–1399. [Google Scholar] [CrossRef]
- Linares, M.B.; Bórnez, R.; Vergara, H. Effect of stunning systems on meat quality of Manchego suckling lamb packed under modified atmospheres. Meat Sci. 2008, 78, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Linares, M.B.; Vergara, H. Effect of gas stunning and modified-atmosphere packaging on the quality of meat from Spanish Manchego light lamb. Small Rumin. Res. 2012, 108, 87–94. [Google Scholar] [CrossRef]
- Grobbel, J.P.; Dikemen, M.E.; Hunt, M.C.; Milliken, G.A. Effects of different packaging atmospheres and injection–enhancement on beef tenderness, sensory attributes, desmin degradation, and display color. J. Anim. Sci. 2008, 86, 2697–2710. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, L.; Toshkov, S.; Brewer, M.S. Effects of carbon monoxide-modified atmosphere packaging and irradiation on E. coli K12 survival and raw beef quality. Meat Sci. 2009, 83, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Suman, S.P.; Konda, M.K.R.; Ramanathan, R. Effect of carbon monoxide packaging and lactate enhancement on the color stability of beef steaks stored at 1 °C for 9 days. Meat Sci. 2009, 81, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Fontes, P.R.; Gomide, L.A.M.; Fontes, E.A.F.; Ramos, E.M.; Ramos, A.L.S. Composition and color stability of carbon monoxide treated dried porcine blood. Meat Sci. 2010, 85, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Raines, C.R.; Hunt, M.C. Headspace Volume and Percentage of Carbon Monoxide Affects Carboxymyoglobin Layer Development of Modified Atmosphere Packaged Beef Steaks. J. Food Sci. 2010, 75, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Claus, J.R. Color stability of ground beef packaged in a low carbon monoxide atmosphere or vacuum. Meat Sci. 2011, 87, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bjørlykke, G.A.; Roth, B.; Sørheim, O.; Kvammeb, B.O.; Slinde, E. Effects of carbon monoxide on Atlantic salmon (Salmo salar L.). Food Chem. 2011, 127, 1706–1711. [Google Scholar] [CrossRef]
- Suman, S.P.; Mancini, R.A.; Joseph, P.; Ramanathan, R.; Konda, M.K.R.; Dady, G.; Yinb, S. Chitosan inhibits premature browning in ground beef. Meat Sci. 2011, 88, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, L.; Toshkov, S.; Brewer, M.S. Effects of irradiation on color and sensory characteristics of carbon monoxide-modified atmosphere packaged beef. J. Food Process. Preserv. 2011, 35, 701–707. [Google Scholar] [CrossRef]
- Pivarnik, LF.; Faustman, C.; Rossi, S.; Suman, S.P.; Palmer, C.; Richard, N.L.; Ellis, P.C.; DiLiberti, M. Quality Assessment of Filtered Smoked Yellowfin Tuna (Thunnus albacares) Steaks. J. Food Sci. 2011, 76, S369–S379. [Google Scholar] [CrossRef] [PubMed]
- Venturini, A.C.; Faria, J.A.F.; Olinda, R.A.; Contreras-Castillo, C.J. Shelf Life of Fresh Beef Stored in Master Packages with Carbon Monoxide and High Levels of Carbon Dioxide. Packag. Technol. Sci. 2014, 27, 29–35. [Google Scholar] [CrossRef]
- Lavieri, N.; Williams, S.K. Effects of packaging systems and fat concentrations on microbiology, sensory and physical properties of ground beef stored at 4 ± 1 °C for 25 days. Meat Sci. 2014, 97, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Yang, X.; Liang, R.; Mao, Y.; Hou, X.; Lu, X.; Luo, X. Potential mechanisms of carbon monoxide and high oxygen packaging in maintaining color stability of different bovine muscles. Meat Sci. 2014, 97, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Concollato, A.; Parisi, G.; Olsen, R.E.; Kvamme, B.O.; Slinde, E.; Dalle Zotte, A. Effect of carbon monoxide for Atlantic salmon (Salmo salar L.) slaughtering on stress response and fillet shelf life. Aquaculture 2014, 433, 13–18. [Google Scholar] [CrossRef]
- Rogers, H.B.; Brooks, J.C.; Martin, J.N.; Tittor, A.; Miller, M.F.; Brashears, M.M. The impact of packaging system and temperature abuse on the shelf life characteristics of ground beef. Meat Sci. 2014, 97, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.D.; Gomide, L.A.M.; Cecon, P.R.; Fontes, E.A.F.; Fontes, P.R.; Ramos, E.M.; Vidigal, J.G. Evaluation of mortadella formulated with carbon monoxide-treated porcine blood. Meat Sci. 2014, 97, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Fontes, P.R.; Gomide, L.A.M.; Costa, N.M.B.; Peternelli, L.A.; Fontes, E.A.F.; Ramos, E.M. Chemical composition and protein quality of mortadella formulated with carbon monoxide-treated porcine blood. LWT-Food Sci. Technol. 2015, 64, 926–931. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhu, L.; Han, M.; Gao, S.; Luo, X. Effect of packaging atmospheres on storage quality characteristics of heavily marbled beef longissimus steaks. Meat Sci. 2016, 117, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sakowska, A.; Guzek, D.; Głąbska, D.; Wierzbicka, A. Carbon monoxide concentration and exposure time effects on the depth of CO penetration and surface color of raw and cooked beef Longissimus lumborum steaks. Meat Sci. 2016, 121, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Shen, K.; Ding, Y.; Ma, X. Effect of pretreatment with carbon monoxide and ozone on the quality of vacuum packaged beef meats. Meat Sci. 2016, 117, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Van Rooyen, L.A.; Allen, P.; Crawley, S.M.; O’Connor, D.I. The effect of carbon monoxide pretreatment exposure time on the colour stability and quality attributes of vacuum packaged beef steaks. Meat Sci. 2017, 129, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Sakowska, A.; Guzek, D.; Sun, D.-W.; Wierzbicka, A. Effects of 0.5% carbon monoxide in modified atmosphere packagings on selected quality attributes of M. Longissimus dorsi beef steaks. J. Food Proc. Eng. 2017, 40, 12517–12527. [Google Scholar] [CrossRef]
- Van Rooyen, L.A.; Allen, P.; O’Connor, D.I. The application of carbon monoxide in meat packaging needs to be re-evaluated within the EU: An overview. Meat Sci. 2017, 132, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Djenane, D.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of varying oxygen concentrations on the shelf-life of fresh pork sausages packaged in modified atmosphere. Food Chem. 2006, 94, 219–225. [Google Scholar] [CrossRef]
- Sanchéz, A.; Torrescano, G.; Djenane, D.; Beltrán, J.A.; Roncalés, P. Stabilisation of colour and odour of beef patties by using lycopene-rich tomato and peppers as a source of antioxidants. J. Sci. Food Agric. 2003, 83, 187–194. [Google Scholar] [CrossRef]
- Ledward, D.A. Post-slaughter influences on the formation of metmyoglobin in beef muscles. Meat Sci. 1985, 15, 149–171. [Google Scholar] [CrossRef]
- Woodruff, R.; Silliker, J. Process and Composition for Producing and Maintaining Good Color in Fresh Meat, Fresh Poultry and Fresh Fish. U.S. Patent No. 4,522,835, 11 June 1985. Available online: http://www.google.com/patents?vid=USPAT4522835 (accessed on 23 January 2008).
- Clydesdale, F.M.; Francis, F.J. The chemistry of meat color. Food Prod. Dev. 1971, 15, 581–584. [Google Scholar]
- Grant, M.; Clay, B. Accidental carbon monoxide poisoning with severe cardiorespiratory compromise in 2 children. Am. J. Crit. Care 2002, 11, 128–131. [Google Scholar] [PubMed]
- Seyfert, M.; Mancini, R.A.; Hunt, M.C.; Tang, J.; Faustman, C. Influence of carbon monoxide in package atmospheres containing oxygen on colour, reducing activity, and oxygen consumption of five bovine muscles. Meat Sci. 2007, 75, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Claus, J.R. Color stability and reversion in carbon monoxide packaged ground beef. Meat Sci. 2010, 85, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Lentz, C.P. Effect of Light Intensity and Other Factors on the Color of Frozen Prepackaged Beef. Can. Inst. Food Sci. Technol. J. 1979, 12, 47–50. [Google Scholar] [CrossRef]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.; Faustman, C. Metmyoglobin reducing activity: Review. Meat Sci. 2005, 71, 407–439. [Google Scholar] [CrossRef] [PubMed]
- Sammel, L.M.; Hunt, M.C.; Kropf, D.H.; Hachmeister, K.A.; Johnson, D.E. Comparison of Assays for Metmyoglobin Reducing Ability in Beef Inside and Outside Semimembranosus Muscle. J. Food Sci. 2002, 67, 978–984. [Google Scholar] [CrossRef]
- King, D.A.; Shackelford, S.D.; Rodriguez, A.B.; Wheeler, T.L. Effect of time of measurement on the relationship between metmyoglobin reducing activity and oxygen consumption to instrumental measures of beef longissimus color stability. Meat Sci. 2011, 87, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Laury, A.; Sebranek, J.G. Use of Carbon Monoxide Combined with Carbon Dioxide for Modified Atmosphere Packaging of Pre- and Postrigor Fresh Pork Sausage To Improve Shelf Life. J. Food Prot. 2007, 70, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Stratakos, A.C. Application of Modified Atmosphere Packaging and Active/Smart Technologies to Red Meat and Poultry: A Review (Review). Food Bioprocess Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- Kudra, L.L.; Sebranek, J.G.; Dickson, J.S.; Mendonca, A.F.; Zhang, Q.; Jackson-Davis, A.; Prusa, K.J. Control of Campylobacter jejuni in Chicken Breast Meat by Irradiation Combined with Modified Atmosphere Packaging Including Carbon Monoxide. J. Food Prot. 2012, 75, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Luño, M.; Beltrán, J.A.; Roncalès, P. Shelf-life extension and colour stabilisation of beef packaged in a low O2 atmosphere containing CO: Loin steaks and ground meat. Meat Sci. 1998, 48, 75–84. [Google Scholar] [CrossRef]
- Gee, D.L.; Brown, W.D. The effect of carbon monoxide on bacterial growth. Meat Sci. 1981, 5, 215–222. [Google Scholar] [CrossRef]
- Clark, D.S.; Lentz, C.P.; Roth, L.A. Use of carbon monoxide for extending shelf-life of prepackaged fresh beef. Can. Inst. Food Sci. Technol. J. 1976, 9, 114–117. [Google Scholar] [CrossRef]
- Viana, E.; Comide, L.; Vanetti, M. Effect of modified atmospheres on microbiological, color and sensory properties of refrigerated pork. Meat Sci. 2005, 71, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.C.; Mancini, R.A.; Hachmeister, K.A.; Kropf, D.H.; Merriman, M.; DelDuca, G.; Milliken, G. Carbon monoxide in modified atmosphere packaging affects color, shelf life, and microorganisms of beef steaks and ground beef. J. Food Sci. 2004, 69, C45–C52. [Google Scholar] [CrossRef]
- Bórnez, R.; Linares, M.B.; Vergara, H. Microbial quality and lipid oxidation of Manchega breed suckling lamb meat: Effect of stunning method and modified atmosphere packaging. Meat Sci. 2009, 83, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Bórnez, R.; Linares, M.B.; Vergara, H. Effect of different gas stunning methods on Manchega suckling lamb meat packed under different modified atmospheres. Meat Sci. 2010, 84, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S. Irradiation effects on meat color—A review. Meat Sci. 2004, 68, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Irradiation effects on meat flavor: A review. Meat Sci. 2009, 81, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, K.; Murano, E.A.; Olson, D.G. Survival of heat-shocked Yersinia enterocolitica after irradiation in ground pork. Int. J. Food Microbiol. 1998, 39, 133–137. [Google Scholar] [CrossRef]
- Seyfert, M.; Mancini, R.A.; Hunt, M.C. Internal premature browning in cooked steaks from enhanced beef round muscles packaged in high oxygen and ultra-low oxygen modified atmospheres. J. Food Sci. 2004, 69, 721–725. [Google Scholar] [CrossRef]
- Mermelstein, N.H. Success or failure? How the food technology industrial achievement award winners have fared over the years. Food Technol. 1977, 31, 36–58. [Google Scholar]
- Kader, A.A. Physiological and biochemical effects of carbon monoxide added to controlled atmospheres on fruits. Acta Hortic. 1983, 138, 221–225. [Google Scholar] [CrossRef]
- Mullan, M.; McDowell, D. Modified atmosphere packaging. In Food Packaging Technology; Coles, R., McDowell, D., Kirwan, M., Eds.; Blackwell Publishing: London, UK, 2003. [Google Scholar]
- Kader, A.A. Advances in CA/MA Applications. Available online: http://ucanr.edu/datastoreFiles/234-105.pdf (accessed on 23 November 2000).
- Cantwell, M. Postharvest Handling Systems: Minimally Processed Fruits and Vegetables; University of California, Cooperative Extension Vegetable Research and Information Center: Oakland, CA, USA, 2005; Available online: http://vric.ucdavis.edu/selectnewtopic.minproc.htm (accessed on 2 December 2007).
- Zhang, S.; Zhu, L.; Dong, X. Combined Treatment of Carbon Monoxide and Chitosan Reduced Peach Fruit Browning and Softening During Cold Storage. Int. J. Nutr. Food Sci. 2015, 4, 477–482. [Google Scholar]
- Zhang, S.; Yu, Y.; Xiao, C.; Wang, X.; Tian, Y. Effect of carbon monoxide on browning of fresh-cut lotus root slice in relation to phenolic metabolism. LWT-Food Sci. Technol. 2013, 53, 555–559. [Google Scholar] [CrossRef]
- Feng Wang, D.S.; Jayas, N.D.G.; White, P.F. Combined effect of carbon monoxide mixed with carbon dioxide in air on the mortality of stored-grain insects. J. Stored Prod. Res. 2009, 45, 247–253. [Google Scholar] [CrossRef]
- Anderson, C.R.; Wu, W.-H. Analysis of carbon monoxide in commercially treated tuna (Thunnus spp.) and mahi–mahi (Coryphaena hippurus) by gas chromatography/mass spectrometry. J. Agric. Food Chem. 2005, 53, 7019–7023. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, B. Tasteless Smoke Sources, Specifications, and Controls. In Modified Atmospheric Processing and Packaging of Fish: Filtered Smokes, Carbon Monoxide, and Reduced Oxygen Packaging; Otwell, W.S., Kristinsson, H.G., Balaban, M.O., Eds.; Blackwell Publishing Professional: Ames, IA, USA, 2006. [Google Scholar]
- Kristinsson, H.; Balaban, M.; Otwell, W.S. Microbial and quality consequences of aquatic foods treated with carbon monoxide or filtered wood smoke. In Modified Atmospheric Processing and Packaging of Fish; Filtered Smokes, Carbon Monoxide Reduced Oxygen Packaging; Otwell, W.S., Kristinsson, H.G., Balaban, M.O., Eds.; Blackwell: Ames, IA, USA, 2006; pp. 65–86. [Google Scholar]
- Kristinsson, H.; Balaban, M.; Otwell, W.S. The influence of carbon monoxide and filtered smoke on fish muscle colour. In Modified Atmospheric Processing and Packaging of Fish; Filtered Smokes, Carbon Monoxide Reduced Oxygen Packaging; Otwell, W.S., Kristinsson, H.G., Balaban, M.O., Eds.; Blackwell: Ames, IA, USA, 2006; pp. 29–52. [Google Scholar]
- Mantilla, D.; Kristinsson, H.G.; Balaban, M.O.; Otwell, W.S.; Chapman, F.A.; Raghavan, S. Carbon monoxide treatments to impart and retain muscle colour in tilapia fillets. J. Food Sci. 2008, 73, C390–C399. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.-J.; Hsieh, P.-P.; Tsai, M.-L.; Chu, Y.-J. Quality changes during iced and frozen storage of tuna flesh treated with carbon monoxide gas. J. Food Drug Anal. 1998, 6, 622–623. [Google Scholar]
- Fraqueza, M.J.; Ferreira, M.F.; Ouakinin, J.S.; Barreto, A.S. Effect of carbon monoxide and argon in sliced turkey meat under modified atmosphere packaging (preliminary assays). In Proceedings of the 46th International Congress of Meat Science and Technology, Buenos Aires, Argentina, 27 August–1 September 2000; pp. 760–761. [Google Scholar]
- Rozbeh, M.; Kalchayanand, N.; Field, R.A.; Johnson, M.C.; Ray, B. The influence of biopreservatives on the bacterial level of refrigerated vacuum packaged beef. J. Food Saf. 1993, 13, 99–111. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Cornforth, D.P.; Whittier, D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001, 57, 359–363. [Google Scholar] [CrossRef]
- Brooks, J.C.; Alvarado, M.; Stephens, T.P.; Kellermeier, J.D.; Tittor, A.W.; Miller, M.F.; Brashears, M.M. Spoilage and safety characteristics of ground beef packaged in traditional and modified atmosphere packages. J. Food Prot. 2008, 71, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Torngren, M.A.; Madsen, N.T. Scandinavian consumer preference for beef steaks packed with or without oxygen. Meat Sci. 2010, 85, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.C.; Sørheim, O.; Slinde, E. Color and heat denaturation of myoglobin forms in ground beef. J. Food Sci. 1999, 64, 847–851. [Google Scholar] [CrossRef]
- Grebitus, C.; Jensen, H.; Roosen, J.; Sebranek, J. Fresh meat packaging: Consumer acceptance of modified atmosphere packaging including carbon monoxide. J. Food Prot. 2013, 76, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Kawasaki, Y.; Kubota, H.; Namiki, T.; Iizuka, T.; Shionoya, N.; Yoshii, N.; Ohara, R.; Tanaka, M.; Kobayashi, H.; et al. Improvement of carbon monoxide analysis in fish meat. J. Food Hyg. Soc. Jpn. 2011, 52, 130–134. [Google Scholar] [CrossRef]
- Droghetti, E.; Bartolucci, G.L.; Focardi, C.; Bambagiotti-Alberti, M.; Nocentini, M.; Smulevich, G. Development and validation of a quantitative spectrophotometric method to detect the amount of carbon monoxide in treated tuna fish. Food Chem. 2011, 128, 1143–1151. [Google Scholar] [CrossRef]
- Bartolucci, G.; Droghetti, E.; Focardi, C.; Bambagiotti-Alberti, M.; Nocentini, M.; Smulevich, G. High throughput headspace GC-MS quantitative method to measure the amount of carbon monoxide in treated tuna fish. J. Mass Spectrom. 2010, 45, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Carbon monoxide. In Environmental Health Criteria, 213; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- EPA. National Ambient Air Quality Standards (NAAQS). Available online: http://www.epa.gov/air/criteria.html (accessed on 9 December 2007).
- Church, N. Developments in modified-atmosphere packaging and related technologies. Trends Food Sci. Technol. 1994, 5, 345–352. [Google Scholar] [CrossRef]
- Madani, I.M.; Khalfan, S.; Khalfan, H.; Jidah, J.; Aladin, M.N. Occupational exposure to carbon monoxide during charcoal meat grilling. Sci. Total Environ. 1992, 114, 141–147. [Google Scholar] [CrossRef]
- European Commission (EC). Health & Consumer Protection Directorate-General. Opinion of the Scientific Committee on Food on the Use of Carbon Monoxide as Component of Packaging Gases IN Modified Atmosphere Packaging. 13 December 2001. Available online: http://europa.eu.int/comm/food/fs/sc/scf/index_en.html (accessed on 18 December 2001).
- Eilert, E.J. New packaging technologies for the 21st century. Meat Sci. 2005, 71, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.A.; Wolfe, S.K.; Brown, W.D. Fate of 14C carbon monoxide in cooked or stored ground beef samples. J. Agric. Food Chem. 1978, 26, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.-J.; Chu, Y.-J. Effect of heating on residual carbon monoxide content in co-treated tuna and myoglobin. J. Food Biochem. 2004, 28, 476–487. [Google Scholar] [CrossRef]
- Soni, S.; Andhare, V.V. Quantitative Analysis of Carbon Monoxide in Frozen Foods. Res. J. Recent Sci. 2015, 4, 76–80. [Google Scholar]
- EPA. Air Trends—Carbon Monoxide—National Trends in CO Levels. Available online: http://www.epa.gov/airtrends/carbon.htm (accessed on 7 December 2007).
- Djenane, D. Antioxidant and Antimicrobial Systems for Shelf-Life Extension of Fresh Beef. Ph.D. Thesis, Department of Animal Production and Food Science, Faculty of Veterinary Sciences, Zaragoza University, Zaragoza, Spain, 24 April 2004; p. 275. [Google Scholar]
- Australia New Zealand Food Standards Code—Standard 1.3.3—Processing Aids. Available online: http://www.foodstandards.gov.au/code/Documents/1.3.3%20Processing%20aids%20v157.pdf (accessed on 1 March 2016).
- Sebranek, J.; Hunt, M.C.; Cornforth, D.P.; Brewer, M.S. Perspective—Carbon monoxide packaging of fresh meat. Food Technol. 2006, 60, 184. [Google Scholar]
- Sebranek, J.; Houser, T. Use of CO for red meats: Current research and recent regulatory approvals. In Modified Atmospheric Processing and Packaging of Fish; Otwell, W.S., Ed.; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- USFDA. GRAS Notice Number GRN 000083; United States Food and Drug Administration: Washington, DC, USA, 2002.
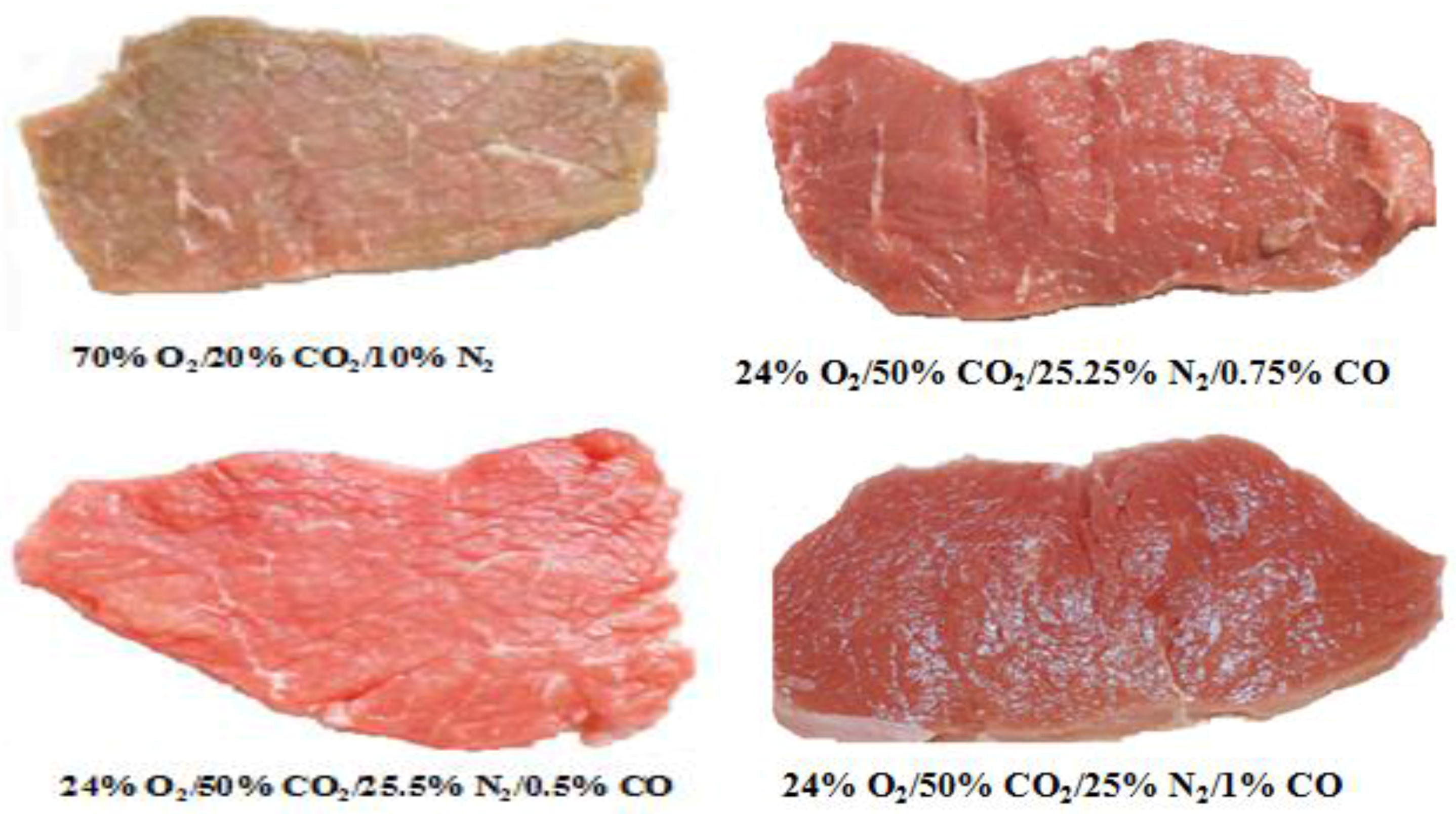
| Publication | Results/Conclusions |
|---|---|
| Luño et al. [88] | The presence of CO and 50% CO2 extends the shelf-life by inhibition of spoilage bacteria growth, delayed metmyoglobin (MetMb) formation; maintains red color and odor of fresh meat and slows down oxidative reactions. CO concentrations of 0.5–0.75% were able to extend shelf life of packaged fresh meat by 5–10 days at 1 °C. |
| Nissen et al. [89] | At 4 °C, the shelf-life of ground beef packed in MA, based on color stability and background flora development, was prolonged for the high CO2 (60%)/low CO (0.4%) mixture compared to high O2 packaging (70% O2/30% CO2), but at 10 °C (abuse temperature), the shelf-life was <8 days for both packaging methods. The growth of Y. enterocolitica and L. monocytogenes in ground beef stored in the high CO2/low CO mixture was not increased as a result of prolonging the shelf-life. However, the growth of strains of Salmonella at 10 °C in this mixture does emphasize the importance of temperature control during storage. |
| Sørheim et al. [85] | By adopting the use of CO in combination with high CO2 for meat packaging under MAP, retailers must adopt packaging systems indicating the deadlines for optimal use of the packaged product. However, as with other perishable foods in MAP, food products must be handled according to strict hygienic standards, and low storage temperatures must be maintained in a continuous chill chain. |
| Jayasingh et al. [90] | Ground beef packaged in 0.5% CO would maintain color stability for several weeks. The penetration of CO and depth of formation of COMb in the meat is dependent on the concentration of CO in the atmosphere, the time of CO exposure and the structure of the meat. For safety issue concerns, the workers were not exposed to dangerous levels of CO during MA packaging, which was verified by CO detectors. |
| Kusmider et al. [91] | The low levels of CO (<1%) incorporated into MAP will maintain a stable, cherry-red color along with extended shelf-life of irradiated ground beef during 28 days of storage, thus countering the potentially negative color effects of irradiation. |
| Krause et al. [92] | 0.5% CO significantly improved color stability and sensory attributes for both injected and non-injected pork chops. The depth (bright red band: COMb) of CO penetration from the surface increases as exposure time increases. The depth of the COMb layer steadily increased from the surface to the interior of the chops during exposure to CO. 0.5% CO packages increased in penetration depth from 5 mm on Day 1 to about 10 mm at 14 days, 15 mm at 28 days and 25 mm at Day 36. |
| John et al. [93] | Raw ground beef packaged in 80% O2 maintained desirable bright red color until 10 days, but began to darken by Day 14 and lost all red color by Day 21. However, ground beef stored in highO2-MAP was very susceptible to premature browning (PB) during cooking. PB is a food safety concern, because the cooked product appears done at temperatures where food poisoning organisms may survive. Raw ground beef held in 0.4% CO remained bright red throughout the 21 days of storage. PB and rancidity associated with ground beef packaged in highO2-MAP were prevented by packaging in 0.4% CO. |
| John et al. [94] | Premature browning and rancidity associated with beef packaged in highO2-MAP were prevented by packaging in 0.4% CO, 30.3% CO2 and 69.3% N2. |
| Mancini et al. [13] | Packaging atmospheres containing high levels of O2 promote beef bone marrow discoloration. Exclusion of O2 from MA packages and the addition of low concentrations of CO (0.4%) minimized this discoloration by limiting hemoglobin oxidation through packaging atmosphere and will promote a bright red lumbar vertebrae color for as long as 6 weeks after packaging. |
| Martínez et al. [95] | The retention of color and odor of fresh pork sausages packaged in MA was better achieved using atmospheres containing low CO2 concentrations (20%). However, increasing concentrations of CO2 (60%) promoted Mb and lipid oxidation, despite the better antimicrobial effects promoted by the high level of CO2. The atmosphere containing 0.3% CO together with 30% CO2 maintained the red color for 20 days, but failed to keep the fresh odor longer than 16 days, in agreement with its small effect on Thiobarbituric Acid Reactive Substances (TBARS) formation and microbial growth. |
| Wilkinson et al. [84] | Use of CO in MAP provides sufficient shelf-life extension of at least 8 weeks of refrigerated retail-ready pork chops in a master-packaging system. The inclusion of CO in the master-packs has not inhibited the growth of pathogenic organisms. However, given the stable fresh color of CO-treated meat and the lack of inhibition of pathogen growth by CO, there is concern that CO-MAP under certain conditions may pose a food safety risk. As such, safe refrigeration and handling must be emphasized with this type of product. |
| Wicklund et al. [96] | Chops packaged in CO-MAP were redder and darker than chops packaged in HiO2-MAP. Based on sensory attributes, the CO-MAP pork was pinker than the HiO2 pork after cooking to an internal temperature of 70 °C. CO-MAP chops also experienced less purge loss than pork in HiO2-MAP, which may have contributed to the increased juiciness perceived by the panelists. |
| Sørheim et al. [97] | CO can be used as an alternative colorant to nitrite in meat products. A gas mixture containing 1% CO was sufficient for achieving a red/pink color of cooked or fermented meat products. Sausages with CO discolored faster during air and light display than nitrite controls. However, discoloration of CO sausages was reduced by anaerobic storage in darkness, showing that absence of O2 is a necessity for optimum color formation and stability of these sausages. |
| De Santos et al. [86] | Enhanced pork chops were packaged in 0.36% CO and stored at 4 °C for 0, 12, 19 or 26 days, displayed for 2 days, then cooked to six endpoint temperatures (54, 60, 63, 71, 77 and 82 °C). As storage time increased, Pork chops packaged in CO-MAP retained their internal pink color even after cooking to 82 °C. |
| Stetzer et al. [98] | Steaks were packaged in 0.4% CO/30% CO2/69.6% N2 or 80% O2/20% CO2, stored in the dark for 12 and 26 days and placed in a lighted retail display case. Steaks were visually evaluated by trained panelists. Steaks were cooked for consumer color evaluation. CO had no effect on flavor or acceptability and minimal effects on other characteristics, such as color, sheen and purge loss. If the CO environment provides microbiological stability through storage, it can be expected that the raw product appearance will not differ from steaks in traditional HiO2-MAP. |
| Aspé et al. [99] | Beef chops (longissimus dorsi) were pre-treated with 5% CO/24 h, vacuum packed and stored at 2 °C. Chops pre-treated with CO were redder during all of the storage period than controls without CO, and microbial shelf-life was 11 weeks. The pre-treatment did not affect pH, water-holding capacity, drip loss or rancidity of the meat stored in vacuum. |
| Mantilla et al. [100] | Color stability of tilapia fillets (Oreochromis niloticus) was significantly improved by pre-mortem CO treatment (CO-euthanized tilapia). The color of CO-treated fillets was also retained during frozen storage compared to untreated fillets. Hence, pre-mortem CO treatment could be used as a valuable method for improving the color of tilapia during storage. |
| Linares et al. [101,102] | The effect of the type of stunning (electrically vs. gas), MA and their interactions on meat quality of suckling lamb of the Spanish Manchego breed was determined at 7, 14 and 21 days of storage. Stunning by CO2 gas prevented the negative effects that electrical systems have on meat quality in lamb apparent during storage. Furthermore, a low CO (30% CO2/69.3% N2/0.7% CO) level could give the best meat quality characteristics, even at 3 weeks of storage in the electrically-stunned group. In addition, in the gas-stunned group, it is possible to obtain a product of better color and more tenderness with a post-packing life of 7 days and possibly 15 days using CO in the gas mixture. |
| Grobbel et al. [103] | Steaks packaged in HiO2 MAP discolored faster and to a greater extent than steaks packaged by vacuum package (VP) or ultra-low O2 with CO (ULO2CO) MAP. Non-enhanced muscles packaged by VP and ULO2CO MAP had more stable display color and very desirable tenderness and flavor compared with those packaged in HiO2 (80% O2/20% CO2). |
| Ramamoorthi et al. [104] | The combined irradiation with CO-MAP showed that, after 14 days of storage, aerobically-packaged beef was visually greener and less red than CO-MAP packaged beef. CO-MAP preserved color until 21 days of storage. CO-MAP could be also used to preserve color of beef irradiated at sufficient doses (~2 kGy) to reduce microbial loads to safe levels during 28 days of storage. |
| Mancini et al. [105] | Packaging steaks in CO (0.4% CO/30% CO2/69.6% N2) did not counteract the darkening effects of lactate enhancement. Nevertheless, CO improved color stability of beef steaks compared with high-oxygen packaging (80% O2/20% CO2). |
| Fontes et al. [106] | Fresh blood saturation with CO produces a dried blood of a pleasant pinkish-red color after 12 weeks of storage when packed in low O2 transmission rates (OTR) bags, with great potential as an additive in meat product formulations. |
| Raines and Hunt [107] | Increased CO concentration in combination with reduced headspace volume has a greater influence on COMb development. Smaller headspaces with higher concentrations of CO (i.e., 0.8% vs. 0.4% CO) optimize the package size while maintaining or improving the appearance of beef packaged in CO-MAP without compromising consumer safety. This would result in greater efficiency of case-ready meat distribution, making the CO-MAP system more economically feasible and advantageous. |
| Jeong and Claus [108] | The color of CO-packaged ground beef upon opening the package deteriorated with display time and became less red. However, the initial rate of color deterioration was faster in vacuum-packaged ground beef when it was opened compared to CO-MAP-packaged product. When a CO-packaged product is opened, this color deterioration would provide consumers with a visual indicator of freshness. |
| Bjørlykke et al. [109] | CO could increase animal welfare when used to slaughter salmon or other fish. Exposure of fish to CO also could improve the quality of products. |
| Suman et al. [110] | The incorporation of chitosan increased the interior redness of ground beef patties stored in CO-MAP (0.4% CO + 19.6% CO2 + 80% N2). This incorporation was also minimizes premature browning (PB) in patties stored under CO-MAP systems instead of under high-O2 MAP. |
| Ramamoorthi et al. [111] | Use of CO in MAP gasses has the potential to allow beef subjected to low doses of irradiation to retain its color. |
| Pivarnik et al. [112] | Filtered smoke (FS) presumably containing high % CO has been used to preserve taste, texture and/or color in tuna (Thunnus albacares). Therefore, a general statement indicating that FS treatments would extend shelf-life of tuna in the studied ways of storage: room temperature (21–22 °C), refrigerated (4–5 °C) and iced (0 °C). |
| Venturini et al. [113] | Packaging under 0.2% CO increased the color stability of beef steaks and ground beef for 28 days at 1 °C, even with residual O2 concentrations that are considered excessive for anaerobic packaging systems (above 0.1%). After 28 days of storage under CO-MAP and 24 h of air exposure, beefsteaks and ground beef maintained an acceptable appearance and a visual color similar or superior to that of fresh meat. However, after 24 h of air exposure, both the appearance and the smell of steaks and ground beef were considered “slightly unpleasant”. |
| Lavieri and Williams [114] | The CO-MAP (0.4% CO) treatment had no effect on maintaining the COMb “cherry red” fresh meat color during meat spoilage. No potential health hazards or deceptions were revealed due to simultaneous onset of spoilage and the presence of COMb “cherry red” fresh meat pigment in the CO-MAP. The CO absorbed in the meat ranged from 0.22–0.46 ppm CO/g of meat on Day 0 and increased to 2.08–2.40 ppm CO/g of meat on Day 25. The maximum level of CO detected in the meat in this study was below the Environmental Protection Agency (EPA) National Ambient Air Quality Standard of 9 ppm. |
| Liu et al. [115] | The CO-MAP (0.4% CO/30% CO2/69.6% N2) significantly increased red color stability of all muscles. Steaks in CO-MAP maintained a higher MetMb reducing activity (MRA) compared with those in HiO2-MAP during storage. After opening packages, the red color of steaks in CO-MAP deteriorated more slowly compared with that of steaks in HiO2-MAP. |
| Concollato et al. [116] | CO-treated fish resulted in an earlier onset of rigor mortis, lower final post-mortem muscle pH and higher drip loss after filleting. The assimilation of CO by Atlantic salmon’s muscles, through injection in the water, slightly increased lightness (L*) and yellowness (b*) values, limited however to the fresh samples. No significant difference in redness (a*) at any considered time was found between CO and the control group, probably because of the content of astaxanthin that may have minimized the color differences amongst the different groups. |
| Rogers et al. [117] | CO-MAP (0.4% CO, 30% CO2, 69.6% N2) exhibited more desirable color and consumer acceptability throughout lighted retail display of ground beef during 20 days. |
| Pereira et al. [118] | Addition of CO-treated blood allows the production of better-colored sausages (mortadella) having lower residual nitrite levels. |
| Fontes et al. [119] | Saturated porcine blood with CO (99%) could substitute meat by up to 20% for mortadella’s processing. Therefore, from the nutritional point of view, meat replacement with up to 20% of CO-treated blood is nutritionally adequate for being used in sausage production. |
| Yang et al. [120] | Aerobically-packaged beef steaks exhibited a bright-red color at the first 4 days. However, discoloration and oxidation became major factors limiting their shelf-life to 8 days. Compared with aerobic packaging, VP extended shelf-life of beef steaks, due to better color stability, together with lower oxidation and microbial populations. Among all packaging methods, CO-MAP (0.4% CO + 30% CO2 + 69.6% N2) had the best preservation for steaks, with more red color than other packaging types. |
| Sakowska et al. [121] | The raw steaks’ CO penetration depth increased as exposure times and CO concentration in gas mixtures increased. However, the COMb that formed did not always turn brown during thermal treatment. In cooked samples treated with 0.3% and 0.5% CO-MAP, a red COMb border was visible at the cross-section, whereas other CO packaging treatments had partial or total browning. To create a red color in raw beef and avoid a red boarder in cooked beef, up to 0.5% CO in VP and only 0.1% for MAP can be recommended. |
| Lyu et al. [122] | The pretreatment of CO combined with O3 at certain concentrations can be a promising technique to maintain the quality of beef meats under vacuum during storage. |
| Van Rooyen et al. [123] | The addition of CO pre-treatments prior to VP may be beneficial to allow a desirable color to be induced while allowing aging to occur within the package and increase meat tenderness. The 5-h CO pretreatment exposure time achieved the desirable color, and discoloration reached unacceptable levels by the use-by date. Therefore, applying 5% CO pretreatments may be a potential solution to current packaging issues within the meat sector for safety and enhancing meat quality. In addition, this anoxic packaging technology should prevent any negative quality issues related to high O2-MAP packaging. |
| Sakowska et al. [124] | Using CO significantly increased the brightness and the redness of beef steaks in both CO-vacuum packaging and CO-MAP systems during storage for 21 days. They evaluated the effects of 0.5% CO exposure in two MAP (0.5% CO + 30% CO2 + 69.5% N2), as compared with conventional VP, on the quality of packaged beef steaks stored for 21 days at 2 °C. The consumers have the greatest desire to purchase the vacuum-packed steaks after exposure in CO. |
| Van Rooyen et al. [125] | CO as a pretreatment applied prior to VP or VSP may play an important role in overcoming some of the challenges the meat industry faces. This technology provides a prolonged storage, improves the tenderness of the meat and prevents the negative problems associated with other packaging technologies (reduces the risk of the cross-linking/aggregation of myosin due to Hi-O2 MA, decreases energy usage, storage facilities and distribution costs). |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djenane, D.; Roncalés, P. Carbon Monoxide in Meat and Fish Packaging: Advantages and Limits. Foods 2018, 7, 12. https://doi.org/10.3390/foods7020012
Djenane D, Roncalés P. Carbon Monoxide in Meat and Fish Packaging: Advantages and Limits. Foods. 2018; 7(2):12. https://doi.org/10.3390/foods7020012
Chicago/Turabian StyleDjenane, Djamel, and Pedro Roncalés. 2018. "Carbon Monoxide in Meat and Fish Packaging: Advantages and Limits" Foods 7, no. 2: 12. https://doi.org/10.3390/foods7020012
APA StyleDjenane, D., & Roncalés, P. (2018). Carbon Monoxide in Meat and Fish Packaging: Advantages and Limits. Foods, 7(2), 12. https://doi.org/10.3390/foods7020012






